Influence of Hydrogen on Steel Components for Clean Energy
Abstract
:1. Introduction
1.1. Aims
1.2. Background
1.2.1. H Economy
1.2.2. Hydrogen Embrittlement
1.2.3. Testing Methodologies
1.3. Significance
1.4. Approach
1.4.1. Hydrogen Characterization
1.4.2. H Influence—Static Strength
1.4.3. H Influence—Fatigue
2. Hydrogen Characterization
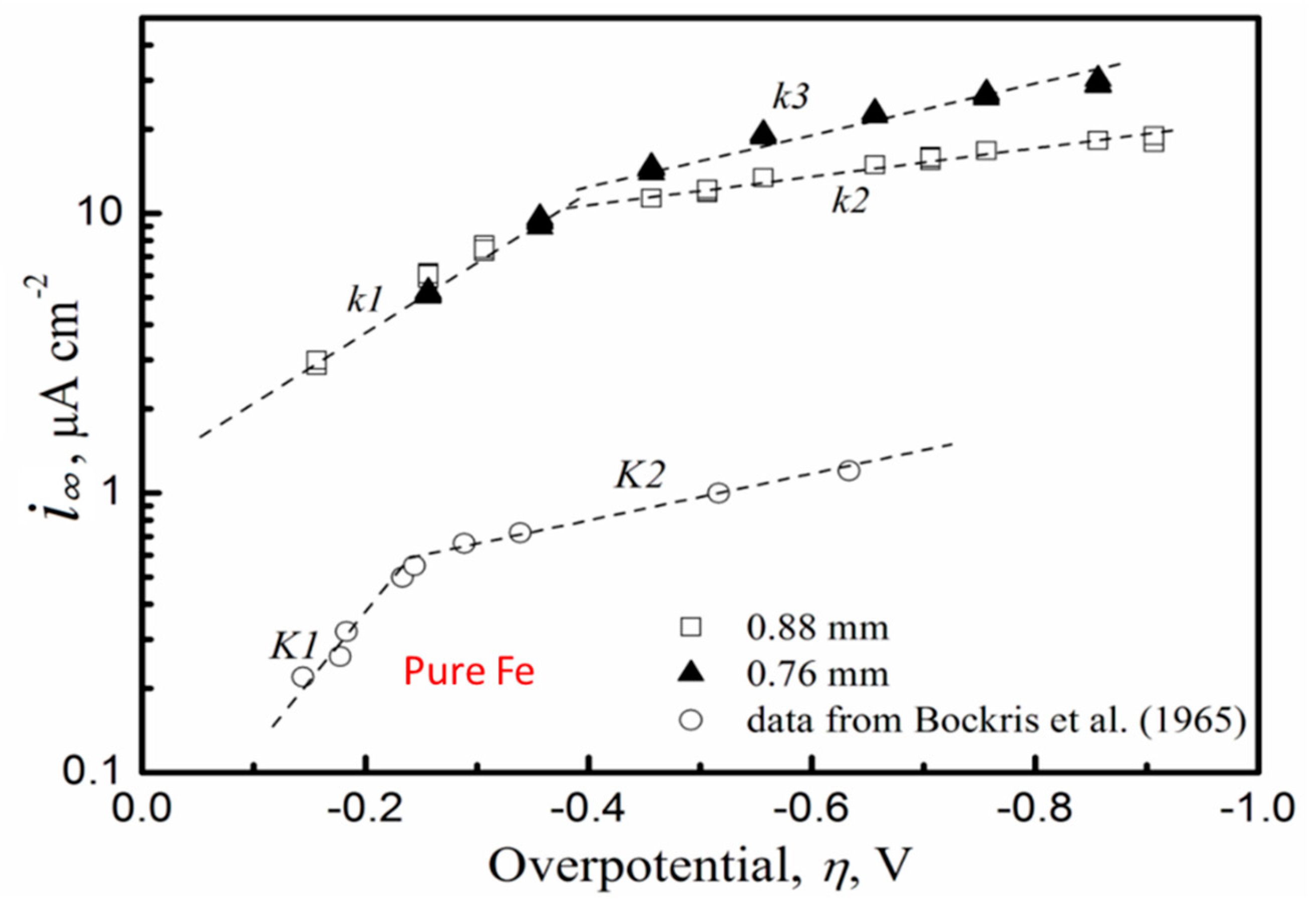
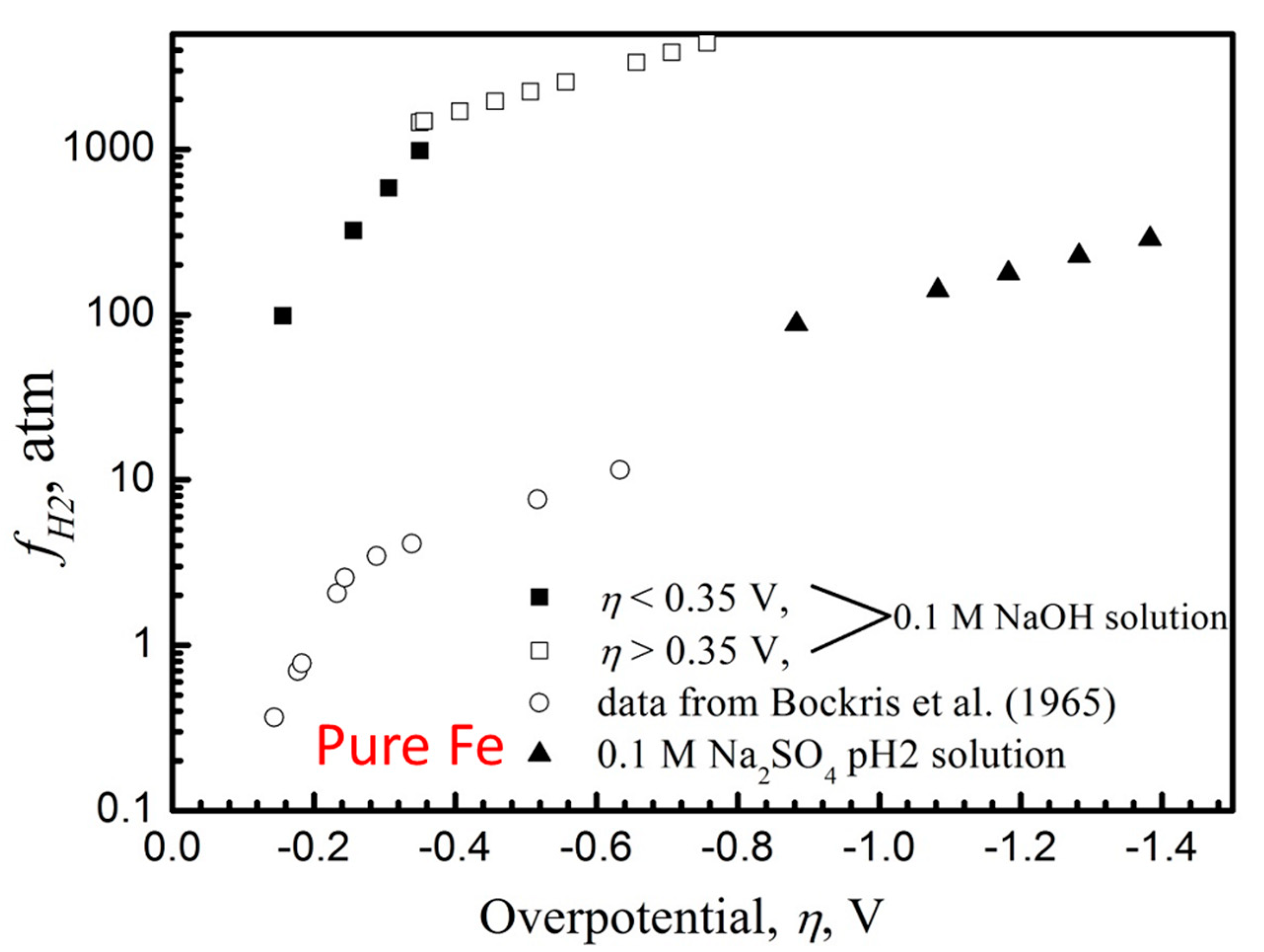
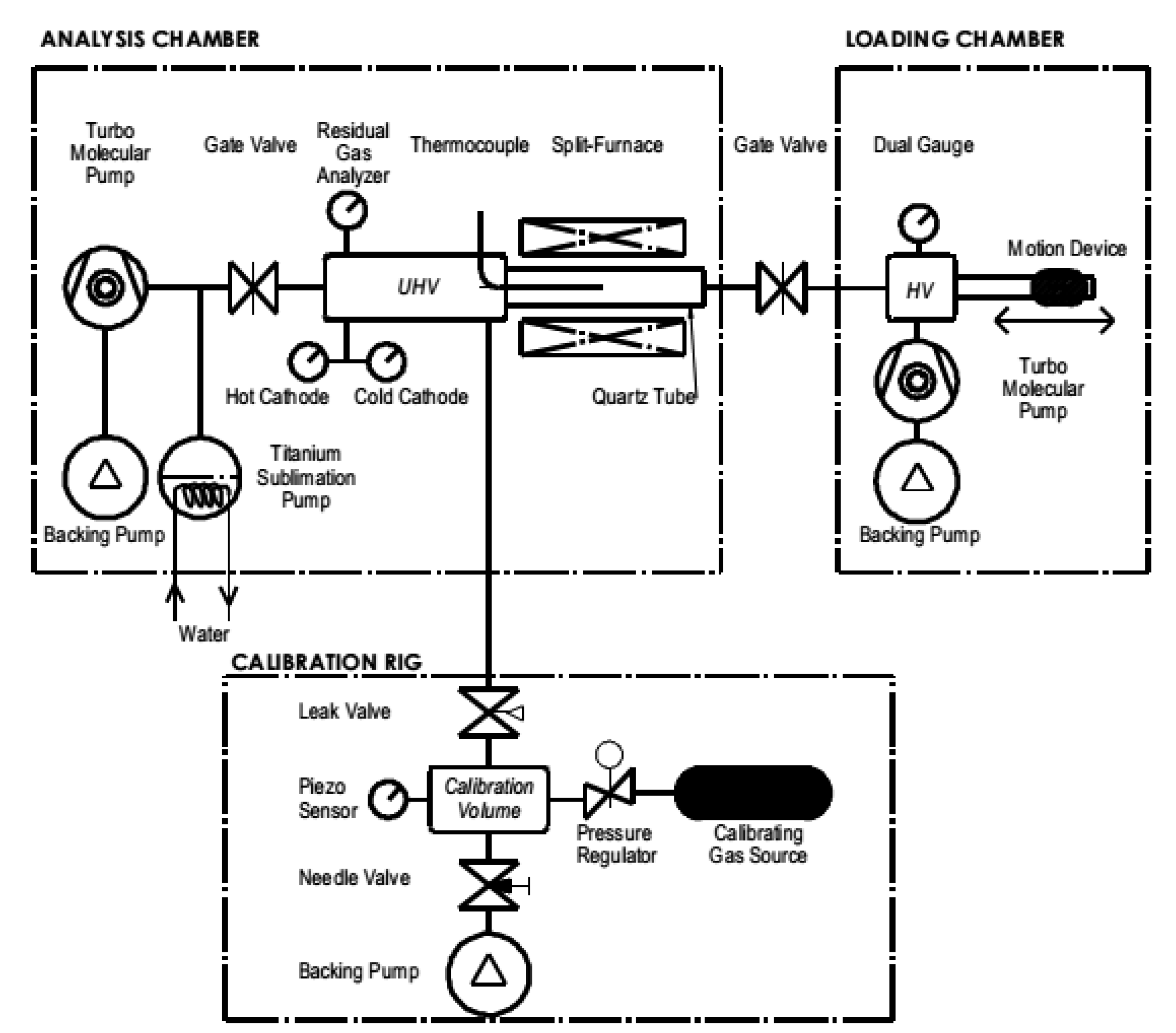
2.1. Permeation Cell
- Preconditioning makes a large difference.
