Transcriptional Profiling and Molecular Characterization of the yccT Mutant Link: A Novel STY1099 Protein with the Peroxide Stress Response and Cell Division of Salmonella enterica Serovar Enteritidis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids and Growth Conditions
2.2. Gene Expression Assay
2.3. Construction of ∆yccT, ∆napB, ∆gutM and ∆ahpCF Salmonella enterica Enteritidis Strains
2.4. Oxidative Stress Killing Assay
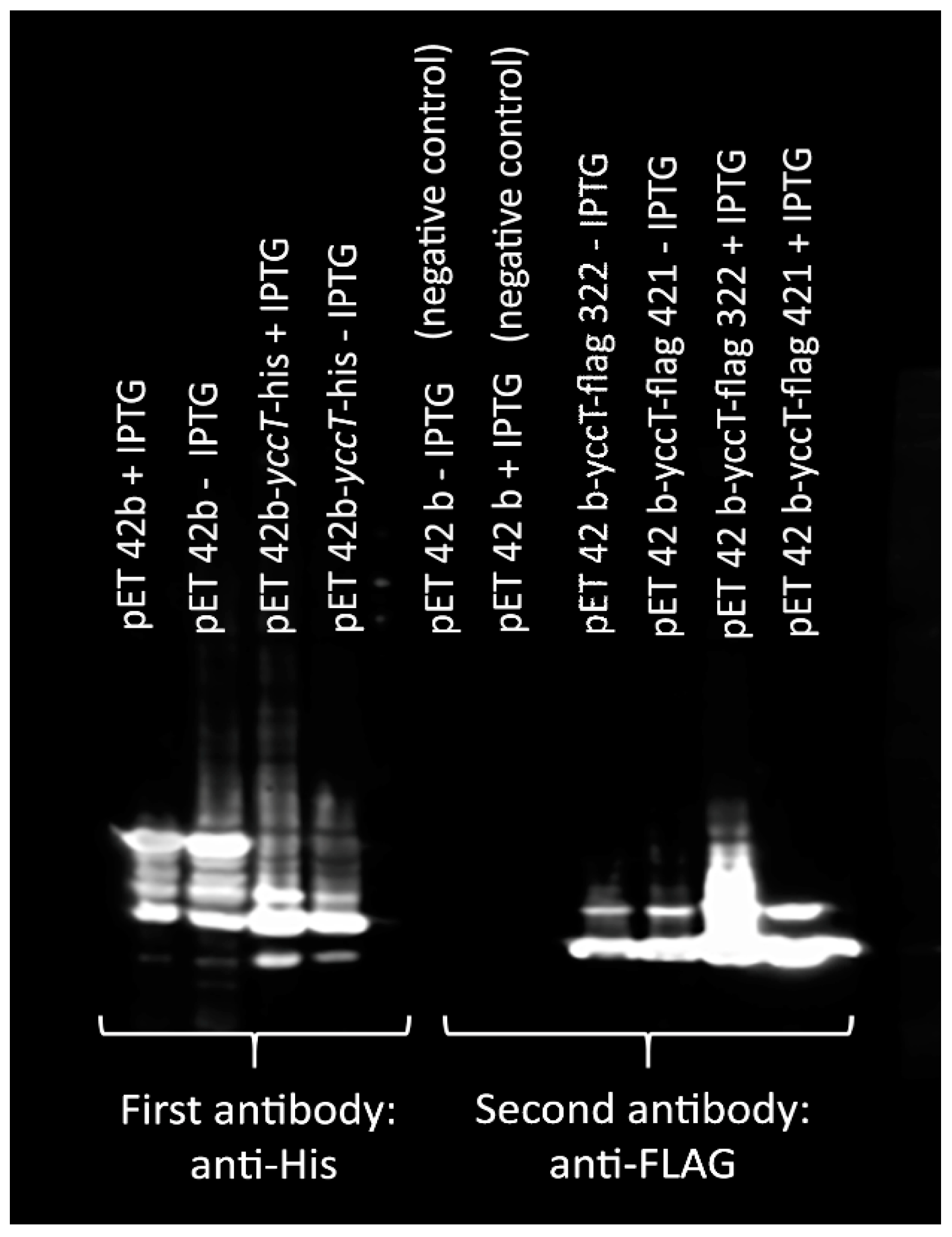
2.5. Complementation Study
2.6. Preparation of Samples for RNA Extraction
2.7. RNA-Seq Analysis
2.8. Real-Time PCR
2.9. Subcellular Localization of STY1099
2.10. Co-Immunoprecipitation of STY1099
2.11. Experimental Replications and Bioinformatics
3. Results
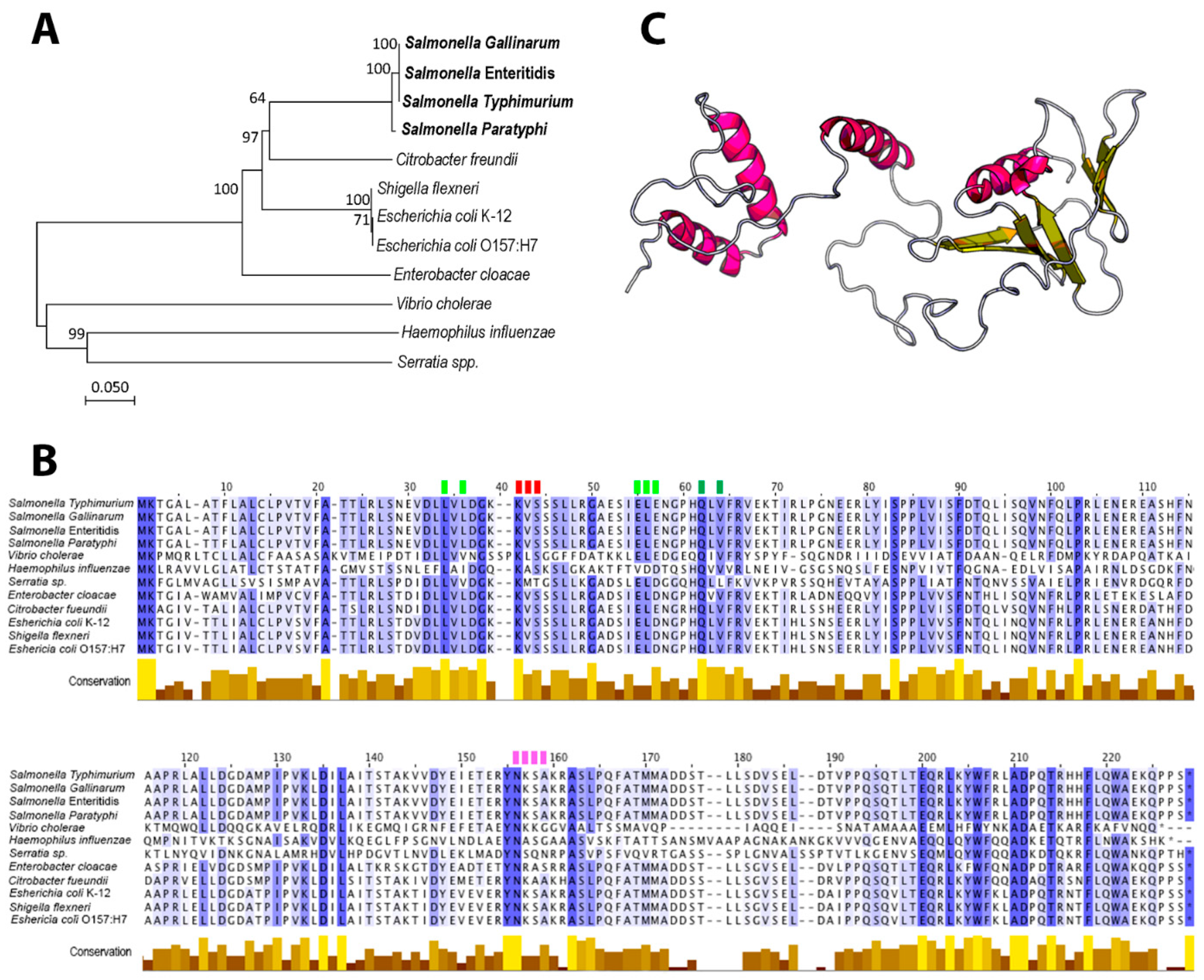
3.1. Phylogeny, Conserved Domains and Structural Characteristics of STY1099 Protein
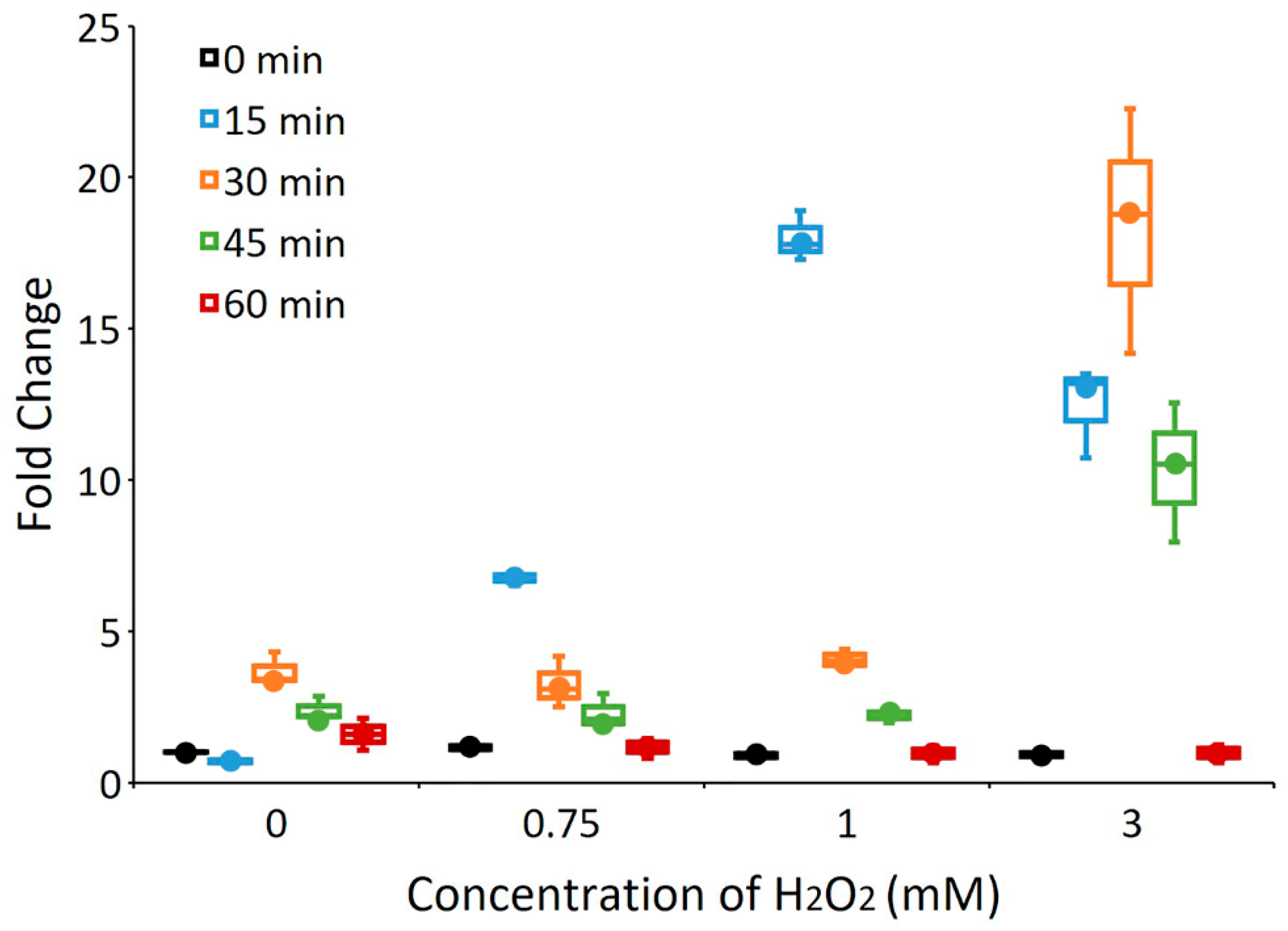
3.2. The yccT Gene Is Highly Inducible upon Exposure to Hydrogen Peroxide, But Not to Parquet
3.3. The ∆yccT Mutant Exhibits Increased Sensitivity to Hydrogen Peroxide
3.4. Subcellular Localization of STY1099
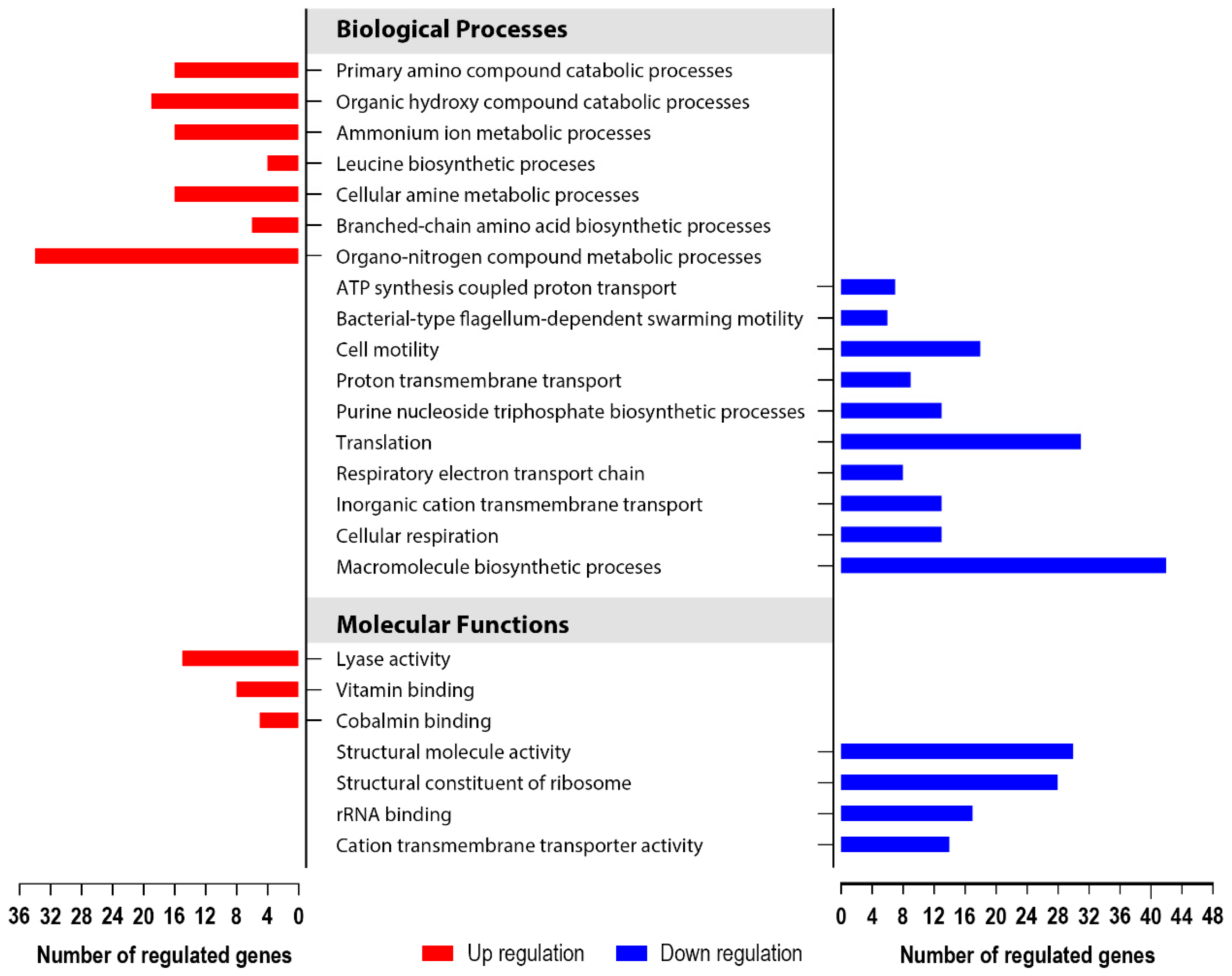
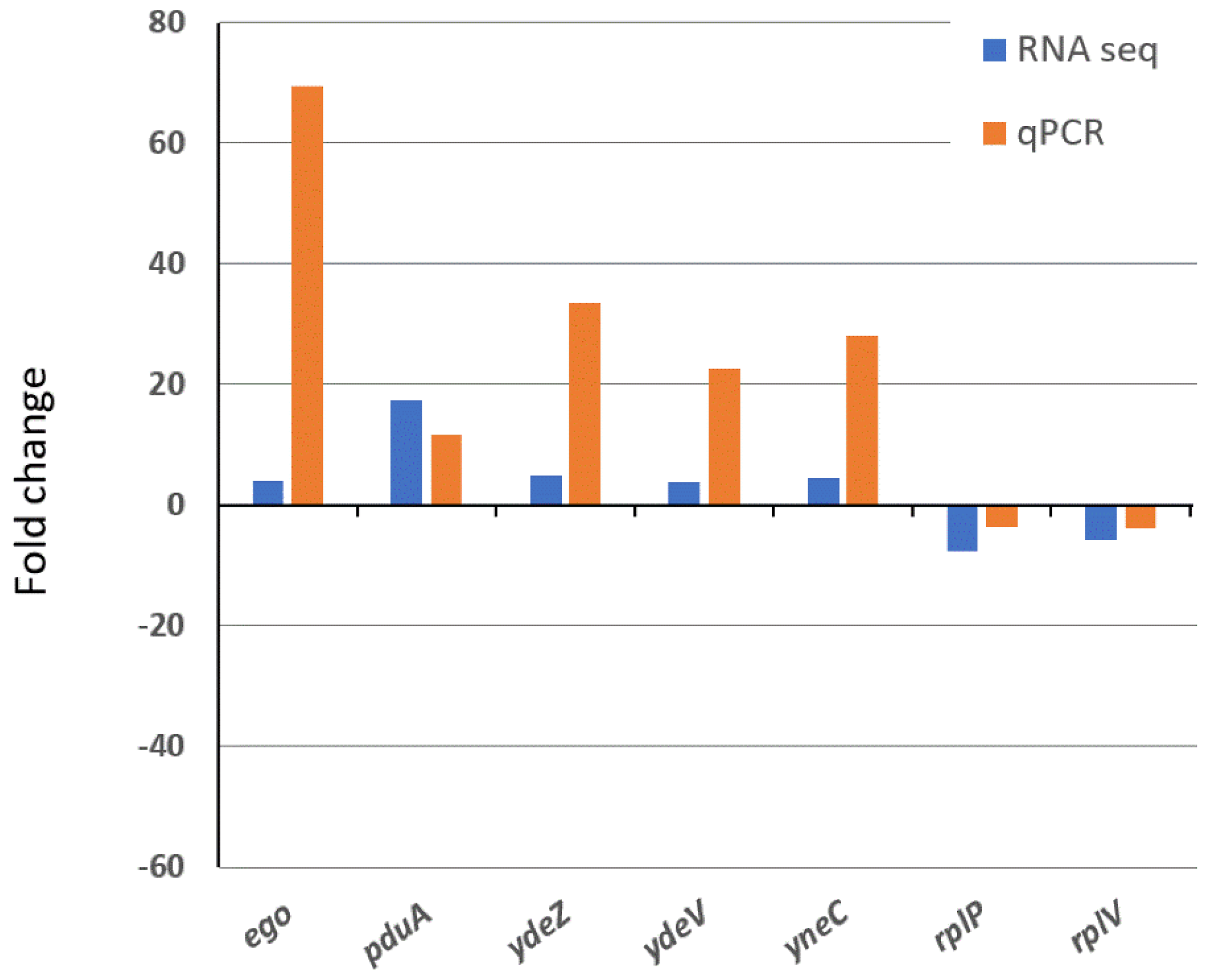
3.5. Global Transcriptomics of the S. enterica Enteritidis ∆yccT Mutant Strain Reveal the STY1099 Regulon
3.6. Determination of STY1099 Interactome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States–major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- de Moraes, M.H.; Desai, P.; Porwollik, S.; Canals, R.; Perez, D.R.; Chu, W.; McClelland, M.; Teplitski, M. Salmonella persistence in tomatoes requires a distinct set of metabolic functions identified by transposon insertion sequencing. Appl. Environ. Microbiol. 2017, 83, e03028-16. [Google Scholar] [CrossRef]
- Parker, W.F.; Mee, B.J. Survival of Salmonella adelaide and fecal coliforms in coarse sands of the swan costal plain, western Australia. Appl. Environ. Microbiol. 1982, 43, 981–986. [Google Scholar] [PubMed]
- Mezrioui, N.; Baleux, B.; Trousselier, M. A microcosm study of the survival of Escherichia coli and Salmonella Typhimurium in brackish water. Water Res. 1995, 29, 459–465. [Google Scholar] [CrossRef]
