The Canady Helios Cold Plasma Scalpel Significantly Decreases Viability in Malignant Solid Tumor Cells in a Dose-Dependent Manner
Abstract
:1. Introduction
2. Materials and Methods
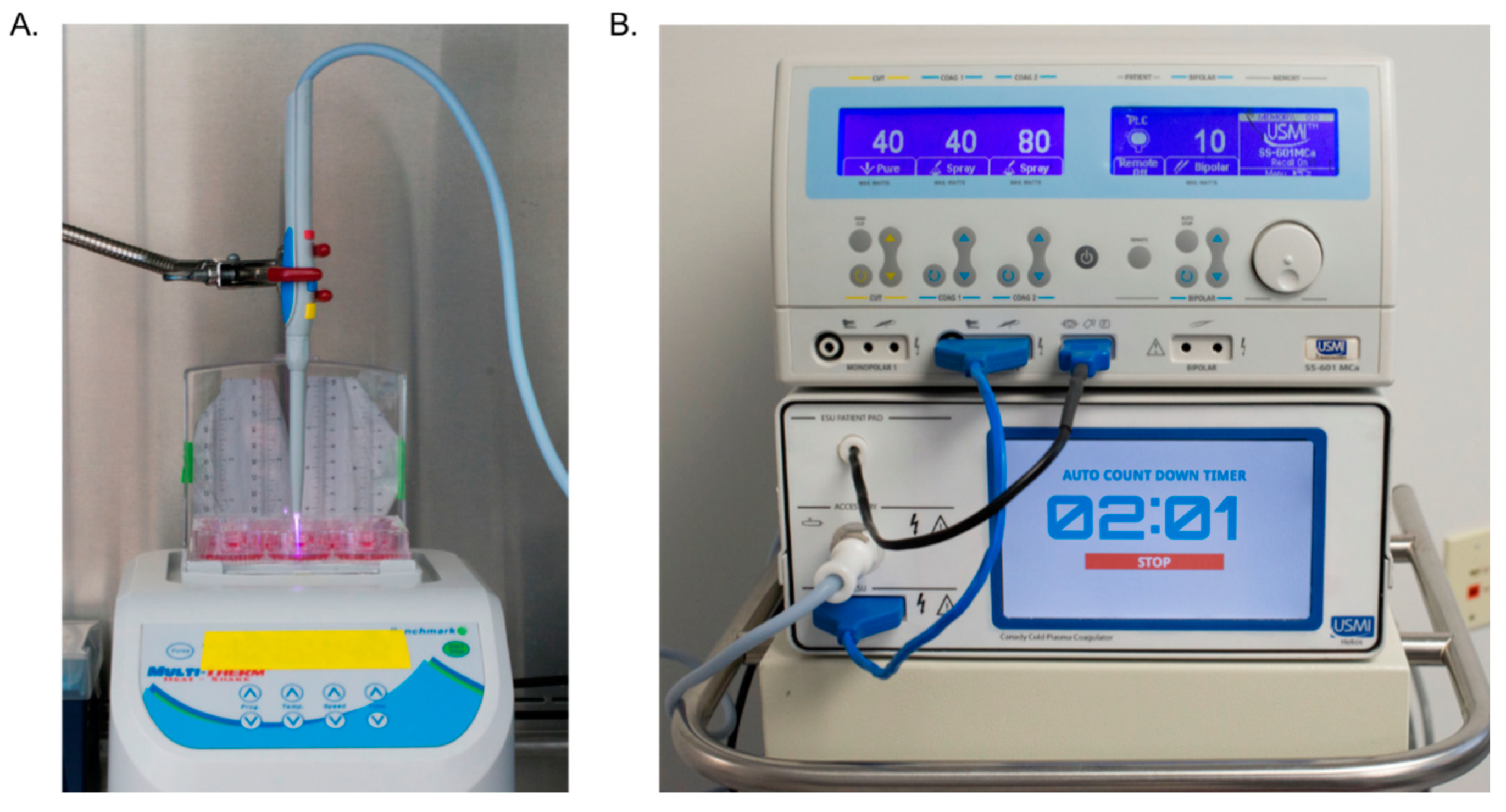
2.1. Cold Plasma Device
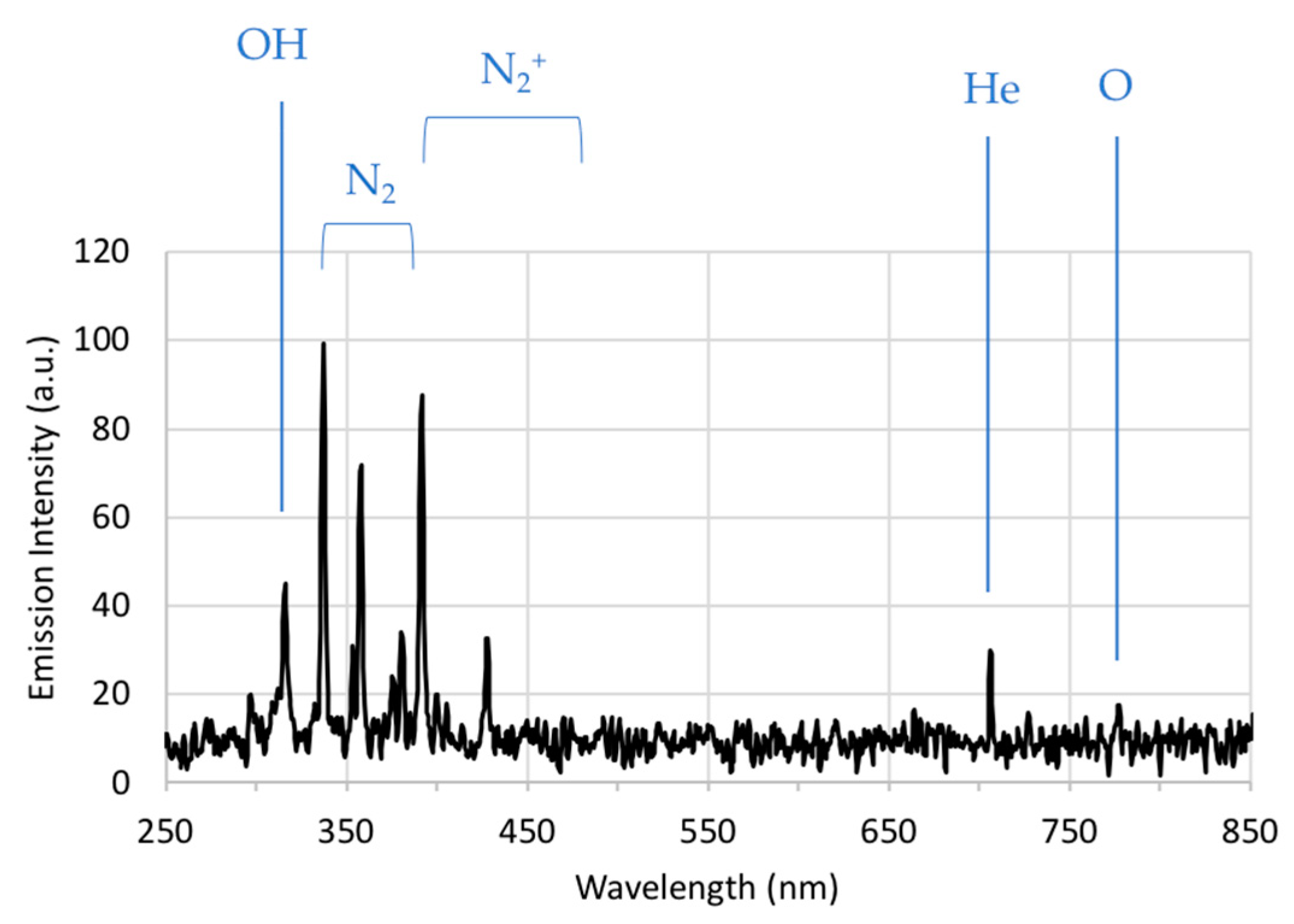
2.2. Optical Emission Spectroscopy
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Statistics
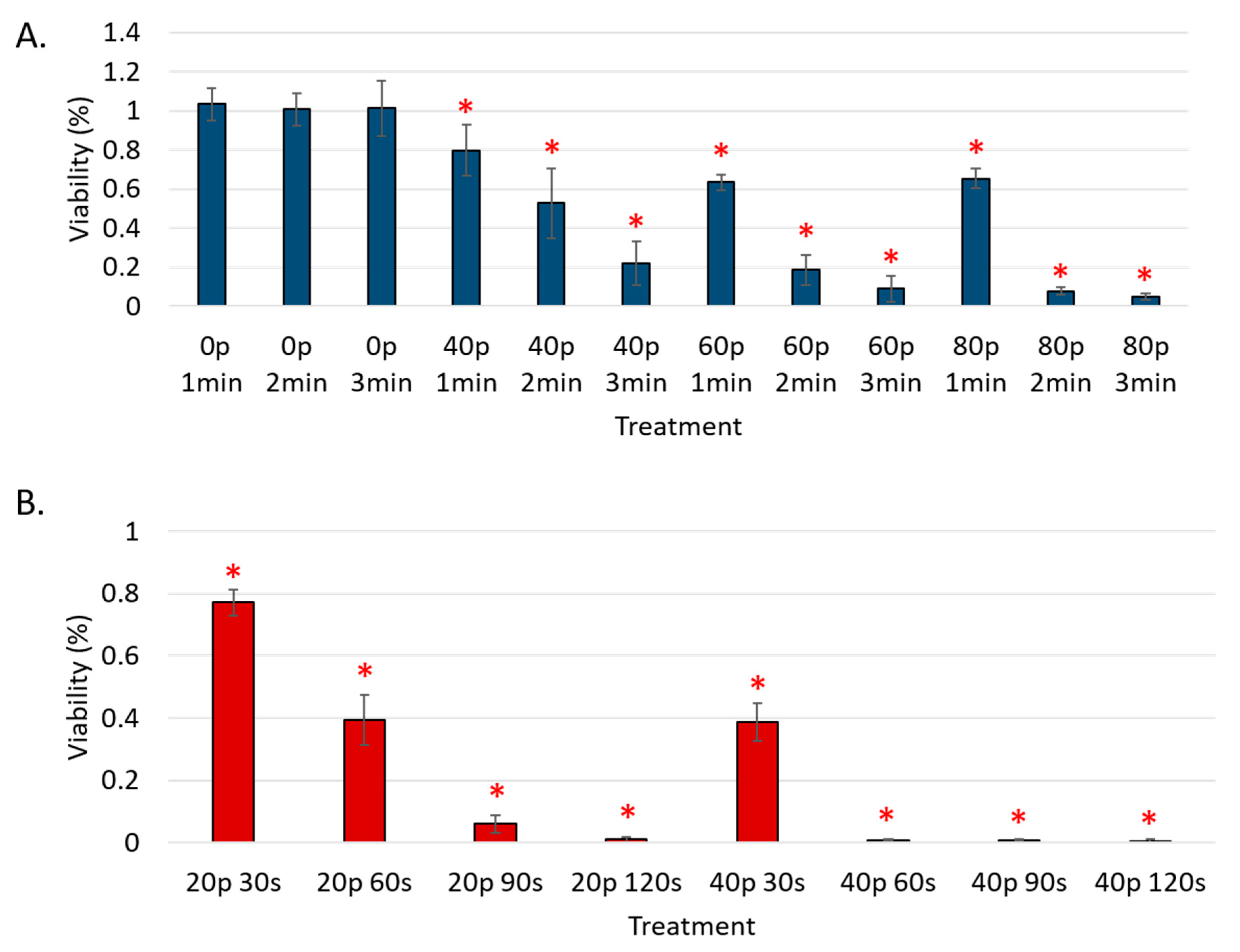
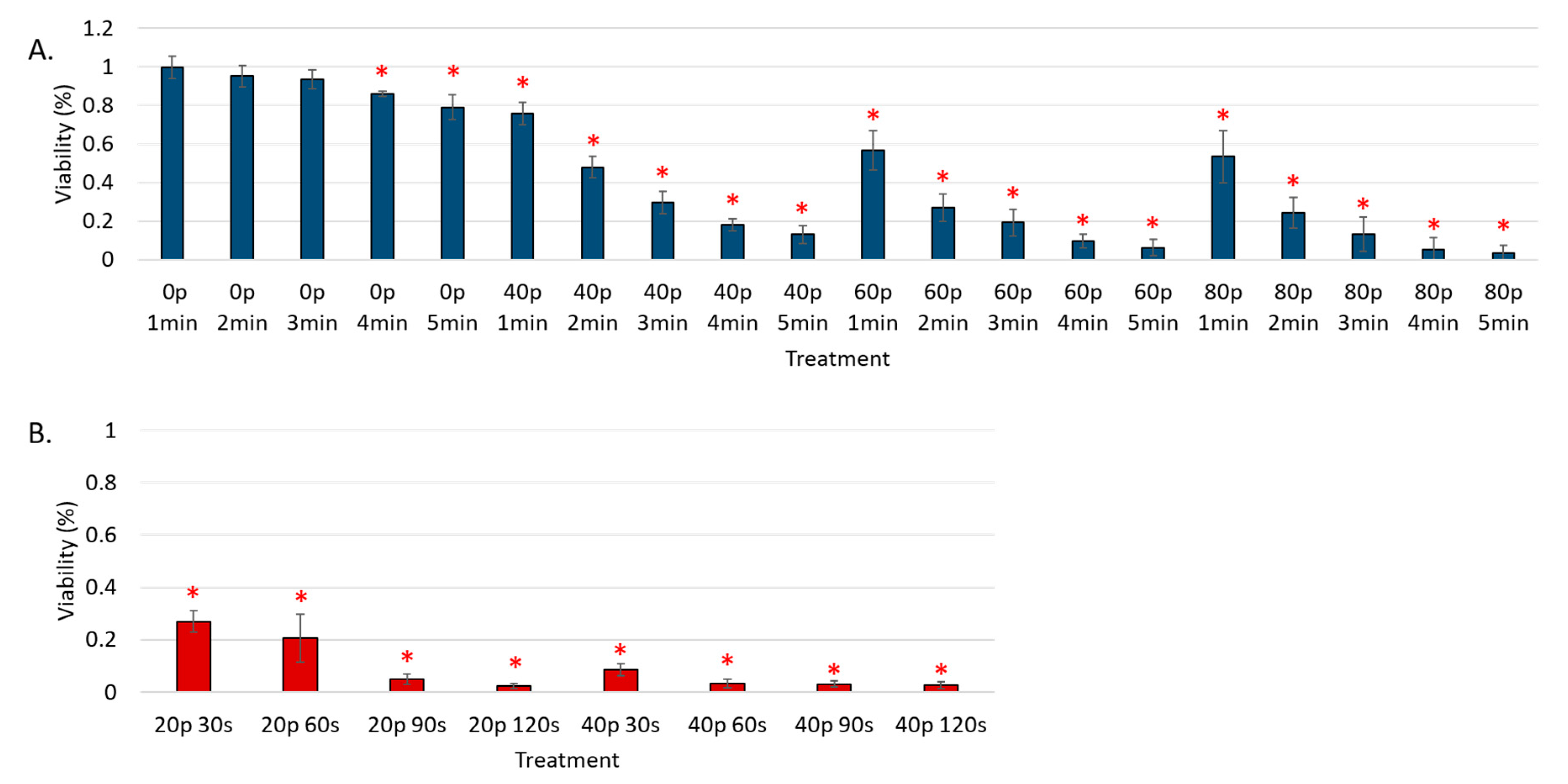
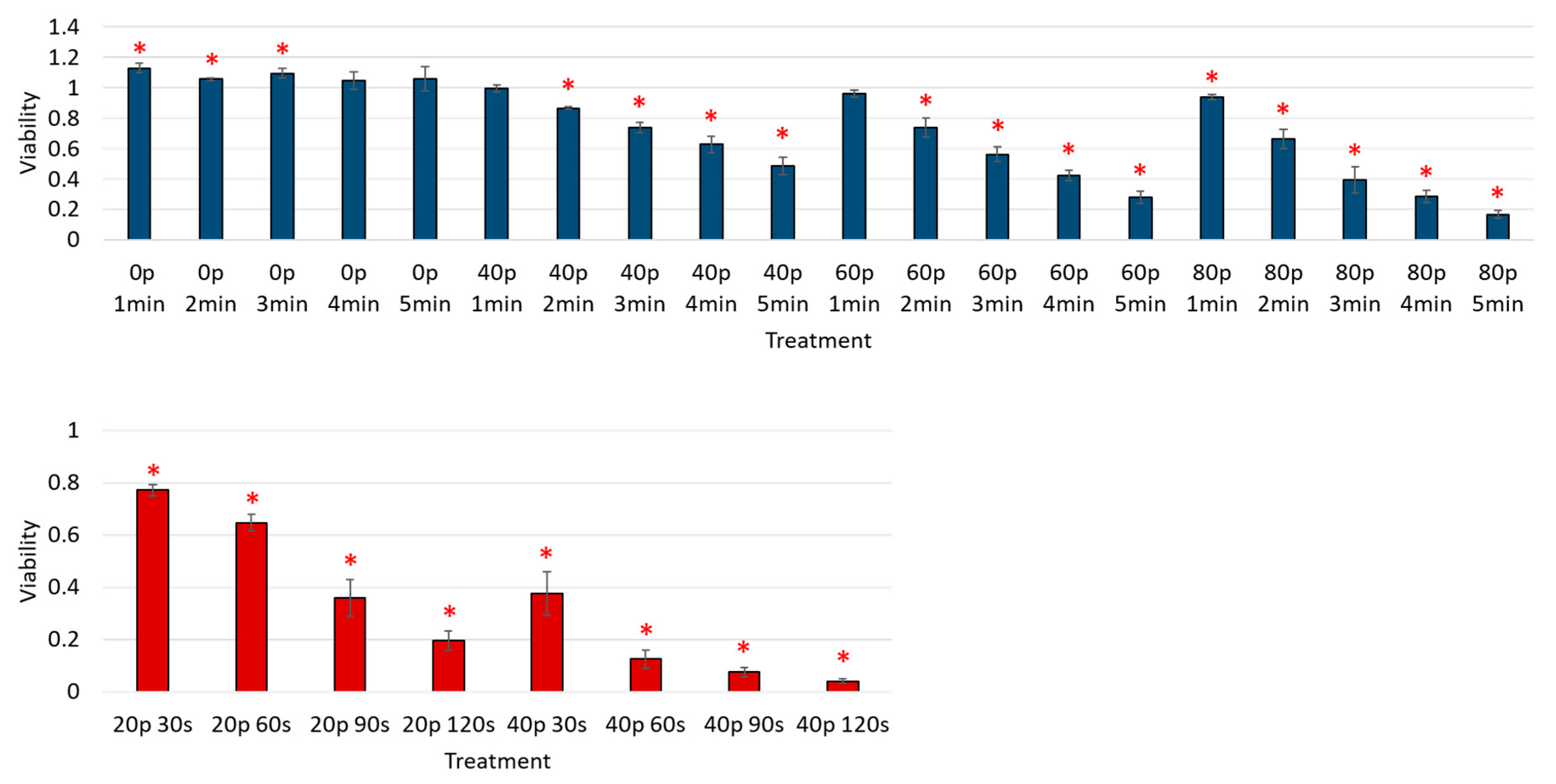
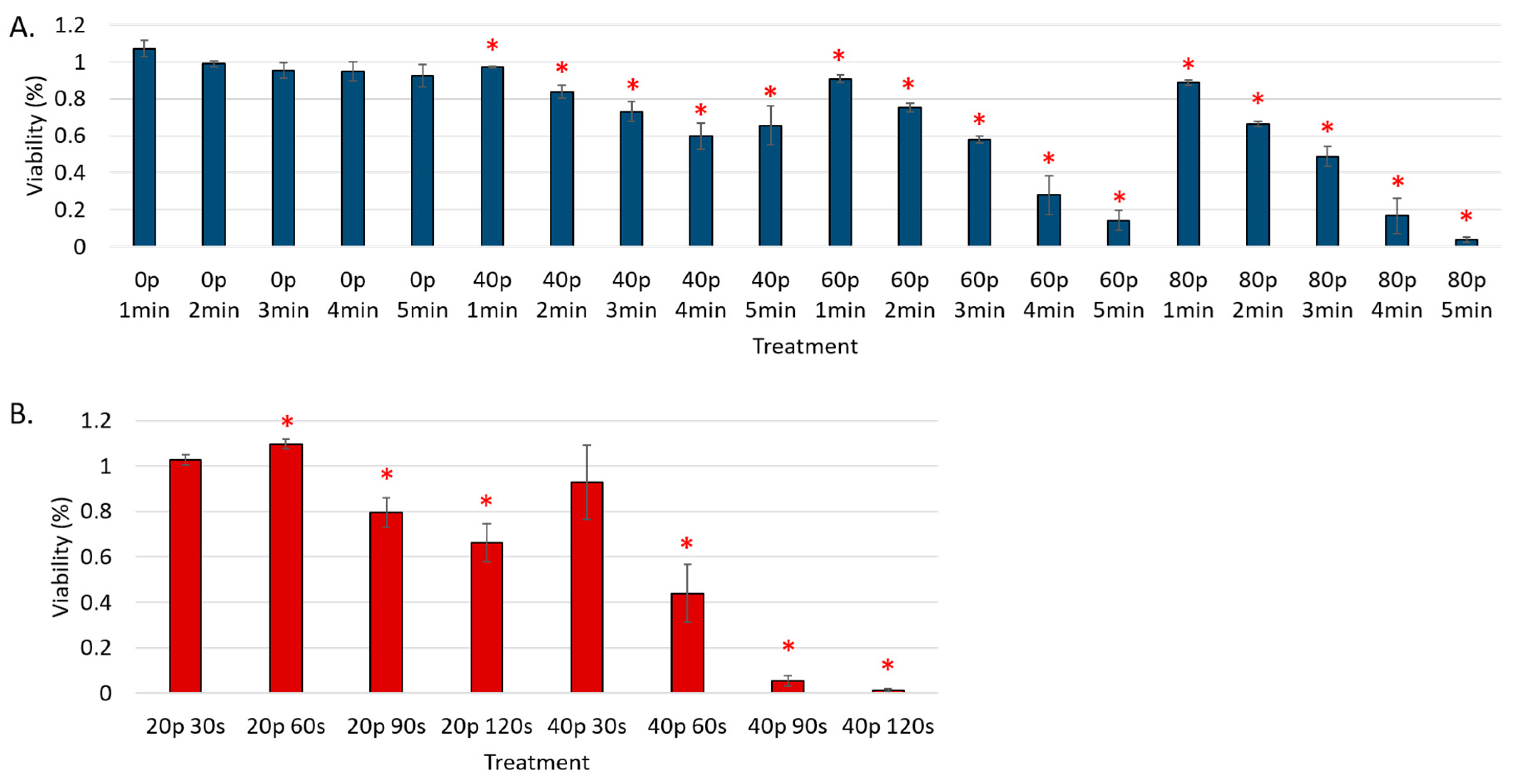
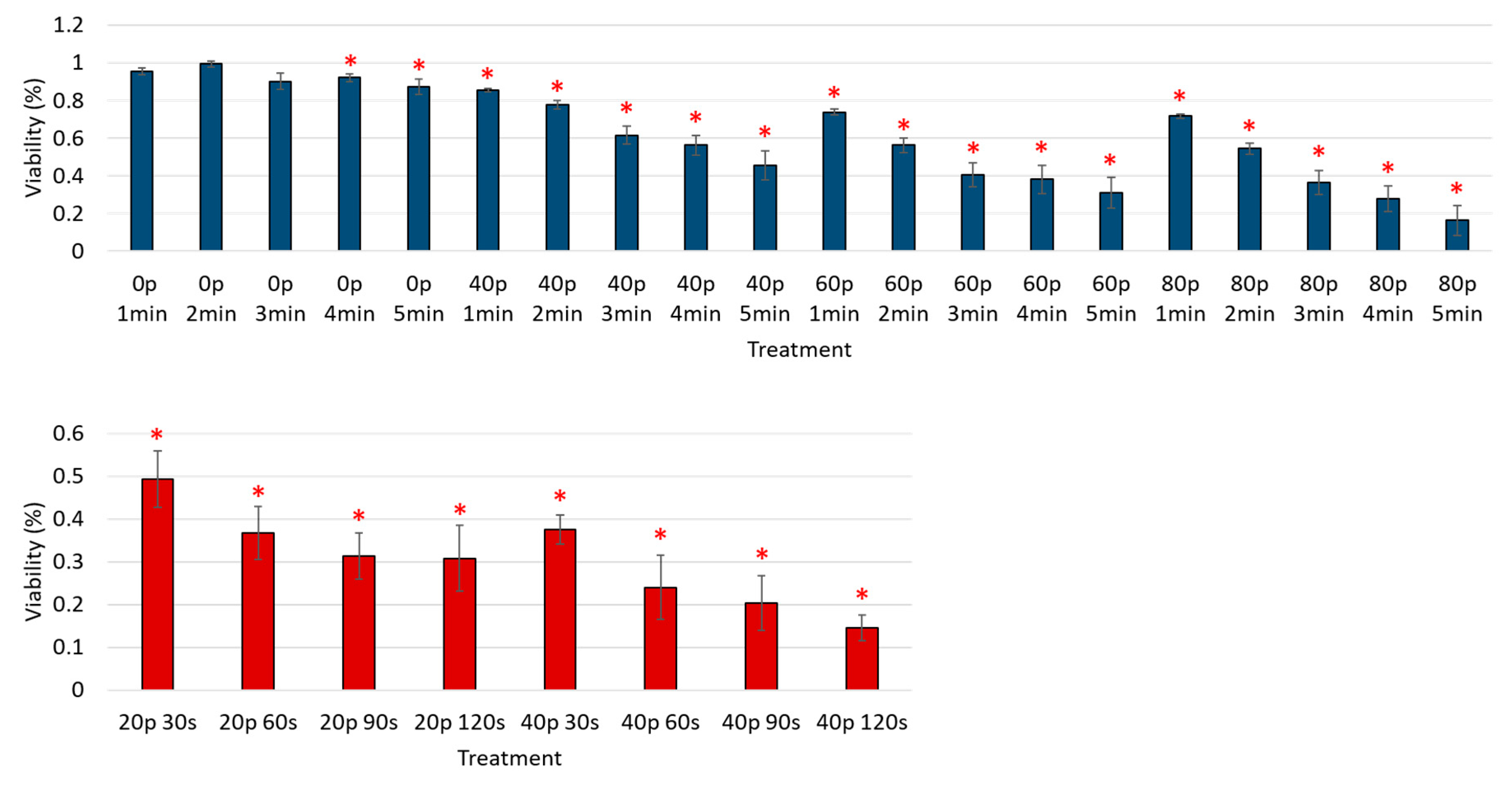
3. Results
Reduction of Cell Viability by CAP in Malignant Solid Tumor Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Turhal, N. Two cases of advanced renal cell cancer with prolonged survival of 8 and 12 years. Jpn. J. Clin. Oncol. 2002, 32, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Dabestani, S.; Beisland, C.; Stewart, G.D.; Bensalah, K.; Gudmundsson, E.; Lam, T.B.; Gietzmann, W.; Zakikhani, P.; Marconi, L.; Fernandez-Pello, S.; et al. Long-term outcomes of follow-up for initially localised clear cell renal cell carcinoma: Recur database analysis. Eur. Urol. Focus 2018. [Google Scholar] [CrossRef] [PubMed]
- Wille-Jorgensen, P.; Syk, I.; Smedh, K.; Laurberg, S.; Nielsen, D.T.; Petersen, S.H.; Renehan, A.G.; Horvath-Puho, E.; Pahlman, L.; Sorensen, H.T.; et al. Effect of more vs less frequent follow-up testing on overall and colorectal cancer-specific mortality in patients with stage ii or iii colorectal cancer: The colofol randomized clinical trial. JAMA 2018, 319, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Benzel, J.; Fendrich, V. Chemoprevention and treatment of pancreatic cancer: Update and review of the literature. Digestion 2018, 97, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Sugimoto, M.; Gotohda, N.; Konishi, M.; Takahashi, S. Efficacy of completion