Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Physical Exercise Sessions
2.3. Lactate Analysis
2.4. Evaluation of Glycogen Content
2.5. Measurement of Reduced Thiol Residues of Total Protein
2.6. Gene Expression by Quantitative PCR
2.7. Antioxidant Enzyme Activity
2.8. NADPH Oxidase Activity
2.9. Statistical Analyses
3. Results
3.1. Strenuous One-Session Exercise
3.2. Biochemical Characterization
3.2.1. Lactate Analysis
3.2.2. Muscle Glycogen Content
3.3. Thiol Content
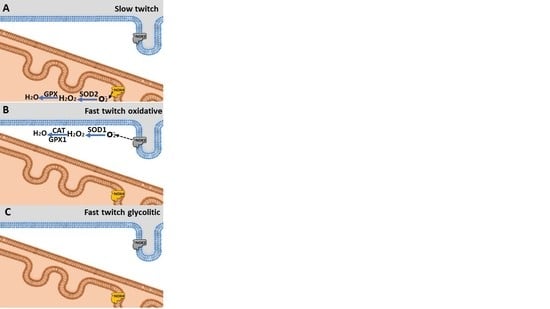
3.4. Effect of Acute Exhaustive Physical Exercise on NOX Activity and mRNA Levels in Different Types of Muscle Fibers
3.5. Antioxidant Enzyme mRNA Levels in Different Types of Muscle Fibers
3.6. Antioxidant Enzyme Activity in Different Types of Muscle Fibers
3.7. PPARGC1α Redox Control and SLC2A4 mRNA Levels
4. Discussion
4.1. Lactate Accumulation and Glycogen Decrease
4.2. Thiol Content
4.3. Antioxidant Capacity and Mitochondrial Function
4.4. PGC-1α Regulation
4.5. NOx Isoforms and Effect on Oxidative Stress
4.6. Potential Application
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urso, M.L.; Clarkson, P.M. Oxidative Stress, Exercise, and Antioxidant Supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kaiany, A.; Zibrik, L.; Mcardle, A.; Jackson, M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.F.; Laitano, O. Regulation of NADPH oxidases in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javesghani, D.; Magder, S.A.; Barreiro, E.; Quinn, M.T.; Hussain, S.N. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am. J. Respir. Crit. Care Med. 2002, 165, 412–418. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Dikalovi, S. Crosstalk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.C.; Lustgarten, M.S.; Liu, Y.; Müller, F.L.; Bhattacharya, A.; Liang, H.; Salmon, A.B.; Brooks, S.V.; Larkin, L.; Hayworth, C.R.; et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010, 24, 1376–1390. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.S.; Callahan, D.M.; Toth, M.J. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front. Physiol. 2014, 5, 369. [Google Scholar] [CrossRef] [Green Version]
- Baldwin-lanier, A. Use of anti-inflammatory drugs following exercise-induced muscle injury. Sports Med. Auckl. 2003, 33, 177–185. [Google Scholar] [CrossRef]
- Fowles, J.R.; Green, H.J.; Ouyang, J. Na+-K+-ATPase in rat skeletal muscle: Content, isoform, and activity characteristics. J. Appl. Physiol. 2004, 96, 316–326. [Google Scholar] [CrossRef] [Green Version]
- Dimauro, I.; Antonioni, A.; Mercatelli, N.; Grazioli, E.; Fantini, C.; Barone, R.; Macaluso, F.; DiFelice, V.; Caporossi, D. The early response of αB-crystallin to a single bout of aerobic exercise in mouse skeletal muscles depend supon fiber oxidative feature. Redox Biol. 2019, 24, 101183. [Google Scholar] [CrossRef] [PubMed]
- Pette, D.; Staron, R.S. Transitions of muscle fiber phenotypic profiles. Histochem. Cell Biol. 2001, 115, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.C.C.; Rego-Monteiro, I.C.; Hortenzi, V.H.; Aguiar, A.P.; Abreu, E.S.; Alburquerque, J.P.C.; Hetchi, F.; Oliveira, A.C.; Ceccatto, V.M.; Fortunato, R.S.; et al. Differential expression of nadph oxidases depends on skeletal muscle fiber type and exercise training in rats. Oxidative Med. Cell. Longev. 2016, 2016, 6738701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, M.; Hepple, R.T.; Burelle, Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: Tailoring the organelle for optimal function. Am. J. Physiol. Cell Physiol. 