- Fang, F.C.; Frawley, E.R.; Tapscott, T.; Vázquez-Torres, A. Bacterial stress responses during host infection. Cell Host Microbe 2016, 20, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Mangalappalli-Illathu, A.K.; Vidovic, S.; Korber, D.R. Differential adaptive response and survival of Salmonella enterica serovar Enteritidis planktonic and biofilm cells exposed to benzalkonium chloride. Antimicrob. Agents Chemother. 2008, 52, 3669–3680. [Google Scholar] [CrossRef]
- Farr, S.B.; Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella Typhimurium. Microbiol. Rev. 1991, 55, 561–585. [Google Scholar] [PubMed]
- Aslund, F.; Zheng, M.; Beckwith, J.; Storz, G. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc. Natl. Acad. Sci. USA 1999, 96, 6161–6165. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Templeton, L.J.; Smulski, D.R.; Larossa, R.A.; Storz, G. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 2001, 183, 4562–4570. [Google Scholar] [CrossRef]
- Altuvia, S.; Weinstein-Fischer, D.; Zhang, A.; Postow, L.; Storz, G. A small, stable RNA induced by oxidative stress: Role as a pleiotropic regulator and antimutator. Cell 1997, 90, 43–53. [Google Scholar] [CrossRef]
- Wu, J.; Weiss, B. Two divergently transcribed genes, soxR and soxS, control a superoxide response regulon in Escherichia Coli. J. Bacteriol. 1991, 173, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Pomposiello, P.J.; Demple, B. Redox-operated genetic switches: The SoxR and OxyR transcription factors. Trends Biotechnol. 2001, 19, 109–114. [Google Scholar] [CrossRef]
- Vidovic, S.; Korber, D. Escherichia coli O157: Insights into the adaptive stress physiology and the influence of stressors on epidemiology and ecology of this human pathogen. Crit. Rev. Microbiol. 2016, 42, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protocol. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Thu, Y.M.; Riper, S.K.V.; Higgins, L.A.; Zhang, T.; Becker, J.R.; Markowski, T.W.; Nguyen, H.D.; Griffin, T.J.; Bielinsky, A.K. Slx5/Slx8 promotes replication stress tolerance by facilitating mitotic progression. Cell Rep. 2016, 15, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- McWilliam, H.; Li, W.; Uludag, M.; Squizzato, S.; Park, Y.M.; Buso, N.; Cowley, A.P.; Lopez, R. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013, 41, W597–W600. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics 2014, 25, 1189–1191. [Google Scholar] [CrossRef]
