Effects of Oxytocin Administration on the Response of Piglets to Weaning
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
2.1. Animals and Treatments
2.2. Weight Measurements
2.3. Behavioral Observations
| Behaviour | Description |
|---|---|
| Maintenance behaviors | |
| Feeding | Head in the feeder with both ears not visible |
| Drinking | Snout in physical contact with the drinker |
| Social behaviors | |
| High aggression | Bout lasts for ≥ 5 sec of head knock, pursuit, parallel push and/or ≥ two bites is delivered to the penmate |
| Mild aggression | Bout lasts for < 5 sec of head knock, pursuit, parallel push and/or one bite is delivered to the penmate |
| Non-aggressive | Snout to snout contact or any other touch with the snout of any penmate’s body parts |
2.4. Physiological Analyses
2.5. Statistical Analyses
3. Results
3.1. Body Weight and Growth
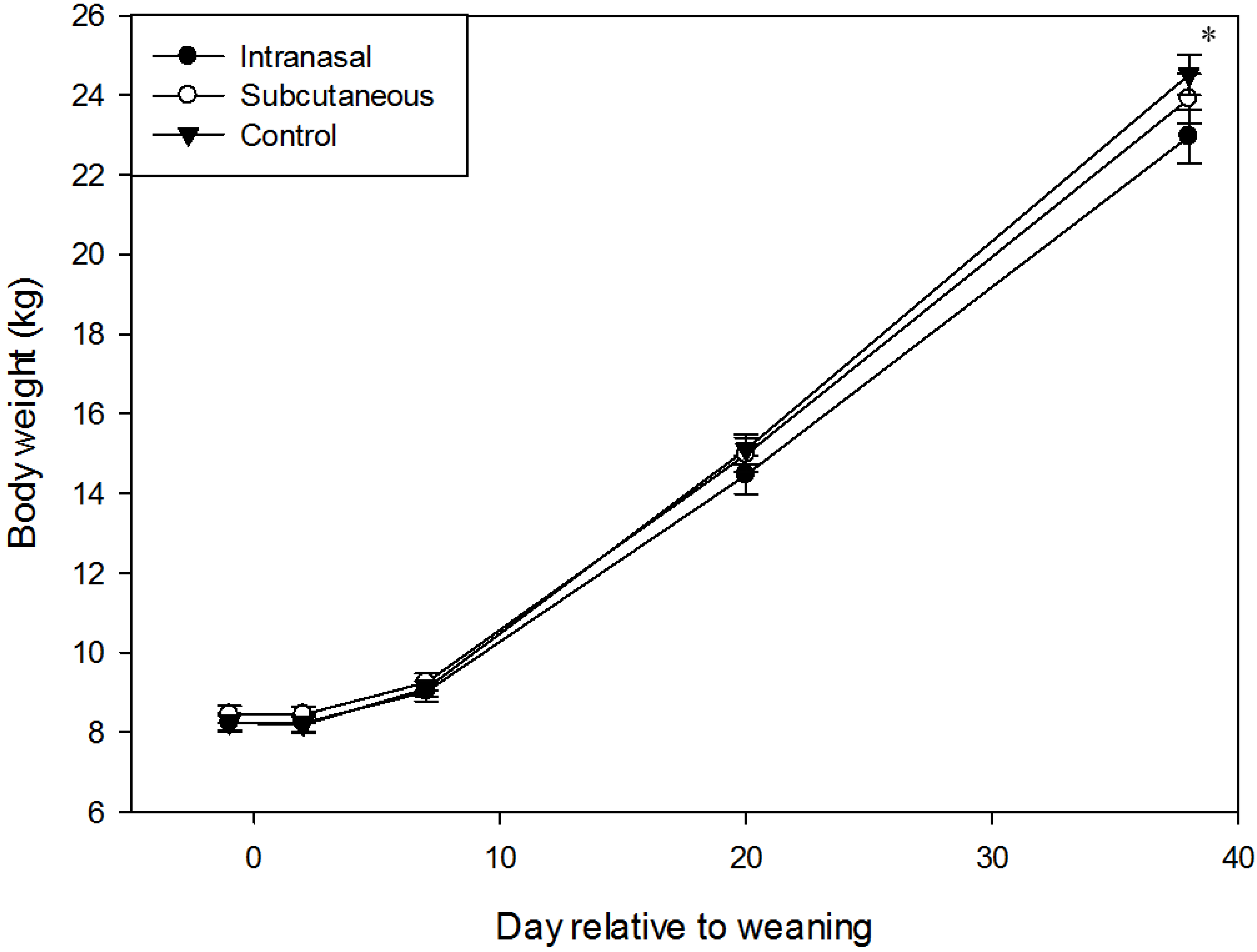
3.2. Physiology
3.2.1. Cortisol
| Variables | 4 h | 28 h | ||||
|---|---|---|---|---|---|---|
| Intranasal oxytocin | Subcutaneous oxytocin | Control | Intranasal oxytocin | Subcutaneous oxytocin | Control | |
| Cortisol (ng/mL) | 111.1 ± 5.3a | 108.1 ± 5.8a | 110.4 ± 5.3a | 22.7 ± 5.3b | 17.1 ± 5.3b | 17.3 ± 5.3b |
| Neutrophil:lymphocyte ratio (arbitrary units) | 3.10 ± 0.26a | 3.85 ± 0.25a | 3.29 ± 0.27a | 0.95 ± 0.25b | 0.85 ± 0.24b | 1.39 ± 0.25b |
| C-reactive protein (mg/mL) | 0.97 ± 0.18 | 1.14 ± 0.19 | 0.88 ± 0.23 | 1.01 ± 0.15 | 0.96 ± 0.15 | 1.02 ± 0.15 |
| Tumor Necrosis Factor α (pg/mL)1 | 40.1 ± 50.7 | 22.6 ± 31.6 | 31.4 ± 47.8 | 121.4 ± 33.8 | 78.3 ± 37.8 | 28.7 ± 34.7 |
3.2.2. Neutrophil:lymphocyte Ratio
3.2.3. C-Reactive Protein
3.2.4. Tumor Necrosis Factor α
3.3. Behavior
3.3.1. Feeding Behavior
| Variables | Intranasal oxytocin | Subcutaneous oxytocin | Control |
|---|---|---|---|
| Feeding frequency (bouts per 4 h) | 16.3 ± 2.2x | 22.2 ± 2.2y | 19.8 ± 2.2xy |
| Feeding duration (sec per 4 h)1 | 305.2 ± 65.8 | 414.2 ± 65.8 | 379.0 ± 65.8 |
| Drinking frequency (bouts per 4 h) | 16.1 ± 3.1 | 11.5 ± 3.1 | 15.3 ± 3.1 |
| Drinking duration (sec per 4 h) | 180.5 ± 34.9 | 117.5 ± 34.9 | 128.9 ± 34.9 |
3.3.2. Drinking Behavior
3.3.3. Social Behavior



4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Jensen, P.; Recen, B. When to wean: observations from free-ranging domestic pigs. Appl. Anim. Behav. Sci. 1989, 23, 49–60. [Google Scholar] [CrossRef]
- Pluske, J.R.; Williams, I.H.; Aherne, F.X. Nutrition of the neonatal pig. In The Neonatal Pig Development and Survival; Varley, M.A., Ed.; CAB International: Wallingford, UK, 1995; pp. 187–235. [Google Scholar]