pancreatectomy for recurrence of adenocarcinoma in the remnant pancreas. J. Surg. Res. 2018, 221, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.M.; Chen, Y.; Wu, Y.K.; Xi, M.R. Pathological characteristics and prognosis of 664 patients with epithelial ovarian cancer: A retrospective analysis. J. Sichuan Univ. Med. Sci. Ed. 2014, 45, 859–862. [Google Scholar]
- Visser, E.; Edholm, D.; Smithers, B.M.; Thomson, I.G.; Burmeister, B.H.; Walpole, E.T.; Gotley, D.C.; Joubert, W.L.; Atkinson, V.; Mai, T.; et al. Neoadjuvant chemotherapy or chemoradiotherapy for adenocarcinoma of the esophagus. J. Surg. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Yang, Y.; Zhang, L.; Yang, H.; Merrell, K.W.; Hallemeier, C.L.; Shen, R.K.; Haddock, M.G.; Hofstetter, W.L.; Maru, D.M.; et al. Multi-institutional analysis of recurrence and survival after neoadjuvant chemoradiotherapy of esophageal cancer: Impact of histology on recurrence patterns and outcomes. Ann. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Arora, V. Cold Atmospheric Plasma (CAP) in dentistry. Dentistry 2013, 4. [Google Scholar] [CrossRef]
- Niemira, B.A.; Boyd, G.; Sites, J. Cold plasma rapid decontamination of food contact surfaces contaminated with salmonella biofilms. J. Food Sci. 2014, 79, M917–M922. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Woedtke, T.V.; Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One year follow-up risk assessment in skh-1 mice and wounds treated with an argon plasma jet. Int. J. Mol. Sci. 2017, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi Rad, Z.; Abbasi Davani, F. Non-thermal atmospheric pressure dielectric barrier discharge plasma source construction and investigation on the effect of grid on wound healing application. Clin. Plasma Med. 2016, 4, 56–64. [Google Scholar] [CrossRef]
