Optimization of Alachlor Photocatalytic Degradation with Nano-TiO2 in Water under Solar Illumination: Reaction Pathway and Mineralization
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Photocatalytic Activity and Analyses
3. Results and Discussion
3.1. UV–Vis Spectral Changes
3.2. Effect of TiO2 Dosages on Photodegradation
3.3. Effect of Temperature on Photodegradation
3.4. Effect of Initial pH on Photodegradation
3.5. Effect of Light Intensity on Photodegradation
3.6. Effect of Illumination Time
3.7. Evolution of the Mineralization
3.8. Photoproducts and Photodegradation Pathway
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Zhang, Y. Degradation of alachlor in aqueous solution by using hydrodynamic cavitation. J. Hazard. Mater. 2009, 161, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Maheswari, S.T. Dissipation of alachlor in cotton plant, soil and water and its bioaccumulation in fish. Chemosphere 2004, 54, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Rivera, K.K.P.; de Luna, M.D.G.; Suwannaruang, T.; Wantala, K. Photocatalytic degradation of reactive red 3 and alachlor over uncalcined Fe–TiO2 synthesized via hydrothermal method. Desalin. Water Treat. 2016, 57, 22017–22028. [Google Scholar] [CrossRef]
- Gabaldón, J.A.; Cascales, J.M.; Maquieira, A.; Puchades, R. Rapid trace analysis of alachlor in water and vegetable samples. J. Chromatogr. A 2002, 963, 125–136. [Google Scholar] [CrossRef]
- Horberg, G. Risk, science and politics, Alachlor regulation in Canada and the United States. Can. J. Political Sci. 1990, 23, 257–277. [Google Scholar] [CrossRef]
- Special Review of Certain Pesticide Products–Alachlor; Document No. PB85-175503; Office of Pesticide Programs, Environmental Protection Agency: Springfield, VA, USA, 1985.
- Osano, O.; Admiraala, W.; Klamerc, H.J.C.; Pastorc, D.; Bleekera, E.A.J. Comparative toxic and genotoxic effects of chloroacetanilides, formamidines and their degradation products on Vibrio fischeri and Chironomus riparius. Environ. Pollut. 2002, 119, 195–202. [Google Scholar] [CrossRef]
- Grizard, G.; Ouchchane, L.; Roddier, H.; Artonne, C.; Sion, B.; Vasson, M.-P.; Janny, L. In vitro alachlor effects on reactive oxygen species generation, motility patterns and apoptosis markers in human spermatozoa. Reprod. Toxicol. 2007, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Qu, J.H.; Liu, H.J. Decomposition of alachlor by ozonation and its mechanism. J. Environ. Sci. 2007, 19, 769–775. [Google Scholar] [CrossRef]
- Bahena, C.L.; Martinez, S.S. Photodegradation of chlorbromuron, atrazine, and alachlor in aqueous systems under solar irradiation. Int. J. Photoenergy 2006, 2006, 81808. [Google Scholar] [CrossRef]
- Katsumata, H.; Kaneco, S.; Suzuki, T.; Ohta, K.; Yobiko, Y. Photo-Fenton degradation of alachlor in the presence of citrate solution. J. Photochem. Photobiol. A Chem. 2006, 180, 38–45. [Google Scholar] [CrossRef]
- Wayment, D.G.; Casadonte, D.J., Jr. Frequency effect on the sonochemical remediation of Alachlor. Ultrason. Sonochem. 2002, 9, 251–257. [Google Scholar] [CrossRef]
- Devipriya, S.; Yesodharan, S. Photocatalytic degradation of pesticide contaminants in water. Sol. Energy Mater. Sol. Cells 2005, 86, 309–348. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.; Tryk, D. Titanium dioxide photocatalysis. J. Photochem. Photobiol. A. Chem. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Kansal, S.K.; Ali, A.H.; Kapoor, S. Photocatalytic decolorization of biebrich scarlet dye in aqueous phase using different nanophotocatalysts. Desalination 2010, 259, 147–155. [Google Scholar] [CrossRef]
- Kenichi, F.; Teijiro, Y.; Chikayoshi, N.; Haruo, S. Molecular-orbital theory of orientation in aromatic, heteroaromatic, and other conjugated molecules. J. Chem. Phys. 1954, 22, 1433–1442. [Google Scholar]
- Behnajady, M.A.; Modirshahla, N.; Hamzavi, R. Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J. Hazard. Mater. B 2006, 133, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Ishiki, R.R.; Ishiki, H.M.; Takashima, K. Photocatalytic degradation of imazethapyr herbicide at TiO2/H2O interface. Chemosphere 2005, 58, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.A.I.; Ahsan, S.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Degradation, kinetics, and mineralization in solar photocatalytic treatment of aqueous amitrole solution with titanium dioxide. Environ. Eng. Sci. 2018, 35, 401–407. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Solar photocatalytic decomposition of Probenazole in water with TiO2 in the presence of H2O2. Energy Sources A Recov. Util. Environ. Eff. 2018, 40, 2432–2441. [Google Scholar] [CrossRef]