- The solution used for cathodic charging makes a large difference.
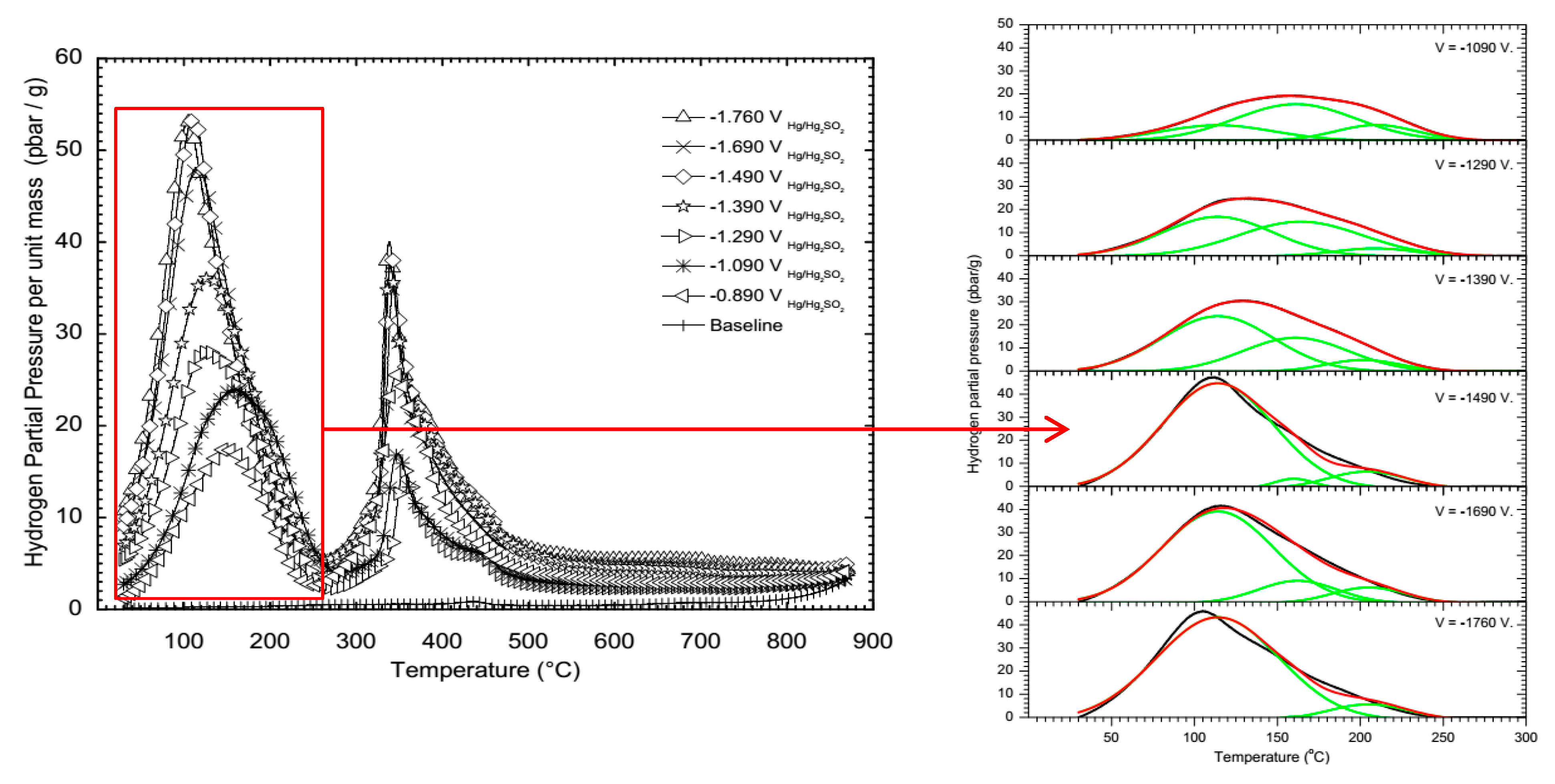
2.2. Thermal Desorption Spectroscopy
2.3. Sieverts’ Law
3. H Influence—Static Strength
4. H Influence—Fatigue
5. Discussion
6. Conclusions
- The relationship was established between the hydrogen charging overpotential and the equivalent hydrogen pressure during cathodic hydrogen charging though the use of electrochemical permeation experiments and thermal desorption spectroscopy.