2012, 302, C629–C641. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.D.; Barp, J.; Ribeiro, J.L.; Belló-Klein, A.; Oliveira, A.R. Oxidative stress after three different intensities of running. Can. J. Appl. Physiol. 2005, 30, 723–734. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Borras, C.; Pallardo, F.V.; Sastre, J.; Ji, L.L.; Vina, J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005, 567, Y20. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, P.S.A. Caracterização Do Treinamento Físico Experimental de Endurance em Esteira Adaptada Através de Marcadores Metabólicos Energéticos. Mestrado Dissertação, Instituto Superior de Ciências Biomédicas, Universidade Estadual do Ceará, Fortaleza, Brazil, 2010. [Google Scholar]
- Rêgo-Monteiro, I.C.C.; Lima, T.I.; Machado, A.A.N.; Ferraz, A.S.M.; Ceccatto, V.M. Physical Training Based on the Maximum Volume Capacity Test: An Ultra-Endurance Rat Model. JEP Online 2011, 14, 40–46. [Google Scholar]
- Lima, T.; Monteiro, I.C.; Valença, S.; Leal-Cardoso, J.H.; Fortunato, R.S.; Carvalho, D.P.; Teodoro, B.G.; Ceccatto, V.M. Effect of exercise training on liver antioxidant enzymes in STZ-diabetic rats. Life Sci. 2015, 128, 64–71. [Google Scholar] [CrossRef]
- Vasconcelos Filho, F.S.L.; Souza, M.B.; Fortes, J.P.A.; Souza, K.C.L.; Carneiro, M.R.A.; Santos-Júnior, F.F.U.; Vânia Marilande, C. Duathlon training model adapted for female rats: Blood glucose-lactate correlations. Rev. Bras. Med. Esporte. 2019, 25, 1. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.K.A.; Gilles, H.J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. Minn. 1956, 28, 350–355. [Google Scholar] [CrossRef]
- Bannister, J.V.; Calabrese, L. Assays for superoxide dismutase. Methods Biochem. Anal. 1987, 32, 279–312. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Flohé, I.; Gunzler, W. Assays of gluthathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Fortunato, R.S.; Lima de Souza, E.C.; Ameziane-ELHassani, R.; Boufraqech, M.; Weyemi, U.; Talbot, M.; Lagente-Chevallier, O.; De Carvalho, D.P.; Bidart, J.M.; Schlumberger, M.; et al. Functional consequences of dual oxidase-thyroperoxidase interaction at the plasma membrane. J. Clin. Endocrinol. Metab. 2010, 95, 5403–5411. [Google Scholar] [CrossRef] [Green Version]
- de Lemos, E.T.; Oliveira, J.; Pinheiro, J.P.; Reis, F. Regular Physical Exercise as a Strategy to Improve Antioxidant and Anti-Inflammatory Status: Benefits in Type 2 Diabetes Mellitus. Oxidative Med. Cell. Longev. 2012, 741545, 1–15. [Google Scholar] [CrossRef]
- Golbidi, S.; Badran, M.; Laher, I. Antioxidant and Anti-Inflammatory Effects of Exercise in Diabetic Patients. Exp. Diabetes Res. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Little, J.P.; Gillen, J.B.; Percival, M.E.; Safdar, A.; Tarnopolsky, M.A.; Punthakee, Z.; Jung, M.E.; Gibala, M.J. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J. Appl. Physiol. 2011, 111, 1554–1560. [Google Scholar] [CrossRef] [Green Version]
- Maiese, K.; Chong, Z.Z.; Li, F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr. Neurovasc. Res. 2005, 2, 425–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumini, J.A.; Magalhães, J.; Oliveira, P.J.; Ascensão, A. Beneficial Effects of Exercise on Muscle Mitochondrial Function in Diabetes Mellitus. Sports Med. 2008, 38, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Atalay, M.; Hanninen, O. Exercise-induced oxidative stress: Glutathione supplementation and deficiency. J. Appl. Physiol. 1994, 77, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Gollnick, P.D.; Jansson, E.D.; Saltin, B.; Stein, E.M. Glycogen depletion pattern in human muscle fibres during distance running. Acta Physiol. Scand. 1973, 89, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, P.D.; Piehl, K.; Saltin, B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J. Physiol. 1974, 241, 45–57. [Google Scholar] [CrossRef]