- Kallberg, M.; Wang, H.; Wang, S.; Peng, J.; Wang, Z.; Lu, H.; Xu, J. Template-based protein structure modeling using the RaptorX web server. Nat. Protocol. 2012, 7, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protocol. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Armenteros, J.J.A.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Vidovic, S.; Elder, J.; Medihala, P.; Lawrence, J.R.; Predicala, B.; Zhang, H.; Korber, D.R. ZnO nanoparticles impose a panmetabolic toxic effect along with strong necrosis, inducing activation of the envelope stress response in Salmonella enterica serovar Enteritidis. Antimicrob. Agents Chemother. 2015, 59, 3317–3328. [Google Scholar] [CrossRef]
- Alhasawi, A.; Auger, C.; Appanna, V.P.; Chahma, M.; Appanna, V.D. Zinc toxicity and ATP production in Pseudomonas fluorescens. J. Appl. Microbiol. 2014, 117, 65–73. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced antimicrobial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Stocks, C.J.; Phan, M.D.; Achard, M.E.S.; Nhu, N.T.K.; Condon, N.D.; Gawthorne, J.A.; Lo, A.W.; Peters, K.M.; McEwan, A.G.; Kapetanovic, R.; et al. Uropathogenic Escherichia coli employs both evasion and resistance to subvert innate immune-mediated zinc toxicity for dissemination. Proc. Natl. Acad. Sci. USA 2019, 116, 6341–6350. [Google Scholar] [CrossRef]
- Ong, C.I.Y.; Walker, M.J.; McEwan, A.G. Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Sci. Rep. 2015, 5, 10799. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivovis, N.; Djediat, S.; Benedetti, M.C.; Fievet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Sparacino-Watkins, C.; Stolz, J.F.; Basu, P. Nitrate and periplasmic nitrate reductases. Chem. Soc. Rev. 2014, 43, 676–706. [Google Scholar] [CrossRef]
- Cruz-Garcia, C.; Murray, A.E.; Klappenbach, J.A.; Stewart, V.; Tiedje, J.M. Respiratory nitrate ammonification by Shewanella oneidensis MR-1. J. Bacteriol. 2007, 189, 656–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegge, C.S.; Brondsted, L.; Li, Y.P.; Bang, D.D.; Ingmer, H. Energy taxis drives Campylobacter jejuni toward the most favorable conditions for growth. Appl. Environ. Microbiol. 