- Weary, D.M.; Jasper, J.; Hotzel, M.J. Understanding weaning distress. Appl. Anim. Behav. Sci. 2008, 110, 24–41. [Google Scholar] [CrossRef]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Fraser, D. Observations on the behavioural development of suckling and early-weaned piglets during the first six weeks after birth. Anim. Behav. 1978, 26, 22–30. [Google Scholar] [CrossRef]
- Dunshea, F.R.; Kerton, D.J.; Eason, P.J.; King, R.H. Supplemental skim milk before and after weaning improves growth performance of pigs. Aust. J. Agr. Res. 1999, 50, 1165–1170. [Google Scholar] [CrossRef]
- Pajor, E.A.; Fraser, D.; Kramer, D.L. Consumption of solid food by suckling pigs: Individual variation and relation to weight gain. Appl. Anim. Behav. Sci. 1991, 32, 139–155. [Google Scholar] [CrossRef]
- de Lange, C.F.M.; Pluske, J.; Gong, J.; Nyachoti, C.M. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 2010, 134, 124–134. [Google Scholar] [CrossRef]
- Lalles, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Weaning—A challenge to gut physiologists. Livest. Sci. 2007, 108, 82–93. [Google Scholar] [CrossRef]
- Kutzer, T.; Bunger, B.; Kjaer, J.B.; Schrader, L. Effects of early contact between non-littermate piglets and of the complexity of farrowing conditions on social behavior and weight gain. Appl. Anim. Behav. Sci. 2009, 121, 16–24. [Google Scholar] [CrossRef]
- Kendrick, K.M.; Keverne, E.B.; Baldwin, B.A. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology 1987, 46, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R. Is social attachment an addictive disorder? Physiol. Behav. 2003, 79, 351–357. [Google Scholar] [CrossRef]
- Carter, C.S.; Grippo, A.J.; Pournajafi-Nazarloo, H.; Ruscio, M.G.; Porges, S.W. Oxytocin, vasopressin and sociality. Progr. Brain Res. 2008, 170, 331–336. [Google Scholar]
- Insel, T.R.; Winslow, J.T. Central administration of oxytocin modulates the infant rats response to social isolation. Eur. J. Pharmacol. 1991, 203, 149–152. [Google Scholar] [CrossRef]
- Kavushansky, A.; Leshem, M. Role of oxytocin and vasopressin in the transitions of weaning in the rat. Dev. Psychobiol. 2004, 45, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Uvnas-Moberg, K.; Alster, P.; Petersson, M. Dissociation of oxytocin effects on body weight in two variants of female Sprague-Dawley rats. Int. Physiol. Behav. Sci. 1996, 31, 44–55. [Google Scholar] [CrossRef]