- Gollnick, P.D.; Piehl, K.; Saubert, I.C.W.; Armstrong, R.B.; Saltin, B. Diet, exercise, and glycogen changes in human muscle fibers. J. Appl. Physiol. 1972, 33, 421–425. [Google Scholar] [CrossRef]
- Reid, M.B. Reactive Oxygen Species as Agents of Fatigue. Med. Sci. Sports Exerc. 2016, 48, 2239–2246. [Google Scholar] [CrossRef]
- Reid, M.B. Redox interventions to increase exercise performance. J. Physiol. 2016, 594, 5125–5133. [Google Scholar] [CrossRef] [Green Version]
- Reid, M.B.; Haack, K.E.; Franchek, K.M.; Valberg, P.A.; Kobzik, L.; West, M.S. Reactive oxygen in skeletal muscle I. Intracellular oxidant kinetics and fatigue in vitro. J. Appl. Physiol. 1992, 73, 1797–1804. [Google Scholar] [CrossRef]
- Picard, M.; Csukly, K.; Robillard, M.E.; Godin, R.; Ascah, A.; Bourcier-Lucas, C.; Burelle, Y. Resistance to Ca2+-induced opening of the permeability transition pore differs in mitochondria from glycolytic and oxidative muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R659–R668. [Google Scholar] [CrossRef]
- Styskal, J.L.; Remmen, H.V.; Richardson, A.; Salmon, A.B. Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic. Biol. Med. 2012, 52, 46–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.J.; Neufer, P.D. Type II skeletal myofibres possess unique properties that potentiate mitochondrial H2O2 generation. Am. J. Physiol. Cell Physiol. 2006, 290, C844–C851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollander, J.; Fiebig, R.; Gore, M.; Bejma, J.; Ookawara, T.; Ohno, H.; Ji, L.L. Superoxide dismutase gene expression in skeletal muscle: Fiber-specific adaptation to endurance training. Am. J. Physiol. 1999, 277, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Oh-ishi, S.; Kizaki, T.; Nagasawa, J.; Izawa, T.; Komabayashi, T.; Nagata, N.; Suzuki, K.; Taniguchi, N.; Ohno, H. Effects of endurance training on superoxide dismutase activity, content and mRNA expression in ratmuscle. Clin. Exp. Pharmcol. Physiol. 1997, 24, 326–332. [Google Scholar] [CrossRef]
- Ohno, H.; Suzuki, K.; Fujii, J.; Yamashita, H.; Kizaki, T.; Oh-ishi, S.; Taniguchi, N. Superoxide Dismutases in Exercise and Disease. In Exercise and Oxygen Toxicity; Sen, C.K., Packer, L., Hanninen, O., Eds.; Elsevier Science: New York, NY, USA, 1994; pp. 127–161. [Google Scholar]
- Wessel, T.V.; de Haan, A.; van der Laarse, W.J.; Jaspers, R.T. The muscle fiber type–fiber size paradox: Hypertrophy or oxidative metabolism? Eur. J. Appl. Physiol. 2010, 110, 665–694. [Google Scholar] [CrossRef] [Green Version]
- Criswell, D.; Powers, S.; Dodd, S.; Lawler, J.; Edwards, W.; Renshler, K.; Grinton, S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med. Sci. Sports Exerc. 1993, 25, 1135–1140. [Google Scholar] [CrossRef]
- Powers, S.K.; Talbert, E.E.; Adhihetty, P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar] [CrossRef]
- Vincent, H.K.; Powers, S.K.; Stewart, D.J.; Demirel, H.A.; Shanely, R.A.; Naito, H. Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur. J. Appl. Physiol. 2000, 81, 67–74. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Hollander, J.; Leichtweis, S.; Griffiths, M.; Gore, M.; Ji, L.L. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997, 272, R363–R369. [Google Scholar] [CrossRef]