- Yang, H.G.; Li, C.Z.; Gu, H.C.; Fang, T.N. Rheological behavior of titanium dioxide suspensions. J. Colloid Interface Sci. 2001, 236, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Mirmasoomi, S.R.; Ghazi, M.M.; Galedari, M. Photocatalytic degradation of diazinon under visible light using TiO2/Fe2O3 nanocomposite synthesized by ultrasonic-assisted impregnation method. Sep. Purif. Technol. 2017, 175, 418–427. [Google Scholar] [CrossRef]
- Bonsen, E.M.; Schroeter, S.; Jacobs, H.; Broekaeet, J.A.C. Photocatalytic degradation of ammonia with TiO2 as photocatalyst in the laboratory and under the use of solar radiation. Chemosphere 1997, 35, 1431–1445. [Google Scholar] [CrossRef]
- Pramauro, E.; Vincenti, M.; Augugliaro, V.; Palmisano, L. Photocatalytic degradation of monuron in aqueous titanium dioxide dispersions. Environ. Sci. Technol. 1993, 27, 1790–1795. [Google Scholar] [CrossRef]
- Sakkas, V.A.; Dimou, A.; Pitarakis, K.; Mantis, G.; Albanis, T. TiO2 photocatalyzed degradation of diazinon in an aqueous medium. Environ. Chem. Lett. 2005, 3, 57–61. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Gharanjig, K. Photocatalytic degradation of agricultural N-heterocyclic organic pollutants using immobilized nanoparticles of titania. J. Hazard. Mater. 2007, 145, 65–71. [Google Scholar] [CrossRef] [PubMed]








| Atom | PC | FED | Atom | PC | FED |
|---|---|---|---|---|---|
| C (1) | −0.074 | 0.174 | H (20) | 0.133 | 0 |
| C (2) | −0.138 | 0.132 | H (21) | 0.139 | 0.001 |
| C (3) | −0.141 | 0.191 | H (22) | 0.042 | 0.012 |
| C (4) | −0.140 | 0.114 | H (23) | 0.042 | 0.012 |
| C (5) | −0.055 | 0.207 | H (24) | 0.122 | 0.003 |
| C (6) | 0.092 | 0.331 | H (25) | 0.122 | 0.003 |
| N (7) | −0.316 | 0. 233 | H (26) | 0.062 | 0 |
| C (8) | 0.149 | 0.116 | H (27) | 0.062 | 0 |
| C (9) | 0.331 | 0.125 | H (28) | 0.114 | 0.004 |
| C (10) | −0.159 | 0.088 | H (29) | 0.102 | 0.010 |
| O (11) | −0.288 | 0.065 | H (30) | 0.102 | 0.010 |
| Cl (12) | −0.083 | 0.062 | H (31) | 0.070 | 0.001 |
| O (13) | −0.273 | 0.023 | H (32) | 0.070 | 0.001 |
| C (14) | −0.068 | 0.022 | H (33) | 0.073 | 0 |
| C (15) | −0.123 | 0.009 | H (34) | 0.105 | 0.015 |
| C (16) | –0.202 | 0.001 | H (35) | 0.105 | 0.015 |
| C (17) | −0.116 | 0.018 | H (36) | 0.067 | 0.001 |
| C (18) | −0.209 | 0.001 | H (37) | 0.067 | 0.001 |
| H (19) | 0.136 | 0.001 | H (38) | 0.078 | 0 |
| Product | Rt (min) | Molecular Weight (m/z) | Characteristic Ions (Abundance, %) | Compound |
|---|---|---|---|---|
| a | 13.1 | 149 | 57 (100) | 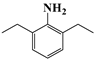 |
| 134 (54) | ||||
| 149 (39) | ||||
| b | 15.8 | 177 | 57 (100) |  |
| 162 (86) | ||||
| 177 (79) | ||||
| c | 17.2 | 223 | 146 (100) |  |
| 174 (56) | ||||
| 223 (40) | ||||
| d | 18.3 | 225 | 147 (18) | 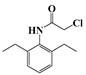 |
| 176 (100) | ||||
| 225 (10) | ||||
| e | 21.7 | 251 | 160 (100) | 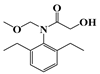 |
| 174 (62) | ||||
| 202 (48) | ||||
| f | 23.0 | 283 | 174 (100) | 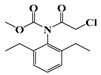 |
| 206 (32) | ||||
| 248 (37) | ||||
| g | 23.6 | 255 | 146 (100) | 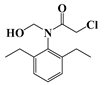 |
| 174 (74) | ||||
| 223 (27) | ||||
| h | 25.7 | 285 | 176 (100) | 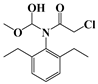 |
| 204 (61) | ||||
| 253 (36) | ||||
| i | 27.1 | 285 | 176 (100) |  |
| 204 (61) | ||||
| 253 (32) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Optimization of Alachlor Photocatalytic Degradation with Nano-TiO2 in Water under Solar Illumination: Reaction Pathway and Mineralization. Clean Technol. 2019, 1, 141-153. https://doi.org/10.3390/cleantechnol1010010
Molla MAI, Furukawa M, Tateishi I, Katsumata H, Kaneco S. Optimization of Alachlor Photocatalytic Degradation with Nano-TiO2 in Water under Solar Illumination: Reaction Pathway and Mineralization. Clean Technologies. 2019; 1(1):141-153. https://doi.org/10.3390/cleantechnol1010010
Chicago/Turabian StyleMolla, Md. Ashraful Islam, Mai Furukawa, Ikki Tateishi, Hideyuki Katsumata, and Satoshi Kaneco. 2019. "Optimization of Alachlor Photocatalytic Degradation with Nano-TiO2 in Water under Solar Illumination: Reaction Pathway and Mineralization" Clean Technologies 1, no. 1: 141-153. https://doi.org/10.3390/cleantechnol1010010