- Uvnas-Moberg, K.; Alster, P.; Petersson, M.; Sohlstrom, A.; Bjorkstrand, E. Postnatal oxytocin injections cause sustained weight gain and increased nociceptive thresholds in male and female rats. Pediatr. Res. 1998, 43, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.-L.; Carter, C.S.; Garner, J.P.; Marchant-Forde, J.N.; Richert, B.T.; Lay, D.C., Jr. Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol. Behav. 2013, 112–113, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.-L.; Ferrari, J.; Pluske, J.R.; Dunshea, F.R. Neonatal oxytocin administration and supplemental milk ameliorate the weaning transition and alter hormonal expression in the gastrointestinal tract in pigs. Dom. Anim. Endocrinol. 2015, 51, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.M.; Johnson, K.G. Stress and Animal Welfare; Chapman and Hall: London, UK, 1993. [Google Scholar]
- St Pierre, N.R. Design and analysis of pen studies in the animal sciences. J. Dairy Sci. 2007, 90, E87–E99. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R.; Winslow, J.T. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000, 25, 284–288. [Google Scholar] [PubMed]
- De Dreu, C.K.W. Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Horm. Behav. 2012, 61, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Bartz, J.A.; Zaki, J.; Bolger, N.; Ochsner, K.M. Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci. 2011, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.J.; Howard, A.L.; Dadds, M.R.; Mitchell, P.; Carson, D.S. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrino 2009, 34, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Mens, W.B.J.; Witter, A.; Van Wimersma Greidanus, T.B. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (csf): Halftimes of disappearance of these neuropeptides from csf. Brain Res. 1983, 262, 143–149. [Google Scholar] [CrossRef]
- Neumann, I.D.; Maloumby, R.; Beiderbeck, D.I.; Lukas, M.; Landgraf, R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrino 2013, 38, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav. Brain Res. 2007, 176, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.; Blackburn, R.; Hoffman, G.; Stricker, E. Establishing behavioral and physiological functions of central oxytocin: insights from studies of oxytocin and ingestive behaviors. Adv. Exp. Med. Biol. 1995, 395, 209–225. [Google Scholar] [PubMed]
- Benelli, A.; Bertolini, A.; Arletto, R. Oxytocin-induced inhibition of feeding and drinking: No sexual dimorphism in rats. Neuropeptides 1991, 20, 57–62. [Google Scholar] [CrossRef]
- Maejima, Y.; Iwasaki, Y.; Yamahara, Y.; Kodaira, M.; Sedbazar, U.; Yada, T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 2011, 3, 1169–1177. [Google Scholar] [PubMed]