- This allows evaluation of the influence of hydrogen on the static and dynamic strength of these steels under hydrogen fugacity values appropriate to service conditions.
- The cathodic hydrogen charging conditions were equivalent to testing in gaseous hydrogen at fugacities of over a thousand bar.
- Under these hydrogen charging conditions, there was no effect of hydrogen up to the yield stress. There was an influence of hydrogen on the final fracture, which occurred at the same stress as for the steels tested in air.
- The influence of hydrogen was on the details of the final fracture: there were some brittle hydrogen induced fracture events, which occurred simultaneously with the overall ductile fracture of the specimen. In some cases, brittle fractures initiated by hydrogen, or DHF: Decohesive hydrogen fracture, initiated the final fracture of the specimen.
- The fisheyes were associated with alumina oxide inclusion, which indicated that these features would be less for a cleaner steel.
- Each fisheye was surrounded by such micro-void coalescence (MVC) fracture. This corresponds to MF: Mixed fracture, wherein a hydrogen microfracture mechanism (i.e., that producing the fisheyes) competed with the ductile MVC fracture.
- There was no subcritical crack growth. There was essentially no influence of hydrogen on ductility for the hydrogen conditions studied.
- At applied stress amplitudes above the threshold, fatigue initiation, for low cycle fatigue, occurred at a lower number of cycles with increasing hydrogen fugacity and increasing stress amplitude. This was caused by a decrease in the fatigue initiation period and by an increase in the crack growth rate. In the presence of hydrogen, there was flat transgranular fracture with vague striations with some intergranular fracture at lower stresses. Mechanical overload occurred when the fatigue crack reached the critical length. There was no significant influence of hydrogen on the final fracture.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Atrens, A. A critical review of the influence of hydrogen on the mechanical properties of medium strength steels. Corros. Rev. 2013, 31, 85–104. [Google Scholar] [CrossRef]
- Liu, Q.; Irwanto, B.; Atrens, A. The influence of hydrogen on 3.5NiCrMoV steel studied using the linearly increasing stress test. Corros. Sci. 2013, 67, 193–203. [Google Scholar] [CrossRef]
- Liu, Q.; Irwanto, B.; Atrens, A. Influence of hydrogen on the mechanical properties of some medium strength Ni-Cr-Mo steels. Mater. Sci. Eng. A 2014, 617, 200–210. [Google Scholar] [CrossRef]
- Liu, Q.; Atrens, A.D.; Shi, Z.; Verbeken, K.; Atrens, A. Determination of the hydrogen fugacity during electrolytic charging of steel. Corros. Sci. 2014, 87, 239–258. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Atrens, A. Reversible hydrogen trapping in a 3.5NiCrMoV medium strength steel. Corros. Sci. 2015, 96, 112–120. [Google Scholar] [CrossRef]
- Liu, Q.; Irwanto, B.; Atrens, A. Steels for the hydrogen economy. In Proceedings of the 5th Baosteel Biennial Academic Conference (BAC2013), Shanghai, China, 4–6 June 2013. [Google Scholar]
- Liu, Q.; Irwanto, B.; Atrens, A. The tensile properties of NiCrMo1 steel under conditions of hydrogen charging studied using the linearly increasing stress test. In Proceedings of the ICF13 13th International Conference on Fracture, Beijing, China, 16–21 June 2013; Yu, S., Feng, X.-Q., Eds.; Science Literature Publishing House: Beijing, China, 2013; Volume 2, pp. 1396–1405. [Google Scholar]
- Liu, Q.; Atrens, A. The influence of hydrogen on the low cycle fatigue behavior of medium strength 3.5NiCrMoV steel studied using notched specimens. Adv. Eng. Mater. 2018, 20, 1700680. [Google Scholar] [CrossRef]
- Tapia-Bastidas, C.V.; Atrens, A.; Gray, E.M. Thermal desorption spectrometer for measuring ppm concentrations of trapped hydrogen. Int. J. Hydrog. Energy 2018, 43, 7600–7617. [Google Scholar] [CrossRef]
- Venezuela, J.; Tapia-Bastidas, C.; Zhou, Q.; Depover, T.; Verbeken, K.; Gray, E.; Liu, Q.; Liu, Q.; Zhang, M.; Atrens, A. Determination of the equivalent hydrogen fugacity during electrochemical charging of 3.5NiCrMoV steel. Corros. Sci. 2018, 132, 90–106. [Google Scholar] [CrossRef]
- Atrens, A.; Venezuela, J.; Liu, Q.; Zhou, Q.; Verbeken, K.; Tapia-Bastidas, C.; Gray, E.; Christien, F.; Wolski, K. Electrochemical and mechanical aspects of hydrogen embrittlement evaluation of martensitic steels. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandel, K., Ed.; Elsevier: New York, NY, USA, 2018; Volume 6, pp. 201–225. [Google Scholar]
- Liu, Q. Influence of Hydrogen on Metallic Components for Clean Energy. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2015. [Google Scholar]
- Tapia-Bastidas, C.V. The Design, Construction and Implementation of a Novel State-of-the-Art Thermal Desorption Spectrometer for the Study of Hydrogen Embrittlement of Medium Strength Steels. Ph.D. Thesis, Griffith University, Nathan, Australia, 2016. [Google Scholar]
- Venezuela, J.; Liu, Q.; Zhang, M.; Zhou, Q.; Atrens, A. A review of hydrogen embrittlement of martensitic advanced high strength steels. Corros. Rev. 2016, 34, 153–186. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Q.; Venezuela, J.; Zhang, M.-X.; Wang, J.Q.; Atrens, A. A review of the influence of hydrogen on the mechanical properties of DP, TRIP and TWIP advanced high strength steels for auto construction. Corros. Rev. 2016, 34, 127–152. [Google Scholar] [CrossRef]