- Volotskova, O.; Dubrovsky, L.; Keidar, M.; Bukrinsky, M. Cold atmospheric plasma inhibits hiv-1 replication in macrophages by targeting both the virus and the cells. PLoS ONE 2016, 11, e0165322. [Google Scholar] [CrossRef] [PubMed]
- Keidar, M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015, 24, 033001. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Cheng, X.; Canady, J.; Sherman, J.; Keidar, M. Principles of using cold atmospheric plasma stimulated media for cancer treatment. Sci. Rep. 2015, 5, 18339. [Google Scholar] [CrossRef] [PubMed]
- Naciri, M.; Dowling, D.; Al-Rubeai, M. Differential sensitivity of mammalian cell lines to non-thermal atmospheric plasma. Plasma Process. Polym. 2014, 11, 391–400. [Google Scholar] [CrossRef]
- Ma, Y.; Ha, C.S.; Hwang, S.W.; Lee, H.J.; Kim, G.C.; Lee, K.W.; Song, K. Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ros stress-response pathways. PLoS ONE 2014, 9, e91947. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. The cellular ros-scavenging function, a key factor determining the specific vulnerability of cancer cells to cold atmospheric plasma in vitro. arXiv, 2017; arXiv:1711.09015. [Google Scholar]
- Lee, H.J.; Shon, C.H.; Kim, Y.S.; Kim, S.; Kim, G.C.; Kong, M.G. Degradation of adhesion molecules of g361 melanoma cells by a non-thermal atmospheric pressure microplasma. New J. Phys. 2009, 11, 115026. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; von Woedtke, T.; Hasse, S. Cell migration and adhesion of a human melanoma cell line is decreased by cold plasma treatment. Clin. Plasma Med. 2015, 3, 24–31. [Google Scholar] [CrossRef]
- Shi, X.-M.; Zhang, G.-J.; Chang, Z.-S.; Wu, X.-L.; Liao, W.-L.; Li, N. Viability reduction of melanoma cells by plasma jet via inducing g1/s and g2/m cell cycle arrest and cell apoptosis. IEEE Trans. Plasma Sci. 2014, 42, 1640–1647. [Google Scholar] [CrossRef]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.S.; Moon, E.; Baek, S.J.; Lee, K.; et al. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: Involvement of DNA-damage-triggering sub-g(1) arrest via the atm/p53 pathway. Arch. Biochem. Biophys. 2014, 545, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, M.; Turrini, E.; Laurita, R.; De Gianni, E.; Ferruzzi, L.; Liguori, A.; Stancampiano, A.; Colombo, V.; Fimognari, C. Atmospheric non-equilibrium plasma promotes cell death and cell-cycle arrest in a lymphoma cell line. Plasma Process. Polym. 2015, 12, 1354–1363. [Google Scholar] [CrossRef]
- Adachi, T.; Tanaka, H.; Nonomura, S.; Hara, H.; Kondo, S.; Hori, M. Plasma-activated medium induces a549 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free Radic. Biol. Med. 2015, 79, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.H.; Bae, S.H.; Leem, S.H. Induction of apoptosis in human breast cancer cells by a pulsed atmospheric pressure plasma jet. Appl. Phys. Lett. 2010, 97, 023702. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuccitelli, R.; Chen, X.; Pakhomov, A.G.; Baldwin, W.H.; Sheikh, S.; Pomicter, J.L.; Ren, W.; Osgood, C.; Swanson, R.J.; Kolb, J.F.; et al. A new pulsed electric field therapy for melanoma disrupts the tumor's blood supply and causes complete remission without recurrence. Int. J. Cancer 2009, 125, 438–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, W.H. Mechanisms of a novel anticancer therapeutic strategy involving atmospheric pressure plasma-mediated apoptosis and DNA strand break formation. Arch. Pharm. Res. 2016, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Canady, J.; Shashurin, A.; Keidar, M.; Zhuang, T. Integrated Cold Plasma and High Frequency Plasma Electrosurgical System and Method. U.S. Patent No. 9,999,462, 19 June 2018. [Google Scholar]
- Okazaki, S.; Kogoma, M.; Uehara, M.; Kimura, Y. Appearance of stable glow discharge in air, argon, oxygen and nitrogen at atmospheric pressure using a 50 hz source. J. Phys. D 1993, 26, 889–892. [Google Scholar] [CrossRef]
