RNA Modifications
A topical collection in Biomolecules (ISSN 2218-273X). This collection belongs to the section "Molecular Genetics".
Viewed by 231880Editors
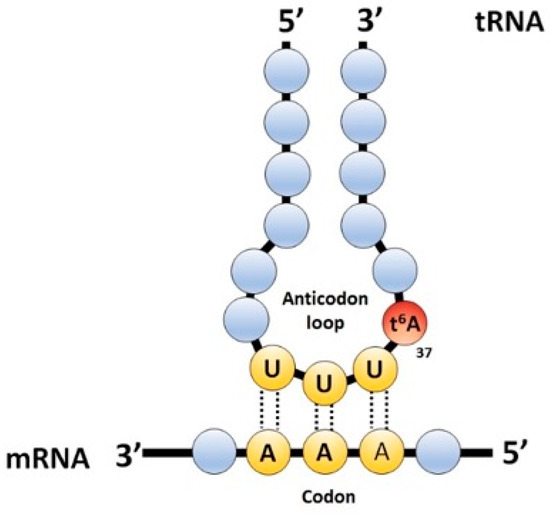
Interests: comparative genomics; gene annotation; vitamin metabolism; tRNA modification pathways; 7-deazaguanosine derivatives; translation accuracy; tRNA metabolism; metabolite repair; bacterial genetics; t6A modification
Topical Collection Information
Dear Colleagues,
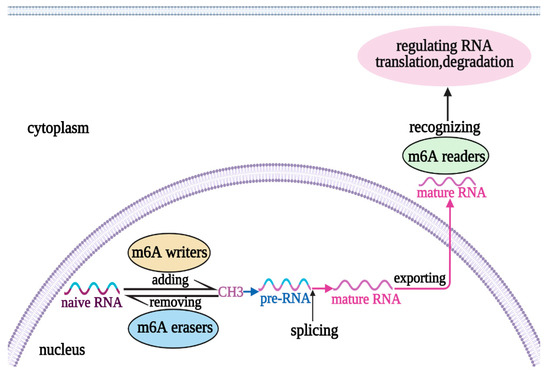
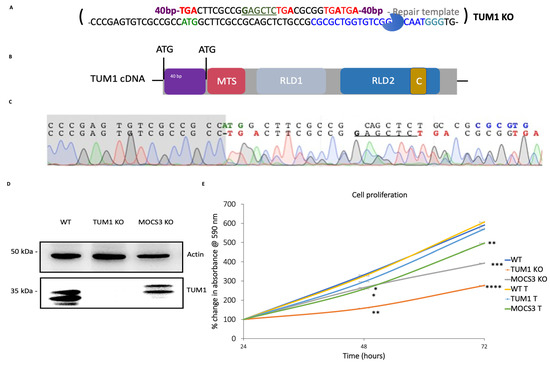
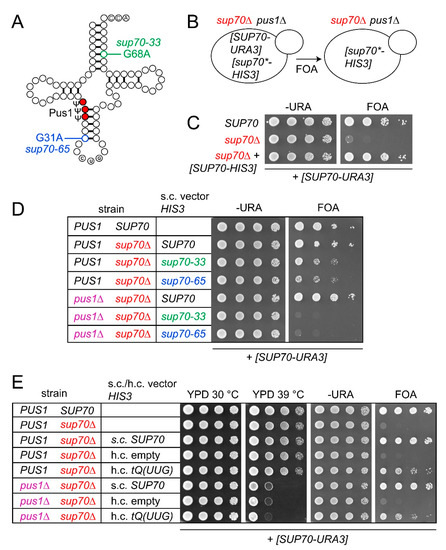
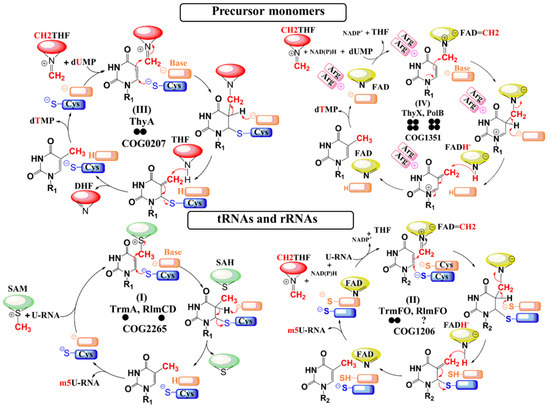
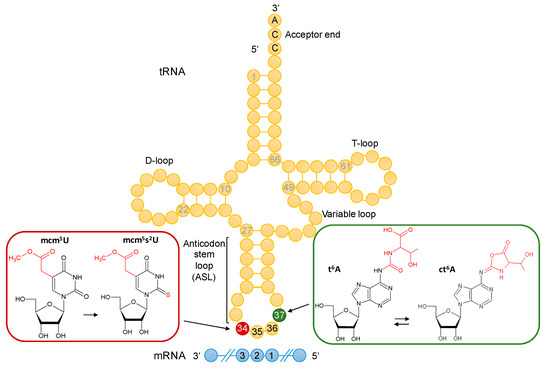
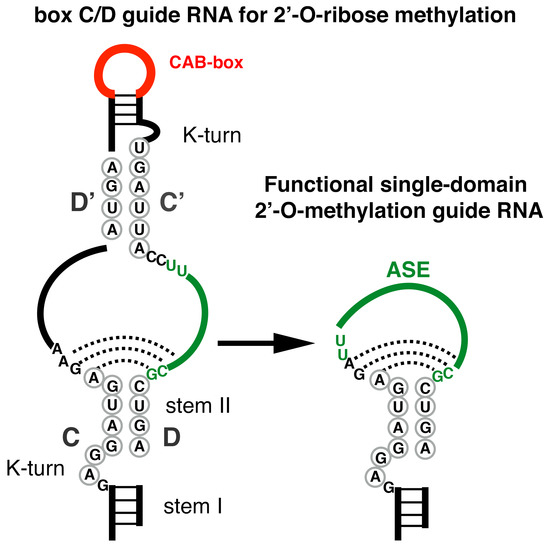
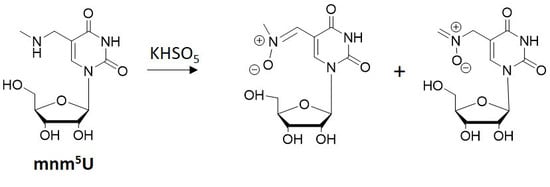
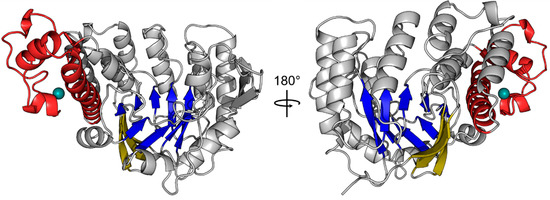
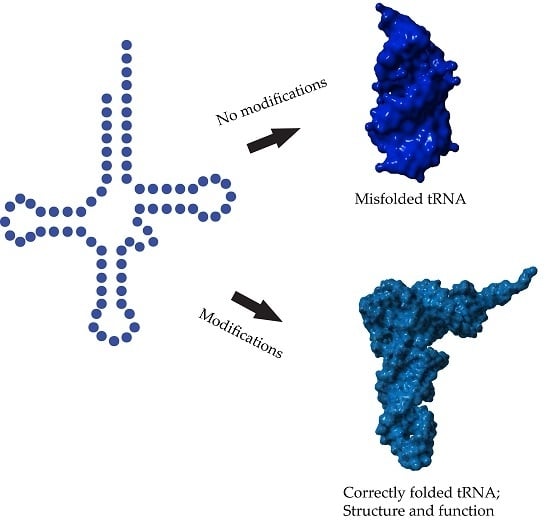
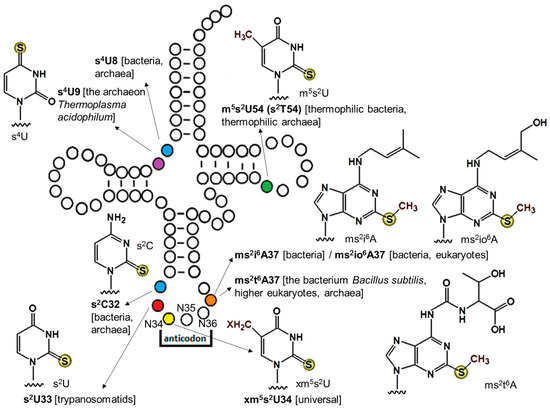
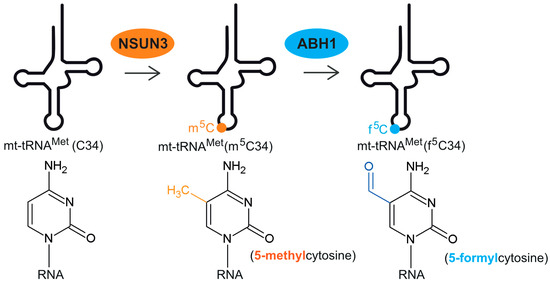
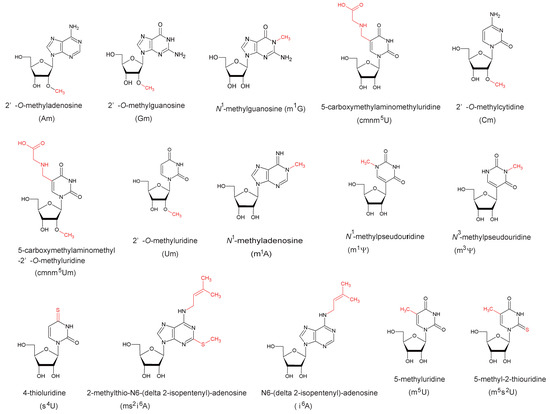
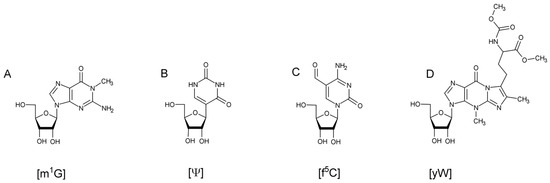
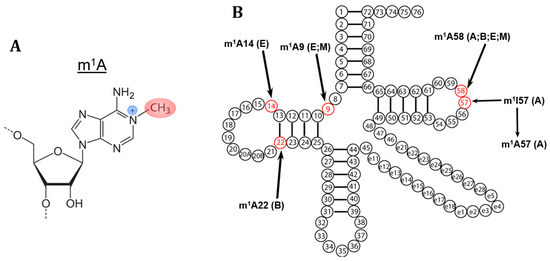