2009, 75, 5308–5314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaguchi, M.; Wittmann, H.G.; Cabezon, T.; DeWilde, M.; Villarroel, R.; Herzog, A.; Bollen, A. Alteration of ribosomal protein S17 by mutation linked to neamine resistance in Escherichia coli. II. Localization of the amino acid replacement in protein S17 from a meaA mutant. J. Mol. Biol. 1976, 104, 617–620. [Google Scholar] [CrossRef]
- Takyar, S.; Hickerson, R.P.; Noller, H.F. mRNA helicase activity of the ribosome. Cell 2005, 120, 49–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, M.; Zavialov, A.; Sengupta, J.; Rawat, U.; Ehrenberg, M.; Frank, J. Locking and unlocking of ribosomal motions. Cell 2003, 114, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Jeter, R.M. Cobalmin-dependent 1,2-propanediol utilization by Salmonella Typhimurium. J. Gen. Microbiol. 1990, 136, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2015, 44, D471–D480. [Google Scholar] [CrossRef]
- Fu, J.; Qi, L.; Hua, M.; Liu, Y.; Yu, K.; Liub, Q.; Liu, X. Salmonella proteomics under oxidative stress reveals coordinated regulation of antioxidant defense with the iron metabolism and bacterial virulence. J. Proteom. 2017, 157, 52–58. [Google Scholar] [CrossRef]
- Shimada, T.; Takada, H.; Yamamoto, K.; Ishihama, A. Expended roles of two-component response regulator OmpR in Escherichia coli: Genomic SELEX search for novel regulation targets. Genes Cells 2015, 20, 915–931. [Google Scholar] [CrossRef]



| Primer Name | Sequence (5′–3′) |
|---|---|
| Primers for yccT, napD, gutM, ahpC and ahpF Deletion | |
| yccT: Forward | CTT CCT TGC TCT CTG TTT GCC GGT GAC TGT TTT TGC CAC AAC GCT CCG TTT GTA GGC TGG AGC TGC TTC G |
| yccT: Reverse | CCC ATT GCA GAA AAT GAT GGC GTG TCT GCG GGT CGG CCA GTC GGA ACC AAC ATA TGA ATA TCC TCC TTA G |
| napD: Forward | ATG CGC ACT AAC TGG CAG GTC TGT AGC CTG GTC GTG CAG GCC AAA AGT CAT GTA GGC TGG AGC TGC TTC G |
| napD: Reverse | TCA TGG TGT TTC CTC ACC TTG CTC ATC CTG CTG GTG ATA AAC CAG CGA CAC ATA TGA ATA TCC TCC TTA G |
| gutM: Forward | ATG GTT TCC ACT CTG ATT ACC GTC GCC GTT ATC GCC TGG TGT GCG CAA CTT GTA GGC TGG AGC TGC TTC G |
| gutM: Reverse | TTA CCC ATG TTT CAG TTT AAG CGC CAA TGA TAG TGC ATT CTG CGC GAG CGC ATA TGA ATA TCC TCC TTA G |