- Sublet, A.; Ding, C.; Dorier, J.L.; Hollenstein, C.; Fayet, P.; Coursimault, F. Atmospheric and sub-atmospheric dielectric barrier discharges in helium and nitrogen. Plasma Sources Sci. Technol. 2006, 15, 627–634. [Google Scholar] [CrossRef]
- Cheng, X.; Rowe, W.J.; Ly, L.; Shashurin, A.; Zhuang, T.; Wigh, S.; Basadonna, G.; Trink, B.; Keidar, M.; Canady, J. Treatment of triple-negative breast cancer cells with the canady cold plasma conversion system: Preliminary results. Plasma 2018. submitted. [Google Scholar]
- Pearse, R.W.B.; Gaydon, A.G. The Identification of Molecular Spectra; Chapman and Hall: London, UK, 1976. [Google Scholar]
- Liu, Z.; Xu, D.; Liu, D.; Cui, Q.; Cai, H.; Li, Q.; Chen, H.; Kong, M.G. Production of simplex rns and ros by nanosecond pulse N2/O2 plasma jets with homogeneous shielding gas for inducing myeloma cell apoptosis. J. Phys. D Appl. Phys. 2017, 50, 195204. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Lunova, M.; Jirsa, M.; Dejneka, A.; Kubinova, S. Chemically different non-thermal plasmas target distinct cell death pathways. Sci. Rep. 2017, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- Ly, L.; Jones, S.; Shashurin, A.; Zhuang, T.; Rowe, W.; Cheng, X.; Wigh, S.; Naab, T.; Keidar, M.; Canady, J. A new cold plasma jet: Performance evaluation of cold plasma, hybrid plasma and argon plasma coagulation. Plasma 2018, submitted. [Google Scholar]
- Xu, X.; Dai, X.; Xiang, L.; Cai, D.; Xiao, S.; Ostrikov, K. Quantitative assessment of cold atmospheric plasma anti-cancer efficacy in triple-negative breast cancers. Plasma Process. Polym. 2018, 15. [Google Scholar] [CrossRef]