The field of RNA modification, that started in 1957 with the discovery of the fifth nucleotide pseudouridine has recently gone through a revival or, as recently discussed by one of the pioneers of the field, Henri Grosjean, a new golden era (RNA. 2015 Apr; 21(4): 625–626). Over 100 different modifications are found in RNAs and in the last 20 years a combination of biochemical, genetic and bioinformatic studies has allowed the identification of many of the enzymes responsible for their synthesis that had been missing for decades. It is now clear that every type of nucleic acid in cells undergoes some type of modification, including, but not limited to, DNA, rRNA, snRNA, miRNA and tRNA. This has led to the discovery of new enzyme families, has revealed a great diversity of RNA recognition modes and allowed to address the functional roles of many modifications. RNA modification detection and quantification methods derived from new generation sequencing techniques as well as new developments in advanced mass spectrometry have recently revolutionized the field. We are just starting to appreciate the multiple roles that RNA modifications have in the cell and how these fundamental processes are linked to human diseases. These recent studies have also shown the strong parallels between RNA editing and RNA modification, between DNA and RNA modifications and between the modification of tRNAs and modifications of other types of RNAs with the different fields cross-fertilizing each other.
This is a very exciting time in the field and we encourage scientists of diverse backgrounds (biochemical, genetic, systems biology) and who use different model systems to contribute original research or review articles that focus on the synthesis or function of RNA modifications.
Prof. Dr. Valérie de Crécy-Lagard
Prof. Dr. Juan Alfonzo
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- miscoding
- translation accuracy
- translational control
- tRNA metabolism
- tRNA modification
- tRNA quality control
- tRNA recognition
- modification tunable transcripts
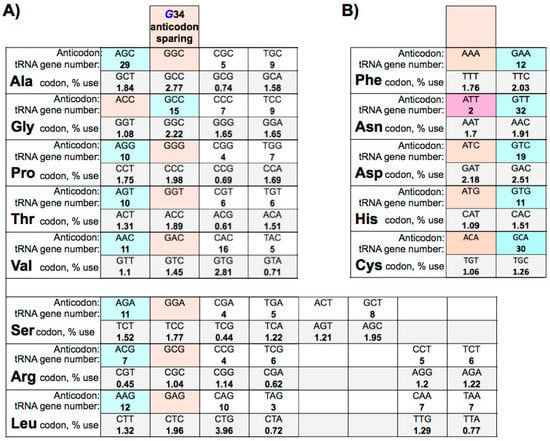
- codon usage
- tRNA fragments
- rRNA modification
- ribosome
- mRNA modification
- mRNA stability
- mRNA splicing
- RNA modification regulation