| ahpC: Forward | ATG TCC TTA ATT AAC ACC AAA ATC AAA CCT TTC AAA AAC CAG GCG TTC AAT GTA GGC TGG AGC TGC TTC G |
| ahpC: Reverse | TTA GAT TTT ACC GAC CAG GTC TAA AGA TGG AGC CAG AGT CGC TTC GCC TTC ATA TGA ATA TCC TCC TTA G |
| ahpF: Forward | ATG CTC GAC ACA AAT ATG AAA ACC CAG CTC AGG GCT TAC CTT GAG AAA CTT GTA GGC TGG AGC TGC TTC G |
| ahpF: Reverse | TTA TGC GAT TTT GGT ACG AAT CAG ATA ATC AAA GGC GCT CAA CGA GGC TTC ATA TGA ATA TCC TCC TTA G |
| Primers for Conformation of yccT, napD, gutM, ahpC and ahpF gene Deletions | |
| yccT mutant F | AAA GTG TAC GAC AAA CCT GAC A |
| yccT mutant R | TGA GCA ACC AAC GCG ATA TGT T |
| napD mutant F | GCT GCC AGG ACA GTT GTG AAC C |
| napD mutant R | CCG TTC CGC AAA AAC GGC ACG G |
| gutM mutant F | TTT ATG CCA GCC CGA AAG CGT C |
| gutM mutant R | GCA TGT GCG TTG GCT TTC CTC G |
| ahpC mutant F | ACT TTA GAT GGC TGA CAG GGC GCA |
| ahpC mutant R | CGC GGG TGC GCC CAT GAC TGA AAC |
| ahpF mutant F | GCC GCC TTA CTC TGA CGT GAA ATA |
| ahpF mutant R | TTA ACA GAC CGT TTC AGA GTA TTG |
| Primers for yccT Mutant Complementation a | |
| yccT-F | TCA CCC GGG ATT CGA TTC CGT AGT TAA CCT |
| yccT-R | GCG GTC GAC AGC AAA ACG CCA AAA TAT GTC |
| Biological Processes and Genes | Locus Tag | Protein Description | Fold Change | FDR p-Value 1 |
|---|---|---|---|---|
| Citrate metabolism | ||||
| citE | SEN0591 | Citryl-CoA lyase | 2.67 | 0.0018 |
| citF | SEN0590 | Citrate CoA-transferase | 4.18 | 0.0307 |
| citT | SEN0587 | Citrate carrier | 2.55 | 0.0413 |
| Oxidoreductase activity/electron transport | ||||
| hycD | SEN2692 | Respiratory-chain NADH dehydrogenase | 2.35 | 0.0274 |
| hycF | SEN2690 | Formate hydrogenlyase complex iron-sulfur | 2.64 | 0.0274 |
| dmsA3 | SEN1552 | Dimethyl sulfoxide reductase | 3.87 | 0.0274 |
| SEN1249 | SEN1249 | Hydrogenases b-type cytochrome | 2.12 | 0.0448 |
| SEN3184 | SEN3184 | Na+-transporting methylmalonyl-CoA/oxaloacetate decarboxylase | 2.06 | 0.0317 |
| rRNA processing/stress response | ||||
| SEN0992 | SEN0992 | Oligogalacturonate-specific porin | 4.66 | 0.0274 |
| rrmJ | SEN3130 | 23S rRNA methyltransferase J | 2.12 | 0.0378 |
| Gluconate transmembrane transport | ||||
| kdgT | SEN0166 | 2-keto-3-deoxygluconate permease | 5.33 | 0.0045 |
| Nitrate metabolism | ||||
| narJ | SEN1277 | Nitrate reductase | −16.96 | 0.0162 |