- Venezuela, J.; Blanch, J.; Zulkiply, A.; Liu, Q.; Zhou, Q.; Zhang, M.; Atrens, A. Further study of the hydrogen embrittlement of martensitic advanced high strength steel in simulated auto service conditions. Corros. Sci. 2018, 135, 120–135. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Q.; Venezuela, J.; Zhang, M.; Atrens, A. The role of microstructure on the influence of hydrogen in some advanced high strength steels. Mater. Sci. Eng. A 2018, 715, 370–378. [Google Scholar] [CrossRef]
- Liu, Q.; Gray, E.; Venezuela, J.; Zhou, Q.; Tapia-Bastidas, C.; Zhang, M.; Atrens, A. Equivalent hydrogen fugacity during electrochemical charging of 980DP steel determined by thermal desorption spectroscopy. Adv. Eng. Mater. 2018, 20, 1700469. [Google Scholar] [CrossRef]
- Venezuela, J.; Zhou, Q.; Liu, Q.; Zhang, M.; Atrens, A. Hydrogen trapping in some automotive martensitic advanced high-strength steels. Adv. Eng. Mater. 2018, 20, 1700468. [Google Scholar] [CrossRef]
- Venezuela, J.; Gray, E.; Liu, Q.; Zhou, Q.; Tapia-Bastidas, C.; Zhang, M.; Atrens, A. Equivalent hydrogen fugacity during electrochemical charging of some martensitic advanced high-strength steels. Corros. Sci. 2017, 127, 45–58. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Q.; Venezuela, J.; Zhang, M.; Atrens, A. Hydrogen influence on some advanced high-strength steels. Corros. Sci. 2017, 125, 114–138. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Q.; Venezuela, J.; Zhang, M.; Atrens, A. Hydrogen concentration in dual phase (DP) and quenched and partitioned (Q&P) advanced high strength steels (AHSS) under simulated service conditions compared with cathodic charging conditions. Adv. Eng. Mater. 2016, 18, 1588–1599. [Google Scholar]
- Liu, Q.; Venezuela, J.; Zhang, M.; Zhou, Q.; Atrens, A. Hydrogen trapping in some advanced high strength steels. Corros. Sci. 2016, 111, 770–785. [Google Scholar] [CrossRef] [Green Version]
- Venezuela, J.; Zhou, Q.; Liu, Q.; Zhang, M.; Atrens, A. Influence of hydrogen on the mechanical and fracture properties of some martensitic advanced high strength steels in simulated service conditions. Corros. Sci. 2016, 111, 602–624. [Google Scholar] [CrossRef]
- Venezuela, J.; Liu, Q.; Zhang, M.X.; Zhou, Q.; Atrens, A. The influence of hydrogen on the mechanical and fracture properties of some advanced high strength steels studied using the linearly increasing stress test. Corros. Sci. 2015, 99, 98–117. [Google Scholar] [CrossRef]
- Husby, H.; Iannuzzi, M.; Kappes, M.; Barnoush, A. Effect of nickel on hydrogen permeation in ferritic/pearlitic low alloy steels. Int. J. Hydrog. Energy 2018, 43, 3845–3861. [Google Scholar] [CrossRef]
- Rogne, B.R.S.; Kheradmand, N.; Deng, Y.; Barnoush, A. In situ micromechanical testing in environmental scanning electron microscope: A new insight into hydrogen-assisted cracking. Acta Mater. 2018, 144, 257–268. [Google Scholar] [CrossRef]
- Deng, Y.; Barnoush, A. Hydrogen embrittlement revealed via novel in situ fracture experiments using notched micro-cantilever specimens. Acta Mater. 2018, 142, 236–247. [Google Scholar] [CrossRef]
- Hajilou, T.; Deng, Y.; Rogne, B.R.; Kheradmand, N.; Barnoush, A. In situ electrochemical microcantilever bending test: A new insight into hydrogen enhanced cracking. Scr. Mater. 2017, 132, 12–21. [Google Scholar] [CrossRef]
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Djuki, M.B.; Bakic, G.M.; Zeravcic, V.S.; Rajicic, B.; Seedmak, A.; Mitrovic, R.; Miskovic, Z. Towards a unified and practical industrial model for prediction of hydrogen embrittlement and damage in steels. Procedia Struct. Integr. 2016, 2, 604–611. [Google Scholar] [CrossRef]
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.; Sedmak, A.; Rajicic, B. Hydrogen embrittlement of low carbon structural steel. Procedia Mater. Sci. 2014, 3, 1167–1172. [Google Scholar] [CrossRef]
- Iannuzzi, M. Environmentally assisted cracking (EAC) in oil and gas production. In Stress Corrosion Cracking; Woodhead Publishing Ltd.: Sawston, UK, 2011; pp. 570–607. [Google Scholar]
- Li, B.; Koyama, M.; Sakurada, E.; Yoshimura, N.; Ushioda, K.; Noguchi, H. Temperature dependence of transgranular fatigue crack resistance in interstitial-free steel and Fe-C steels with supersaturated carbon: Effects of dynamic strain aging and dynamic precipitation. Int. J. Fatigue 2018, 110, 1–9. [Google Scholar] [CrossRef]
- Mohammadi, A.; Koyama, M.; Gerstein, G.; Maier, H.J.; Noguchi, H. Hydrogen-assisted failure in a bimodal twinning-induced plasticity steel: Delamination events and damage evolution. Int. J. Hydrog. Energy 2018, 43, 2492–2502. [Google Scholar] [CrossRef]
- Li, B.; Koyama, M.; Hamada, S.; Noguchi, H. Threshold stress intensity factor range of a mechanically-long and microstructually-short crack perpendicular to an interface with plastic mismatch. Eng. Fract. Mech. 2017, 182, 287–302. [Google Scholar] [CrossRef]
- Yoshimura, N.; Ushioda, K.; Yonemura, M.; Koyama, M.; Tanaka, M.; Noguchi, H. Effect of the state of carbon on ductility in Fe-0.017mass%C ferritic steel. Mater. Sci. Eng. A 2017, 701, 120–128. [Google Scholar] [CrossRef]
- Koyama, M.; Onishi, Y.; Noguchi, H. Characteristics of hydrogen assisted intergranular fatigue crack growth in interstitial free steel: Role of plastic strain localization. Int. J. Fract. 2017, 206, 123–130. [Google Scholar] [CrossRef]
- Jemblie, L.; Olden, V.; Maincon, P.; Akselsen, O.M. Cohesive zone modelling of hydrogen induced cracking on the interface of clad steel pipes. Int. J. Hydrog. Energy 2017, 42, 28622–28634. [Google Scholar] [CrossRef]
- Yu, H.; Olsen, J.S.; Olden, V.; Alvaro, A.; He, J.Y.; Zhang, Z. Cohesive zone simulation of grain size and misorientation effects on hydrogen embrittlement in nickel. Eng. Fail. Anal. 2017, 81, 79–93. [Google Scholar] [CrossRef]