- Sablina, A.A.; Budanov, A.V.; Ilyinskaya, G.V.; Agapova, L.S.; Kravchenko, J.E.; Chumakov, P.M. The antioxidant function of the p53 tumor suppressor. Nat. Med. 2005, 11, 1306–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, P.; Zhong, J.; Tan, M.; Ge, J.; Tao, L.; Li, Y.; Zhu, Y.; Wu, L.; Qiu, J.; et al. The platelet isoform of phosphofructokinase contributes to metabolic reprogramming and maintains cell proliferation in clear cell renal cell carcinoma. Oncotarget 2016, 7, 27142–27157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, J.; Ito, K.; Fujita, T.; Matsuzaki, Y.; Asano, T.; Hayakawa, M.; Asano, T.; Kawakami, Y. Progression of human renal cell carcinoma via inhibition of rhoa-rock axis by PARG1. Transl. Oncol. 2017, 10, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Hu, C.; Zhang, H.; Qu, Z.; Cen, J.; Qiu, Z.; Li, C.; Ren, H.; Li, Y.; He, X.; et al. Mir-27b synergizes with anticancer drugs via p53 activation and CYP1B1 suppression. Cell Res. 2015, 25, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Bamford, S.; Dawson, E.; Forbes, S.; Clements, J.; Pettett, R.; Dogan, A.; Flanagan, A.; Teague, J.; Futreal, P.A.; Stratton, M.R.; et al. The cosmic (catalogue of somatic mutations in cancer) database and website. Br. J. Cancer 2004, 91, 355–358. [Google Scholar] [CrossRef] [PubMed]
- O'Connor, P.M.; Jackman, J.; Bae, I.; Myers, T.G.; Fan, S.; Mutoh, M.; Scudiero, D.A.; Monks, A.; Sausville, E.A.; Weinstein, J.N.; et al. Characterization of the p53 tumor suppressor pathway in cell lines of the national cancer institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997, 57, 4285–4300. [Google Scholar] [PubMed]
- Antoun, S.; Atallah, D.; Tahtouh, R.; Alaaeddine, N.; Moubarak, M.; Khaddage, A.; Ayoub, E.N.; Chahine, G.; Hilal, G. Different tp53 mutants in p53 overexpressed epithelial ovarian carcinoma can be associated both with altered and unaltered glycolytic and apoptotic profiles. Cancer Cell Int. 2018, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Yaginuma, Y.; Westphal, H. Abnormal structure and expression of the p53 gene in human ovarian carcinoma cell lines. Cancer Res. 1992, 52, 4196–4199. [Google Scholar] [PubMed]