| narH | SEN1276 | 4Fe–4S ferredoxin, respiratory nitrate reductase | −15.31 | 0.0178 |
| narI | SEN1278 | Nitrate reductase, gamma subunit | −11.24 | 0.0178 |
| narG | SEN1275 | Respiratory nitrate reductase, subunit alpha | −10.98 | 0.0162 |
| narK | SEN1274 | Nitrite extrusion protein | −7.48 | 0.0162 |
| nirC | SEN3303 | Nitrite transporter | −3.58 | 0.0274 |
| Unknown | ||||
| SEN0167 | SEN0167 | Hypothetical protein | 4.75 | 0.0274 |
| SEN0271 | SEN0271 | Hypothetical protein | 2.33 | 0.0306 |
| yycT2 | SEN0942 | STY1099 | −1525.68 | 9.0780 × 10−56 |
| SEN1163 | SEN1163 | Phage membrane protein | −8.98 | 0.0008 |
| SEN0541 | SEN0541 | Protein of unknown function DUF1471 | −2.31 | 0.0274 |
| Biological Processes and Protein Names | Accession Number | Identification Probability (%) | Molecular Weight | Identified Unique Peptide Sequences |
|---|---|---|---|---|
| Cell Division and Shape | ||||
| Septum site-determining protein MinD | YP_005216705.1 | 100 | 29,509.3 | ASNQGEPVILDATADAGK; AYADTVDR; IIVVTSGK; IKLVGVIPEDQSVLR; LVGVIPEDQSVLR; TENLFILPASQTR; TTSSAAIATGLAQK |
| Cell division protein FtsH | ZP_12129674.1 | 100 | 70,784.5 | QKLESQISTLYGGR; QVVVGLPDVR |
| Rod shape-determining protein MreB | ZP_11739057.1 | 100 | 36,934.4 | DGVIADFFVTEK; GMVLTGGGALLR; IKHEIGSA YPGDEVR; IKHEIGSAYPGD EVREIEVR; NLAEG VPR; NYGSLIGEATAER; RNYGSLIGEATAER; VL VCVPVGATQVER |
| Oxidoreductase Activity/Electron Transport | ||||
| Biotin carboxylase of acetyl-CoA carboxylase | ZP_12119137.1 | 97.0 | VVEEAPAPGITPELRR; YLENPR | |
| Multifunctional fatty acid oxidation complex subunit alpha | ZP_15867656.1 | 100 | 79,596.2 | DFSDDEIIAR; KEEDAAVDDLLASVSQTKR; QAI TGDLDWR |
| Glutamate dehydrogenase | ZP_15809056.1 | 100 | 45,978.8 | CAALNLPYGGAK; LFAGAGAR; RANIAVEGAR; TAAYIVACER; VAVQGFGNVGSEAAR |
| Precorrin-4 C11-methyltransferase | ZP_11737843.1 | 100 | 28,358.5 | EQGEELTR; GTLADISDKVR; LQTGDVSLYGSVR |
| Short chain dehydrogenase | ZP_13073001.1 | 100 | 27,852.2 | ADVRDFASVQAAVAR; AKETEGRIDILVNNAG VCR; SLAVEYAQSGIR; TALITGASQGIGEGIAR; TPMAESIAR; VNAICPGYVR |
| Aminoacyl-histidine dipeptidase | YP_005235990.1 | 100 | 52,419.9 | EAVPAGFACFK; FLAGHAEELDLR; LLNATPNGVIR |
| Cytochrome d terminal oxidase subunit 1 | ZP_15859114.1 | 99.9 | 58,265.5 | AYELLEQLR; DLGYGLLLKR |