- Alvaro, A.; Jensen, I.; Kheradmand, N.; Løvvik, O.M.; Olden, V. Hydrogen embrittlement in nickel, visited by first principles modeling, cohesive zone simulation and nanomechanical testing. Int. J. Hydrog. Energy 2015, 40, 16892–16900. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. Thermal desorption spectroscopy study of the hydrogen trapping ability of W based precipitates in a Q&T matrix. Int. J. Hydrog. Energy 2018, 43, 5760–5769. [Google Scholar]
- Depover, T.; Verbeken, K. The effect of TiC on the hydrogen induced ductility loss and trapping behavior of Fe-C-Ti alloys. Corros. Sci. 2016, 112, 308–326. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. Evaluation of the effect of V4C3 precipitates on the hydrogen induced mechanical degradation in Fe-C-V alloys. Mater. Sci. Eng. A 2016, 675, 299–313. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. Evaluation of the role of Mo2C in hydrogen induced ductility loss in Q&T FeCMo alloys. Int. J. Hydrog. Energy 2016, 41, 14310–14329. [Google Scholar]
- Depover, T.; Verbeken, K. Hydrogen trapping and hydrogen induced mechanical degradation in lab cast Fe-C-Cr alloys. Mater. Sci. Eng. A 2016, 669, 134–149. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. Hydrogen induced mechanical degradation in tungsten alloyed steels. Mater. Charact. 2018, 136, 84–93. [Google Scholar] [CrossRef]
- Fan, Y.H.; Zhang, B.; Yi, H.L.; Hao, G.S.; Sun, Y.Y.; Wang, J.Q.; Han, E.H.; Ke, W. The role of reversed austenite in hydrogen embrittlement fracture of S41500 martensitic stainless steel. Acta Mater. 2017, 139, 188–195. [Google Scholar] [CrossRef]
- Zafra, A.; Peral, L.B.; Belzunce, J.; Rodríguez, C. Effect of hydrogen of on the tensile properties of 42CrMo4 steel quenched and tempered at different temperatures. In. J. Hydrog. Energy 2018, 43, 9068–9082. [Google Scholar] [CrossRef]
- Shin, D.H.; Lee, T.; Lee, J.; Lee, H.J.; Yoo, J.Y.; Lee, C.S. Increased resistance to hydrogen embrittlement in high-strength steels composed of granular bainite. Mater. Sci. Eng. A 2018, 700, 473–480. [Google Scholar] [CrossRef]
- García, T.E.; Arroyo, B.; Rodríguez, C.; Belzunce, F.J.; Álvarez, J.A. Small punch test methodologies for the analysis of the hydrogen embrittlement of structural steels. Theor. Appl. Fract. Mech. 2016, 86A, 89–100. [Google Scholar] [CrossRef]
- Sanchez, J.; Martin-Rengel, S.F.L.M.A.; Fullea, J.; Andrade, C.; Ruiz-Herevias, J. Measurement of hydrogen and embrittlement of high strength steels. Eng. Fail. Anal. 2016, 59, 467–477. [Google Scholar] [CrossRef]
- Ichii, K.; Koyama, M.; Tasan, C.C.; Tsuzaki, K. Comparative study of hydrogen embrittlement in stable and metastable high-entropy alloys. Scr. Mater. 2018, 150, 74–77. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Sijacki Zeravcic, V.; Sedmak, A.; Rajicic, B. Hydrogen Embrittlement of Industrial Components: Prediction, Prevention, and Models. Corrosion 2016, 72, 943–961. [Google Scholar] [CrossRef]
- Popov, B.N.; Lee, J.; Djukic, M.B. Chapter 7: Hydrogen Permeation and Hydrogen Induced Cracking. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; Elsevier: New York, NY, USA, 2018. [Google Scholar]
- Iannuzzi, M.; Barnoush, A.; Johnsen, R. Materials and Corrosion Trends in Offshore and Subsea Oil and Gas Production. Mater. Degrad. 2017, 1, 2. [Google Scholar] [CrossRef]
- Barnoush, A.; Yang, B.; Vehoff, H. Chapter: Effect of Hydrogen and Grain Boundaries on Dislocation Nucleation and Multiplication Examined with a NI-AFM. In Advances in Solid State Physics; Springer: Berlin, Germany, 2008; Volume 47, pp. 253–269. [Google Scholar]
- Koyama, M.; Akiyama, E.; Lee, Y.-K.; Raabe, D.; Tsuzaki, K. Overview of hydrogen embrittlement in high-Mn steels. Int. J. Hydrog. Energy 2017, 42, 12706–12723. [Google Scholar] [CrossRef]
- Koyama, M.; Rohwerder, M.; Tasan, C.C.; Bashir, A.; Akiyama, E.; Takai, K.; Raabe, D.; Tsuzaki, K. Recent progress in microstructural hydrogen mapping in steels: Quantification, kinetic analysis, and multi-scale characterization. Mater. Sci. Technol. 2017, 33, 1481–1496. [Google Scholar] [CrossRef]
- Jemblie, L.; Olden, V.; Akselsen, O.M. A review of cohesive zone modelling as an approach for numerically assessing hydrogen embrittlement of steel structures. Philos. Trans. R. Soc. A 2017, 375, 20160411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannuzzi, M. Chapter 15: Environmentally assisted cracking (EAC) in oil and gas production. In Stress Corrosion Cracking: Theory and Practice; Raja, V.S., Shoji, T., Eds.; Woodhead: Sawston, UK, 2011; pp. 570–607. [Google Scholar]
- Myers, S.; Baskes, M.; Birnbaum, H.; Corbett, J.; DeLeo, G.; Estreicher, S.K.; Haller, E.E.; Jena, P.; Johnson, N.M.; Kirchheim, R.; et al. Hydrogen interactions with defects in crystalline solids. Rev. Mod. Phys. 1992, 64, 559–617. [Google Scholar] [CrossRef]
- Serebrinsky, A.; Carter, E.A.; Ortiz, M. A quantum-mechanically informed continuum model of hydrogen embrittlement. J. Mech. Phys. Solids 2004, 52, 2403–2430. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Pundt, A.; Kirchheim, R. Hydrogen in metals: Microstructural aspects. Annu. Rev. Mater. Res. 2006, 36, 555–608. [Google Scholar] [CrossRef]
- Bhadeshia, H.K. Prevention of hydrogen embrittlement in steels. ISIJ Int. 2016, 56, 24–36. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen embrittlement understood. Metall. Mater. Trans. B 2015, 46, 1085–1103. [Google Scholar] [CrossRef]