- Chen, D.; Niu, M.; Jiao, X.; Zhang, K.; Liang, J.; Zhang, D. Inhibition of AKT2 enhances sensitivity to gemcitabine via regulating puma and NF-κB signaling pathway in human pancreatic ductal adenocarcinoma. Int. J. Mol. Sci. 2012, 13, 1186–1208. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, H.; Yan, X.G.; Zhou, Z.W.; Yi, Z.G.; He, Z.X.; Pan, S.T.; Yang, Y.X.; Wang, Z.Z.; Zhang, X. Alisertib induces cell cycle arrest and autophagy and suppresses epithelial-to-mesenchymal transition involving PI3K/AKT/mTOR and sirtuin 1-mediated signaling pathways in human pancreatic cancer cells. Drug Des. Dev. Ther. 2015, 9, 575–601. [Google Scholar]
- Ruggeri, B.; Zhang, S.Y.; Caamano, J.; DiRado, M.; Flynn, S.D.; Klein-Szanto, A.J. Human pancreatic carcinomas and cell lines reveal frequent and multiple alterations in the p53 and Rb-1 tumor-suppressor genes. Oncogene 1992, 7, 1503–1511. [Google Scholar] [PubMed]
- Liu, D.S.; Read, M.; Cullinane, C.; Azar, W.J.; Fennell, C.M.; Montgomery, K.G.; Haupt, S.; Haupt, Y.; Wiman, K.G.; Duong, C.P.; et al. APR-246 potently inhibits tumour growth and overcomes chemoresistance in preclinical models of oesophageal adenocarcinoma. Gut 2015, 64, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Brulle, L.; Vandamme, M.; Ries, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.M.; Le Pape, A. Effects of a non thermal plasma treatment alone or in combination with gemcitabine in a MIA PaCa2-luc orthotopic pancreatic carcinoma model. PLoS ONE 2012, 7, e52653. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Cheng, Y.-C.; Cheng, Y.-J. Synergistic effects of plasma-activated medium and chemotherapeutic drugs in cancer treatment. J. Phys. D 2018, 51, 13LT01. [Google Scholar] [CrossRef] [Green Version]