| Anaerobic glycerol-3-phosphate dehydrogenase subunit A | YP_005397674.1 | 100 | 57,893.3 | ACEAAGIR; AEAIDPQQAR; EGATVCGVHVR; H DIATGATGR; HGDRTPGWLSEGR; IAEYADLSI R; IISLPAPLR; INQHVINR |
| Bifunctional acetaldehyde-CoA/alcohol dehydrogenase | ZP_15844816.1 | 96.9 | 96,199.8 | AVTNVAELNALVER; AAALAAADAR |
| Transcription and Translation | ||||
| 30S ribosomal protein S3 | ZP_15868039.1 | 100 | 25,965.5 | EFADNLDSDFKVR; EGRVPLHTLR; GIKVEVSGR; IVIERPAK; KPELDAK; KVEVSGR; KVVADIAGPAQINIAEVR; LGGAEIAR; LVADSITSQLER; VPLH TLR |
| DNA-binding transcriptional regulator PhoP | ZP_15835390.1 | 99.7 | 25,483.5 | IQAQYPHDVITTVR; VLVVEDNALLR |
| 30S ribosomal protein S9 | YP_002638940.1 | 100 | 14,790.5 | AENQYYGTGR; GGGISGQAGAIR; SLEQYFGR |
| 30S ribosomal protein S11 | NP_462321.1 | 100 | 13,812.8 | ALNAAGFR; CADAVKEYGIK; STPFAAQVAAER |
| LSU ribosomal protein L6p | ZP_12153653.1 | 100 | 18,841.3 | APVVVPAGVDVK; DGYADGWAQAGTAR; GA DKQVIGQVAADLR; KLQLVGVGYR; YADEVVR |
| 30S ribosomal protein S4 | ZP_09771019.1 | 99.6 | 23,467.7 | AALELAEQR; LSDYGVQLR; VKAALELAEQR |
| Threonyl-tRNA synthetase | YP_002226745.1 | 100 | 73,887.2 | ALNAYLQR; IYGTAWADKK; LSASYVGEDNER; PVITLPDGSQR |
| 50S ribosomal protein L5 | YP_005183280.1 | 100 | 20,300.6 | GLDITITTTAK; ITLNMGVGEAIADKK; LITIAVPR; QGYPIGCK |
| cAMP-regulatory protein | ZP_09770968.1 | 100 | 23,595.9 | QEIGQIVGCSR; VGNLAFLDVTGR |
| 50S ribosomal protein L21 | NP_457683.1 | 95.7 | 11,560 | MYAVFQSGGK; VSEGQTVR |
| Stress Response | ||||
| ATP-dependent protease ATP-binding subunit ClpX | ZP_15869131.1 | 100 | 46,159.1 | KHPQQEFLQVDTSK; LLYCSFCGK; SNILLIGPT GSGK |
| Two-component response regulator OmpR | YP_005215137.1 | 99.8 | 27,317.4 | SIDVQISR; SVANAEQMDR |
| DNA-binding ATP-dependent protease La | YP_002225562.1 | 100 | 87,395.7 | DIHVHVPEGATPK; LGINPDFYEKR |
| DnaK protein (heat shock protein 70) | YP_005211303.1 | 99.9 | 69,241.2 | FQDEEVQR; RFQDEEVQR |
| Carbohydrate Metabolism | ||||
| Phosphoenolpyruvate synthase | ZP_15868324.1 | 100 | 87,132.5 | AAAIVTNR; AVIEELAR; IEDVPQQQR |
| Dihydrolipoamide succinyltransferase | ZP_15861596.1 | 100 | 43,825.8 | APAVEPAAQPALGAR; LLAEHNLEASAIKGTGV GGR; QYGEVFEKR |