- Song, J.; Curtin, W.A. Atomic mechanism and prediction of hydrogen embrittlement in iron. Nat. Mater. 2013, 12, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Oriani, R.A. Award lecture-1987: Hydrogen-the versatile embrittler. Corrosion 1987, 43, 390–397. [Google Scholar] [CrossRef]
- Dadfarnia, M.; Nagao, A.; Wang, S.; Martin, M.L.; Somerday, B.P.; Sofronis, P. Recent advances on hydrogen embrittlement of structural materials. Int. J. Fract. 2015, 196, 223–243. [Google Scholar] [CrossRef]
- Nagao, A.; Dadfarnia, M.; Somerday, B.P.; Sofronis, P.; Ritchie, R.O. Hydrogen-enhanced-plasticity mediated decohesion for hydrogen-induced intergranular and “quasi-cleavage” fracture of lath martensitic steels. J. Mech. Phys. Solids 2018, 112, 403–430. [Google Scholar] [CrossRef]
- Nagao, A.; Smith, C.D.; Dadfarnia, M.; Sofronis, P.; Robertson, I.M. The role of hydrogen in hydrogen embrittlement fracture of lath martensitic steel. Acta Mater. 2012, 60, 5182–5189. [Google Scholar] [CrossRef]
- Barrera, O.; Bombac, D.; Chen, Y.; Daff, T.D.; Galindo-Nava, E.; Gong, P.; Haley, D.; Horton, R.; Katzarov, I.; Kermode, J.R.; et al. Understanding and mitigating hydrogen embrittlement of steels: A review of experimental, modelling and design progress from atomistic to continuum. J. Mater. Sci. 2018, 53, 6251–6290. [Google Scholar] [CrossRef]
- Katz, Y.; Tymiak, N.; Gerberich, W.W. Nanomechanical probes as new approaches to hydrogen/deformation interaction studies. Eng. Fract. Mech. 2001, 68, 619–646. [Google Scholar] [CrossRef]
- Nagumo, M. Hydrogen related failure of steels-a new aspect. Mater. Sci. Technol. 2004, 20, 940–950. [Google Scholar] [CrossRef]
- Ramamurthy, R.; Atrens, A. Stress Corrosion Cracking of High Strength Steels. Corros. Rev. 2013, 31, 1–31. [Google Scholar] [CrossRef]
- Ganglof, R.P.; Somerday, B.P. Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Woodhead: Sawston, UK, 2012. [Google Scholar]
- Dietzel, W.; Atrens, A.; Barnoush, A. Mechanics of modern test methods and quantitative-accelerated testing for hydrogen embrittlement. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Ganglof, R.P., Somerday, B.P., Eds.; Woodhead: Sawston, UK, 2012; Chapter 8; pp. 237–273. [Google Scholar]
- Villalba, E.; Atrens, A. Hydrogen Embrittlement and Rock Bolt Stress Corrosion Cracking. Eng. Fail. Anal. 2009, 16, 164–175. [Google Scholar] [CrossRef]
- Villalba, E.; Atrens, A. Metallurgical Aspects of Rock Bolt Stress Corrosion Cracking. Mater. Sci. Eng. A 2008, 491, 8–18. [Google Scholar] [CrossRef]
- Villalba, E.; Atrens, A. SCC of Commercial Steels Exposed to High Hydrogen Fugacity. Eng. Fail. Anal. 2008, 15, 617–641. [Google Scholar] [CrossRef]
- Villalba, E.; Atrens, A. An Evaluation of Steels Subjected to Rock Bolt SCC Conditions. Eng. Fail. Anal. 2007, 14, 1351–1393. [Google Scholar] [CrossRef]
- Gamboa, E.; Atrens, A. Material influence on the stress corrosion cracking of rock bolts. Eng. Fail. Anal. 2005, 12, 201–225. [Google Scholar] [CrossRef]
- Gamboa, E.; Atrens, A. Environmental Influence on the Stress Corrosion Cracking of Rock Bolts. Eng. Fail. Anal. 2003, 10, 521–558. [Google Scholar] [CrossRef]
- Gamboa, E.; Atrens, A. Stress Corrosion cracking fracture mechanisms in rock bolts. J. Mater. Sci. 2003, 38, 3813–3829. [Google Scholar] [CrossRef]
- Atrens, A.; Mezzanotte, D.; Fiore, N.F.; Genshaw, M.A. Electrochemical Studies of Hydrogen Diffusion and Permeability in Ni. Corros. Sci. 1980, 20, 673–684. [Google Scholar] [CrossRef]
- Atrens, A.; Wang, J.Q.; Stiller, K.; Andren, H.O. Atom probe field ion microscope measurements of carbon segregation at an grain boundary and service failures by intergranular stress corrosion cracking. Corros. Sci. 2006, 48, 79–92. [Google Scholar] [CrossRef]
- Wang, J.Q.; Atrens, A. Analysis of Service Stress Corrosion Cracking in a Natural Gas Transmission Pipeline, Active or Dormant? Eng. Fail. Anal. 2004, 11, 3–18. [Google Scholar] [CrossRef]
- Wang, Z.F.; Atrens, A. Initiation of Stress Corrosion Cracking for Pipeline Steels in a Carbonate-Bicarbonate Solution. Metall. Mater. Trans. A 1996, 27, 2686–2691. [Google Scholar] [CrossRef]
- Rieck, R.M.; Atrens, A.; Smith, I.O. The Role of Crack Tip Strain Rate in the Stress Corrosion Cracking of High Strength Steels in Water. Metall. Trans. A 1989, 20, 889–895. [Google Scholar] [CrossRef]
- Kinaev, N.N.; Cousens, D.R.; Atrens, A. The Crack Tip Strain Field of AISI 4340 Part III Hydrogen Influence. J. Mater. Sci. 1999, 34, 4931–4936. [Google Scholar] [CrossRef]
- Ramamurthy, R.; Atrens, A. The Stress Corrosion Cracking of As-Quenched 4340 and 3.5NiCrMoV Steels Under Stress Rate Control in Distilled Water at 90C. Corros. Sci. 1993, 34, 1385–1402. [Google Scholar] [CrossRef]
- Atrens, A.; Brosnan, C.C.; Ramamurthy, S.; Oehlert, A.; Smith, I.O. Linearly Increasing Stress Test (LIST) for SCC Research. Meas. Sci. Technol. 1993, 4, 1281–1292. [Google Scholar] [CrossRef]
- Skogsmo, J.; Atrens, A. Analytic Electron Microscopy of Grain Boundaries in High Strength Steel. Acta Metall. Mater. 1994, 42, 1139–1146. [Google Scholar] [CrossRef]
- Oehlert, A.; Atrens, A. Initiation and Propagation of Stress Corrosion Cracking in AISI 4340 and 3.5NiCrMoV Rotor Steel in Constant Load Tests. Corros. Sci. 1996, 38, 1159–1170. [Google Scholar] [CrossRef]