- Huang, H.; Michetti, C.; Busnelli, M.; Manago, F.; Sannino, S.; Scheggia, D.; Giancardo, L.; Sona, D.; Murino, V.; Chini, B.; et al. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology 2014, 39, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Bales, K.L.; van Westerhuyzen, J.A.; Lewis-Reese, A.D.; Grotte, N.D.; Lanter, J.A.; Carter, C.S. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm. Behav. 2007, 52, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Pié, S.; Lalles, J.P.; Blazy, F.; Laffitte, J.; Seve, B.; Oswald, I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [PubMed]
- Iseri, S.O.; Sener, G.; Saglam, B.; Gedik, N.; Ercan, F.; Yegen, B.C. Oxytocin ameliorates oxidative colonic inflammation by a neutrophil-dependent mechanism. Peptides 2005, 26, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.; Bissonauth, V.; Gao, L.; Gangal, M.; Wang, D.; Danalache, B.; Wang, Y.; Stoyanova, E.; Cloutier, G.; Blaise, G.; Gutkowska, J. Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Res. Cardiol. 2010, 105, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Gauldie, J. The acute phase response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef]
- Eckersall, P.D.; Saini, P.K.; McComb, C. The acute phase response of acid soluble glycoprotein, α1-acid glycoprotein, ceruloplasmin, haptoglobin and C-reactive protein, in the pig. Vet. Immunol. Immunopathol. 1996, 51, 377–385. [Google Scholar] [CrossRef]
- Parra, M.D.; Fuentes, P.; Tecles, F.; Martinez-Subiela, S.; Martinez, J.S.; Munoz, A.; Ceron, J.J. Porcine acute phase protein concentrations in different diseases in field conditions. J. Vet. Med. B. 2006, 53, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Salamano, G.; Mellia, E.; Candiani, D.; Ingravalle, F.; Bruno, R.; Ru, G.; Doglione, L. Changes in haptoglobin, C-reactive protein and pig-MAP during a housing period following long distance transport in swine. Vet. J. 2008, 177, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.W.; Carroll, J.A.; Allee, G.L.; Zanelli, M.E. The effects of thermal environment and spray-dried plasma on the acute-phase response of pigs challenged with lipopolysaccharide. J. Anim. Sci. 2003, 81, 1166–1176. [Google Scholar] [PubMed]
- Widowski, T.; Curtis, S.; Graves, C. The neutrophil:lymphocyte ratio in pigs fed cortisol. Can. J. Anim. Sci. 1989, 69, 501–504. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rault, J.-L.; Dunshea, F.R.; Pluske, J.R. Effects of Oxytocin Administration on the Response of Piglets to Weaning. Animals 2015, 5, 545-560. https://doi.org/10.3390/ani5030371
Rault J-L, Dunshea FR, Pluske JR. Effects of Oxytocin Administration on the Response of Piglets to Weaning. Animals. 2015; 5(3):545-560. https://doi.org/10.3390/ani5030371
Chicago/Turabian StyleRault, Jean-Loup, Frank R. Dunshea, and John R. Pluske. 2015. "Effects of Oxytocin Administration on the Response of Piglets to Weaning" Animals 5, no. 3: 545-560. https://doi.org/10.3390/ani5030371