- Masur, K.; von Behr, M.; Bekeschus, S.; Weltmann, K.-D.; Hackbarth, C.; Heidecke, C.-D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Synergistic inhibition of tumor cell proliferation by cold plasma and gemcitabine. Plasma Process. Polym. 2015, 12, 1377–1382. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Jeong, D.; Ham, J.; Park, S.; Choi, E.H.; Kim, S.J. Cold atmospheric plasma restores tamoxifen sensitivity in resistant MCF-7 breast cancer cell. Free Radic. Biol. Med. 2017, 110, 280–290. [Google Scholar] [CrossRef] [PubMed]
| Cell Name | Cell Type | p53 Status | P53 Expression | Reference |
|---|---|---|---|---|
| 769-P | Renal adenocarcinoma | WT | Positive | [42,43,44,45] |
| HCT-116 | Colorectal carcinoma | WT | Positive | [19,46] |
| SK-OV-3 | Ovarian adenocarcinoma | MUT/NULL | Negative | [45,46,47,48] |
| BxPC-3 | Pancreatic adenocarcinoma | MUT | Positive | [49,50,51] |
| OE33 | Esophageal adenocarcinoma | MUT | Positive | [45,52] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowe, W.; Cheng, X.; Ly, L.; Zhuang, T.; Basadonna, G.; Trink, B.; Keidar, M.; Canady, J. The Canady Helios Cold Plasma Scalpel Significantly Decreases Viability in Malignant Solid Tumor Cells in a Dose-Dependent Manner. Plasma 2018, 1, 177-188. https://doi.org/10.3390/plasma1010016
Rowe W, Cheng X, Ly L, Zhuang T, Basadonna G, Trink B, Keidar M, Canady J. The Canady Helios Cold Plasma Scalpel Significantly Decreases Viability in Malignant Solid Tumor Cells in a Dose-Dependent Manner. Plasma. 2018; 1(1):177-188. https://doi.org/10.3390/plasma1010016
Chicago/Turabian StyleRowe, Warren, Xiaoqian Cheng, Lawan Ly, Taisen Zhuang, Giacomo Basadonna, Barry Trink, Michael Keidar, and Jerome Canady. 2018. "The Canady Helios Cold Plasma Scalpel Significantly Decreases Viability in Malignant Solid Tumor Cells in a Dose-Dependent Manner" Plasma 1, no. 1: 177-188. https://doi.org/10.3390/plasma1010016