| Phosphoenolpyruvate carboxylase | YP_005214624.1 | 100 | 99,020.2 | GEAASNPEVIAR; GGAPAHAALLSQPPGSLK; H VLLLSR |
| Carbohydrate Metabolism Continued | ||||
| S-adenosylmethionine synthetase | ZP_09770040.1 | 100 | 42,437.9 | HGGGAFSGKDPSKVDR; KIIVDTYGGMAR; QSPDINQGVDR; TDKAQLLR |
| Amino Acid Metabolism | ||||
| Carbamate kinase | ZP_11788746.1 | 100 | 33,332.7 | DHLVICNGGGGVPVVEK; KNIELAAR; NHLPER; RGEPLEADIQRK; RIVENDAIR; TLVVALGGNALLK; VTACAEFVSHCR; YIGPIYDEAQAR |
| Glutamate-1-semialdehyde aminotransferase | YP_002242369.1 | 100 | 45,417.4 | ELIPGGVNSPVR; HTLTCTYNDLTSVR; NAVIEA AER; SKSENLYSAAR |
| Arginine deiminase | ZP_15827530.1 | 99.4 | 45,571.6 | AGEEHDIFANTLR; LGPTFAADIR |
| Lipid Metabolism | ||||
| 3-oxoacyl-(acyl carrier protein) synthase II | ZP_11731244.1 | 98.6 | 41,678.9 | ASTPLGVGGFGAAR; SVFGDAASR |
| Cellular Transport | ||||
| Maltose/maltodextrin transporter ATP-binding protein | ZP_14274887.1 | 100 | 40,780.7 | FVAGFIGSPK; MNDIPPAER; MNFLPVK; QQI WLPVESR; RLHQEPGV; TLVAEPR; VAQVGKPL ELYHYPADR; VTATAIEQVQVELPNR |
| Oligopeptide ABC transporter ATP-binding protein OppF | ZP_15831204.1 | 100 | 37,252.3 | HAVSCLKVDPL; LYEGETLGVVGESGCGK; VGLLPNLINR |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidovic, S.; Liu, X.; An, R.; Mendoza, K.M.; Abrahante, J.E.; Johny, A.K.; Reed, K.M. Transcriptional Profiling and Molecular Characterization of the yccT Mutant Link: A Novel STY1099 Protein with the Peroxide Stress Response and Cell Division of Salmonella enterica Serovar Enteritidis. Biology 2019, 8, 86. https://doi.org/10.3390/biology8040086
Vidovic S, Liu X, An R, Mendoza KM, Abrahante JE, Johny AK, Reed KM. Transcriptional Profiling and Molecular Characterization of the yccT Mutant Link: A Novel STY1099 Protein with the Peroxide Stress Response and Cell Division of Salmonella enterica Serovar Enteritidis. Biology. 2019; 8(4):86. https://doi.org/10.3390/biology8040086
Chicago/Turabian StyleVidovic, Sinisa, Xiaoying Liu, Ran An, Kristelle M. Mendoza, Juan E. Abrahante, Anup K. Johny, and Kent M. Reed. 2019. "Transcriptional Profiling and Molecular Characterization of the yccT Mutant Link: A Novel STY1099 Protein with the Peroxide Stress Response and Cell Division of Salmonella enterica Serovar Enteritidis" Biology 8, no. 4: 86. https://doi.org/10.3390/biology8040086