- Oehlert, A.; Atrens, A. SCC Propagation in Aermet 100. J. Mater. Sci. 1998, 33, 775–781. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Dietzel, W.; Song, G.; Kainer, K.U. The Fractography of Stress Corrosion Cracking (SCC) of Mg-Al Alloys. Metall. Mater. Trans. A 2008, 39, 1157. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Song, G.; Ghali, E.; Dietzel, W.; Kainer, K.U.; Hort, N.; Blawert, C. A critical review of the stress corrosion cracking (SCC) of magnesium alloys. Adv. Eng. Mater. 2005, 7, 659–693. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Dietzel, W.; Song, G.; Kainer, K.U. Evaluation of the Delayed Hydride Cracking Mechanism for Transgranular Stress Corrosion Cracking of Magnesium Alloys. Mater. Sci. Eng. A 2007, 466, 18–31. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Dietzel, W.; Song, G.; Kainer, K.U. Comparison of the Linearly Increasing Stress Test and the Constant Extension Rate Test in the Evaluation of Transgranular Stress Corrosion Cracking of Magnesium. Mater. Sci. Eng. A 2008, 472, 97–106. [Google Scholar] [CrossRef]
- Bobby Kannan, M.; Dietzel, W.; Blawert, C.; Atrens, A.; Lyon, P. Stress corrosion cracking of rare-earth-containing magnesium alloys ZE41, QE22, and Elektron 21 (EV31A) compared with AZ80. Mater. Sci. Eng. A 2008, 480, 529–539. [Google Scholar] [CrossRef]
- Cann, C.D.; Atrens, A. A Metallographic Study of the Terminal Solubility of Hydrogen in Zirconium at Low Hydrogen Concentrations. J. Nucl. Mater. 1980, 88, 42–50. [Google Scholar] [CrossRef]
- Atrens, A.; Dannhaeuser, G.; Baero, G. Stress Corrosion Cracking of Zircaloy-4 Cladding Tubes, Part 1. Threshold in the presence of iodine. J. Nucl. Mater. 1984, 126, 91–102. [Google Scholar] [CrossRef]
- Song, R.G.; Dietzel, W.; Zhang, B.J.; Liu, W.J.; Tseng, M.K.; Atrens, A. Stress corrosion cracking and hydrogen embrittlement of an Al-Zn-Mg-Cu alloy. Acta Mater. 2004, 52, 4727–4743. [Google Scholar] [CrossRef]
- Atrens, A. Service performance of engineering materials. J. Mater. Sci. Technol. 2005, 21, 1–5. [Google Scholar]
- Gamboa, E.; Atrens, A. relationship of rock bolt SCC to service failures of rock bolts. In Hydrogen Effects on Material Behavior and Corrosion Deformation Interactions; TMS/AIME: Pittsburgh, PA, USA, 2003; pp. 647–661. [Google Scholar]
- Winzer, N.; Atrens, A.; Dietzel, W.; Kainer, K.U. The role of hydrogen in the stress corrosion cracking of Mg-Al alloys. In Effects of Hydrogen on Materials; TMS: Pittsburgh, PA, USA, 2009; pp. 259–262. [Google Scholar]
- Mezzanotte, D.A.; Fiori, N.F.; Kargol, J.A.; Atrens, A. Hydrogen mobility in a Ni-base alloy. J. Met. 1979, 31, F13. [Google Scholar]
- McElligott, J.; Shi, Z.; Li, Y.; Wen, C.; Atrens, A. Corrosion of Ti35Zr28Nb in Hanks’ solution and 3.5wt% NaCl solution. Mater. Corros. 2018, 69, 197–206. [Google Scholar] [CrossRef]
- Rodger, J.; Bartlett, S.; Atrens, A. Corrosion of the galvanizing of galvanized-steel electricity towers. Mater. Corros. 2017, 68, 902–910. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, K.; Zhang, L.; Atrens, A.; Yu, J.; Li, X. Solidification of Mg-Zn-Y alloys at 6 GPa pressure: Nanostructure, phases formed, and their stability. JMEP 2016, 25, 3830–3837. [Google Scholar] [CrossRef]
- Atrens, A.; Wang, Z.F. Stress Corrosion Cracking. Mater. Forum 1995, 19, 9–34. [Google Scholar]
- Faller, M.; Richner, P. Materials selection of safety relevant components in indoor swimming pools. Mater. Corros. 2003, 54, 331–338. [Google Scholar] [CrossRef]
- Degrève, F.; Jardin, C. New methods for the determination of hydrogen content of aluminum and its alloys: Part II. Rapid determination by the nitrogen carrier fusion method. Metall. Trans. B 1975, 6, 545–550. Available online: http://www.leco.com/products/inorganic/hydrogen/dh_603/dh_603.html (accessed on 11 June 2018). [CrossRef]
- Smith, S.W.; Scully, J.R. The identification of hydrogen trapping states in an Al-Li-Cu-Zr alloy using thermal desorption spectroscopy. Metall. Mater. Trans. A 2000, 31, 179–193. [Google Scholar] [CrossRef]
- Atrens, A. Initiation of fatigue cracks in duplex stainless steel X4CrMnNiMoN2664 in 4N NaCl at 80(C, pH = 2 and 7. Met. Technol. 1982, 9, 117–121. [Google Scholar] [CrossRef]
- Addach, H.; Bercot, P.; Rezrazi, M.; Takadoum, J. Study of the electrochemical permeation of iron. Corros. Sci. 2009, 51, 263–267. [Google Scholar] [CrossRef]
- Winzer, N.; Rott, O.; Thiessen, R.; Thomas, I.; Mraczek, K.; Hoche, T.; Wright, L.; Mrovec, M. Hydrogen diffusion anf trapping in Ti-modified advanced high strength steels. Mater. Des. 2016, 92, 450–461. [Google Scholar] [CrossRef]
- Wu, T.; Yan, M.; Zeng, D.; Xu, J.; Sun, C.; Yu, C.; Ke, W. Hydrogen permeation of X80 steel with superficial stress in presence of sulphate reducing bacteria. Corros. Sci. 2015, 91, 86–94. [Google Scholar] [CrossRef]
- Flis-Kabulska, I.; Flis, J.; Zakroczymski, T. Enhanced hydrogen entry into iron from 0.1 M NaOH at definite potentials. Electrochim. Acta 2008, 53, 3094–3101. [Google Scholar] [CrossRef]
- Flis-Kabulska, I.; Flis, J.; Zakroczymski, T. Promotion of hydrogen entry into iron from NaOH by iron-oxygen species. Electrochim. Acta 2007, 52, 7158–7165. [Google Scholar] [CrossRef]
- Flis-Kabulska, I.; Zakroczymski, T.; Flis, J. Accelerated entry of hydrogen into iron fromNaOH solutions at low cathodic and low anodic polarisations. Electrochim. Acta 2007, 52, 2966–2977. [Google Scholar] [CrossRef]
- Zakroczymski, T. Adaption of the electrochemical permeation technique for studying entry, transport and trapping of hydrogen in metals. Electrochim. Acta 2006, 51, 2261–2266. [Google Scholar] [CrossRef]
- Qian, S.Y.; Conway, B.E.; Jekiewicz, G. Kineric rationalisation of catalyst effect of cathodic H sorption into metals: Relation of enhancement and inhibition to H coverage. J. Chem. Soc. Faraday Trans. 1998, 94, 2945–2954. [Google Scholar] [CrossRef]