- Laughlin, M.H.; Simpson, T.; Sexton, W.L.; Brown, O.R.; Smith, J.K.; Korthuis, R.J. Skeletal muscle oxidative capacity, antioxidant enzymes, exercise training. J. Appl. Physiol. 1990, 68, 2337–2343. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Handschin, C.; Choi, C.S.; Chin, S. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic βcell crosstalk. J. Clin. Investig. 2007, 117, 3463–3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wende, A.R.; Schaeffer, P.J.; Parker, G.J.; Zechner, C.; Han, D.H.; Chen, M.M.; Hancock, C.R.; Lehman, J.J.; Huss, J.M.; McClain, D.A.; et al. A role for the transcriptional coactivator PGC-1α in muscle refueling. J. Biol. Chem. 2007, 50, 36642–36651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, C.; Ji, L.L. Role of PGC-1α signaling in skeletal muscle health and Disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Mitochondrial oxygen radical generation and leak: Sites of production in states 4 and 3, organ specificity, relation to aging and longevity. J. Bioenerg. Biomembr. 1999, 31, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Liu, Y.; Van Remmen, H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004, 279, 49064–49073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijles, D.N.; Fan, L.M.; Howlin, B.J.; Li, J.M. Molecular insights of p47phox phosphorylation dynamics in the regulation of NADPH oxidase activation and superoxide production. J. Biol. Chem. 2014, 289, 22759–22770. [Google Scholar] [CrossRef] [Green Version]
- Hawley, J.A. Adaptations of skeletal muscle to prolonged, intense endurance training. Clin. Exp. Pharmacol. Physiol. 2002, 29, 218–222. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; Van Essen, M.; Wilkin, G.P.; Burgomaster, K.A.; Safdar, A.; Tarnopolsky, M.A. Short-term sprint interval versus traditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. J. Physiol. 2006, 575, 901–911. [Google Scholar] [CrossRef]
- da Justa Pinheiro, C.H.; Silveira, L.R.; Nachbar, R.T.; Vitzel, K.F.; Curi, R. Regulation of glycolysis and expression of glucose metabolism-related genes by reactive oxygen species in contracting skeletal muscle cells. Free Radic. Biol. Med. 2010, 48, 953–960. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osório Alves, J.; Matta Pereira, L.; Cabral Coutinho do Rêgo Monteiro, I.; Pontes dos Santos, L.H.; Soares Marreiros Ferraz, A.; Carneiro Loureiro, A.C.; Calado Lima, C.; Leal-Cardoso, J.H.; Pires Carvalho, D.; Soares Fortunato, R.; et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants 2020, 9, 57. https://doi.org/10.3390/antiox9010057
Osório Alves J, Matta Pereira L, Cabral Coutinho do Rêgo Monteiro I, Pontes dos Santos LH, Soares Marreiros Ferraz A, Carneiro Loureiro AC, Calado Lima C, Leal-Cardoso JH, Pires Carvalho D, Soares Fortunato R, et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants. 2020; 9(1):57. https://doi.org/10.3390/antiox9010057
Chicago/Turabian StyleOsório Alves, Juliana, Leonardo Matta Pereira, Igor Cabral Coutinho do Rêgo Monteiro, Luiz Henrique Pontes dos Santos, Alex Soares Marreiros Ferraz, Adriano Cesar Carneiro Loureiro, Crystianne Calado Lima, José Henrique Leal-Cardoso, Denise Pires Carvalho, Rodrigo Soares Fortunato, and et al. 2020. "Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle" Antioxidants 9, no. 1: 57. https://doi.org/10.3390/antiox9010057