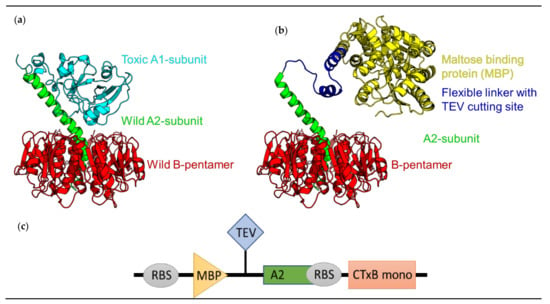
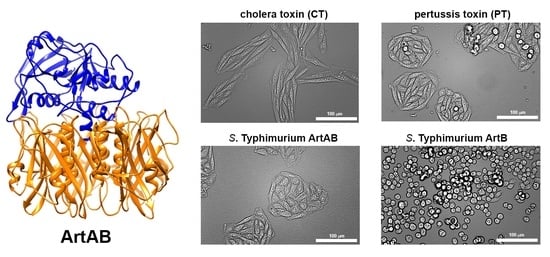
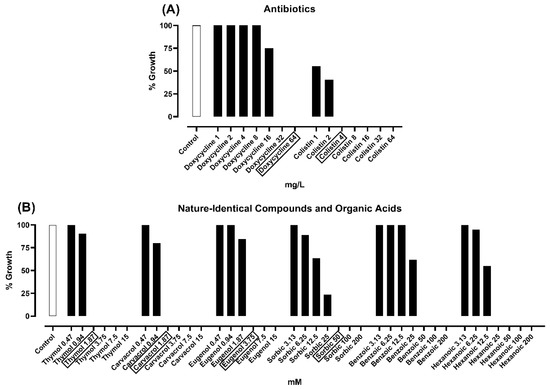
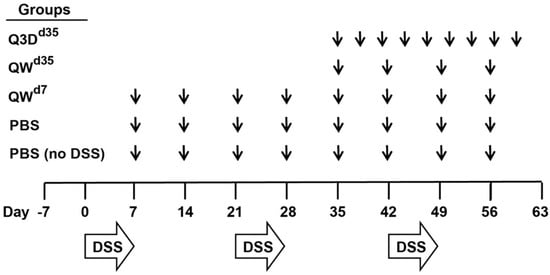
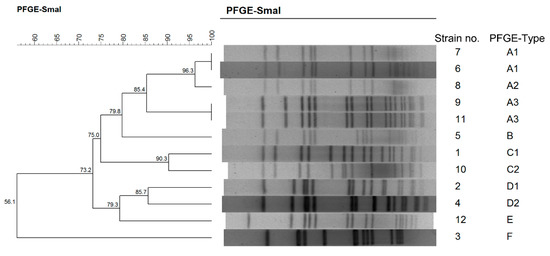
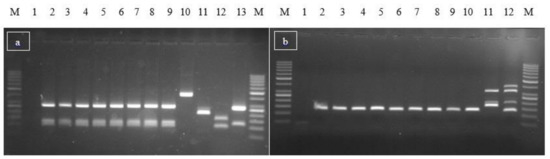
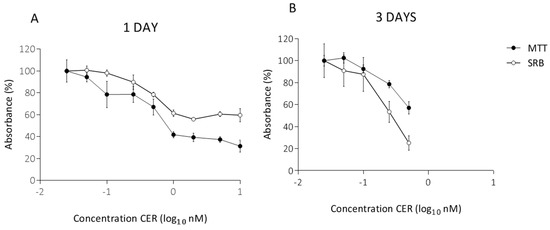
Bacterial Enterotoxins (Closed)
A topical collection in Toxins (ISSN 2072-6651). This collection belongs to the section "Bacterial Toxins".
Viewed by 34908Editor
Interests: Bacillus cereus enterotoxins; emetic toxin; cereulide; Staphylococcal enterotoxins; Clostridium botulinum neurotoxins; Clostridium perfringens enterotoxin; shiga toxin; food safety; beauvericin; enniatins; food safety in Serbia
Topical Collection Information
Dear Colleagues,
Bacterial enterotoxins, in the broadest sense, are proteins or peptides that exert their effect on intestines, causing a plethora of gastrointestinal disease manifestations. Enterotoxins are produced by both gram-positive and gram-negative pathogens, both in food and in host’s gastro intestinal tract. In Europe, data collected by Rapid and Alert System for Food and Feed safety about 10% of all outbreaks is found to be caused by bacterial toxins, and only toxins taken here into account were those of Bacillus, Clostridium, and Staphylococcus, which were found in almost all types of food. In USA and UK, enterotoxins produced by C. perfringens are even reported as the top two causative agents of foodborne bacterial poisoning. Bacterial enterotoxins are for many foodborne pathogens the major virulence factor responsible for the symptoms of food poisoning. This is the case among others for Bacillus cereus, Clostridium perfringens, enterotoxigenic Escherichia coli, and Staphylococcus aureus. Many pathogens produce different toxins, and often one strain can produce multiple toxins. The path from the presence of genes encoding for toxin production and actual protein expression, or non-ribosomal peptide synthesis, is not always straight forward, and final toxin production depends on many factors. Understanding these factors is detrimental to food safety assurance. Also, there is great need for a better understanding of the threat to public health from exposure to a single toxin arising from multiple pathways or to multiple toxins that have the same mechanism of toxicity. The aim of the current Special Issue is to gather the most recent cutting-edge research on the topic of bacterial enterotoxins, including, but not limited to, in-vitro, in-vivo and in-silico toxicity, their roles in host-pathogen interactions, the mode and mechanisms of action, the regulation of production, the structure-activity relationship, the exposure assessment, and the detection and control relevant to food safety. This Special Issue aims to become a reference for a new body of knowledge on bacterial toxins, from microbial genes to the human gut.
Prof. Dr. Andreja Rajkovic
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a double-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Toxins is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- host–pathogen interaction
- food safety
- toxin
- expression
- exposure
- toxicity