- Frappart, S.; Feagas, X.; Creus, J.; Thebault, F.; Delattre, L.; Marchebois, H. Study of the hydrogen diffusion and segregation into Fe–C–Mo martensitic HSLA steel using electrochemical permeation test. J. Phys. Chem. Solids 2010, 71, 1467–1479. [Google Scholar] [CrossRef] [Green Version]
- Frappart, S.; Feagas, X.; Creus, J.; Thebault, F.; Delattre, L.; Marchebois, H. Hydrogen solubility, diffusivity and trapping in a tempered Fe-C-Cr martensitic steel under various mechanical stress states. Mater. Sci. Eng. A 2012, 534, 384–393. [Google Scholar] [CrossRef]
- Dos Santos, D.S.; de Mirna, P.E.V. Hydrogen solubility in amorphous and crystalline materials. Int. J. Hydrog. Energy 1998, 23, 1011–1017. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Beck, W.; Genshaw, M.A.; Subramanyan, P.A.; Williams, F.S. The effect of stress on the chemical potential of hydrogen in iron and steel. Acta Metall. 1971, 19, 1209–1218. [Google Scholar] [CrossRef]
- Mezzanotte, D.A.; Kargol, J.A.; Fiore, N.F. Hydrogen transport in nickel base alloys. Metall. Trans. A 1982, 13, 1181–1186. [Google Scholar] [CrossRef]
- Brass, A.M.; Chene, J. Influence of tensile straining on the permeation of hydrogen in low alloy Cr-Mo steels. Corros. Sci. 2006, 48, 481–497. [Google Scholar] [CrossRef]
- Brass, A.M.; Chene, J. Influence of deformation on the hydrogen behaviour in iron and nickel base alloys: A review of experimental data. Mater. Sci. Eng. A 1998, 242, 210–221. [Google Scholar] [CrossRef]
- Lan, L.; Kong, X.; Hu, Z.; Qiu, C.; Zhao, D.; Du, L. Hydrogen permeation behaviour in relation to microstructural evolution of low carbon banitic steel weldments. Corros. Sci. 2016, 112, 180–193. [Google Scholar] [CrossRef]
- Xiong, X.L.; Tao, X.; Zhou, Q.J.; Li, J.X.; Volinsky, A.A.; Su, Y.J. Hydrostatic pressure effects on hydrogen permeation in A514 steel during galvanostatic hydrogen charging. Corros. Sci. 2016, 112, 86–93. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, T.; Zhao, Y.; Sun, J.; Wang, Y. Hydrogen permeation and emrittlement susceptibility of X80 welded joint under high-pressure gas environment. Corros. Sci. 2016, 111, 84–97. [Google Scholar] [CrossRef]
- Marcus, P. (Ed.) Corrosion Mechanisms in Theory and Practice, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Flis, J.; Zakroczymski, T.; Kleshnya, V.; Kobiela, T.; Duś, R. Changes in hydrogen entry rate and in surface of iron during cathodic polarisation in alkaline solutions. Electrochim. Acta 1999, 44, 3989–3997. [Google Scholar] [CrossRef]
- McBreen, J.; Nonis, L.; Beck, W. A method for determination of the permeation rate of hydrogen through metal membranes. J. Electrochem. Soc. 1966, 113, 1218–1222. [Google Scholar] [CrossRef]
- Bockris, J.; McBreen, J.; Nanis, L. The hydrogen evolution kinetics and hydrogen entry into alpha-iron. J. Electrochem. Soc. 1965, 112, 1025–1031. [Google Scholar] [CrossRef]
- Sezgin, J.B.; Bosch, C.; Montouchet, A.; Perrin, G.; Wolski, K. Modelling of hydrogen induced pressurization of internal cavities. Int. J. Hydrog. Energy 2017, 42, 15403–15414. [Google Scholar] [CrossRef]
- Sezgin, J.B. Modelization de la formation des decohesions dues a l’hydrogren dans l’acier 18MND5, These de doctorate de l’universite de Lyon (No 488); L’Ecole des mines des Saint-Etienne: Saint-Étienne, France, 2017. [Google Scholar]











| Steel | Composition, wt % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Cr | Ni | Mo | V | Cu | Si | Al | S | P | Nb or Ti | |
| NiCrMo1 | 0.09 | 0.88 | 0.52 | 1.04 | 0.60 | 0.01 | 0.06 | 0.31 | 0.017 | 0.01 | 0.01 | <0.01 |
| 0.08 | 0.91 | 0.48 | 0.98 | 0.60 | 0.01 | 0.06 | 0.31 | 0.018 | 0.01 | 0.01 | <0.01 | |
| 3.5NiCrMoV | 0.22 | 0.23 | 1.67 | 2.77 | 0.43 | 0.09 | 0.09 | 0.08 | <0.005 | 0.01 | 0.01 | <0.01 |
| 0.21 | 0.22 | 1.65 | 2.75 | 0.41 | 0.09 | 0.09 | 0.07 | <0.005 | 0.01 | 0.01 | <0.01 | |
| 27NiCrMoV15-6 | 0.25 | 0.24 | 1.52 | 3.50 | 0.40 | 0.11 | 0.08 | 0.08 | <0.005 | 0.01 | 0.01 | <0.01 |
| 0.27 | 0.24 | 1.55 | 3.50 | 0.41 | 0.12 | 0.08 | 0.08 | <0.005 | 0.01 | 0.01 | <0.01 | |
| 34CrNiMo6 | 0.38 | 0.59 | 1.61 | 1.5 | 0.18 | 0.01 | 0.18 | 0.23 | 0.025 | 0.01 | 0.01 | <0.01 |
| 0.38 | 0.60 | 1.63 | 1.49 | 0.18 | 0.01 | 0.18 | 0.24 | 0.026 | 0.01 | 0.01 | <0.01 | |
| Air or Applied Potential, mVAg/AgCl | Applied Stress Rate MPa/s | σY, MPa | σF, MPa | RA, % |
|---|---|---|---|---|
| Air | 0.2 | 650 | 770 | 79 |
| Air | 0.2 | 681 | 796 | 79 |
| Air | 0.02 | 671 | 793 | 78 |
| Air | 0.002 | 656 | 772 | 79 |
| Ecorr | 0.2 | 719 | 841 | 81 |
| Ecorr | 0.2 | 719 | 850 | 80 |
| Ecorr | 0.02 | 710 | 818 | 80 |
| Ecorr | 0.002 | 695 | 807 | 82 |
| −950 | 0.02 | 737 | 840 | 36 |
| −950 | 0.02 | 685 | 788 | 78 |
| −950 | 0.002 | 693 | 802 | 73 |
| −1200 | 0.02 | 702 | 816 | 64 |
| −1200 | 0.02 | 678 | 784 | 75 |
| −1200 | 0.002 | 687 | 798 | 72 |
| −1400 | 0.02 | 685 | 792 | 64 |
| −1700 | 0.02 | 680 | 774 | 50 |
| −1700 | 0.2 | 688 | 811 | 72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atrens, A.; Liu, Q.; Tapia-Bastidas, C.; Gray, E.; Irwanto, B.; Venezuela, J.; Liu, Q. Influence of Hydrogen on Steel Components for Clean Energy. Corros. Mater. Degrad. 2020, 1, 3-26. https://doi.org/10.3390/cmd1010002
Atrens A, Liu Q, Tapia-Bastidas C, Gray E, Irwanto B, Venezuela J, Liu Q. Influence of Hydrogen on Steel Components for Clean Energy. Corrosion and Materials Degradation. 2020; 1(1):3-26. https://doi.org/10.3390/cmd1010002
Chicago/Turabian StyleAtrens, Andrej, Qian Liu, Clotario Tapia-Bastidas, Evan Gray, Bartolomeus Irwanto, Jeff Venezuela, and Qinglong Liu. 2020. "Influence of Hydrogen on Steel Components for Clean Energy" Corrosion and Materials Degradation 1, no. 1: 3-26. https://doi.org/10.3390/cmd1010002








