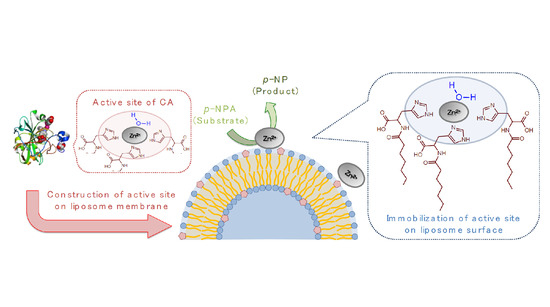
Hydrolase-Like Activity Provided by Zinc(II) and Oleoyl-Histidine at Liposome Membrane Surface
Abstract
:1. Introduction
2. Results and Discussion
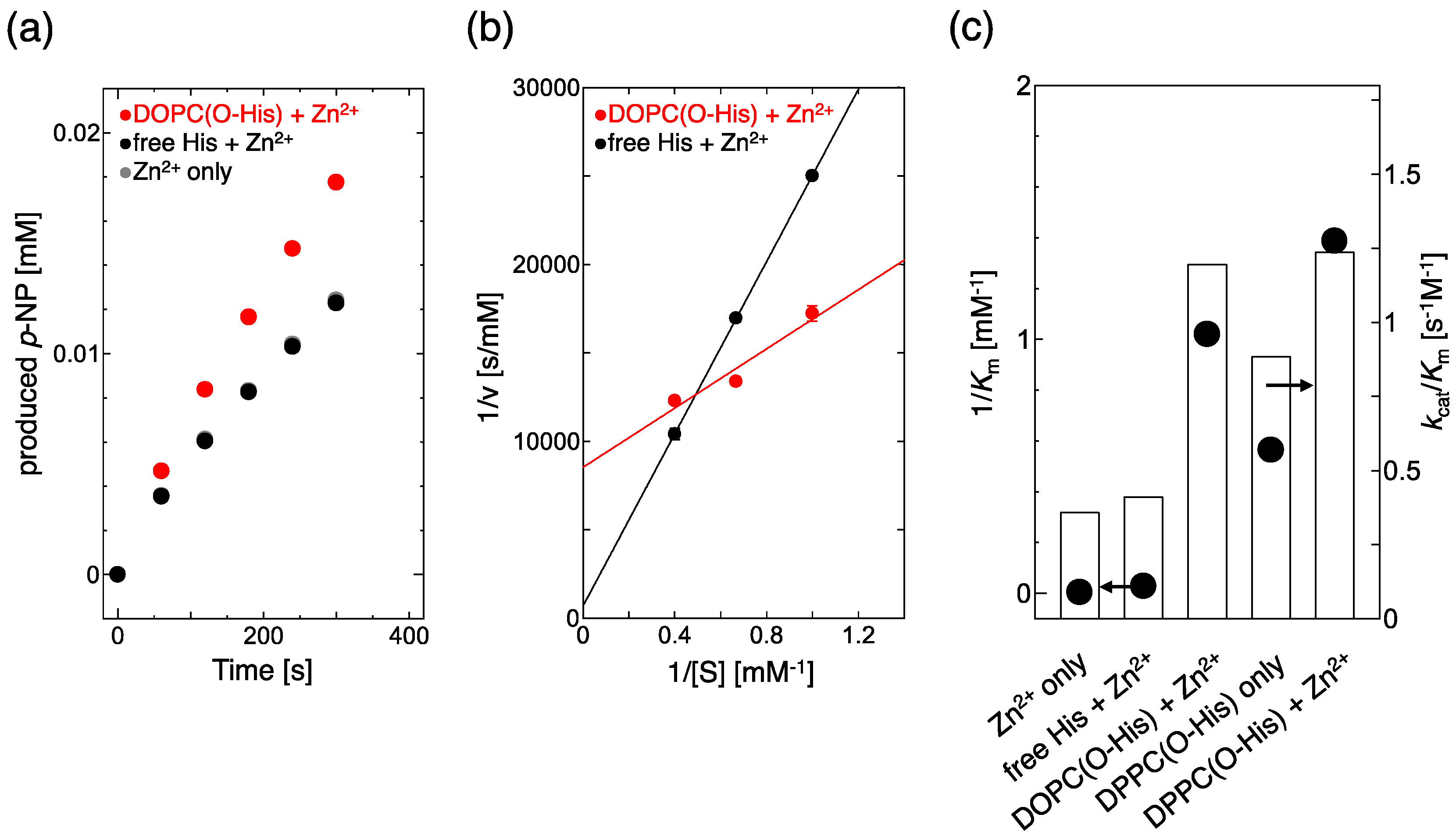
2.1. Evaluation of Hydrolysis Catalytic Activity
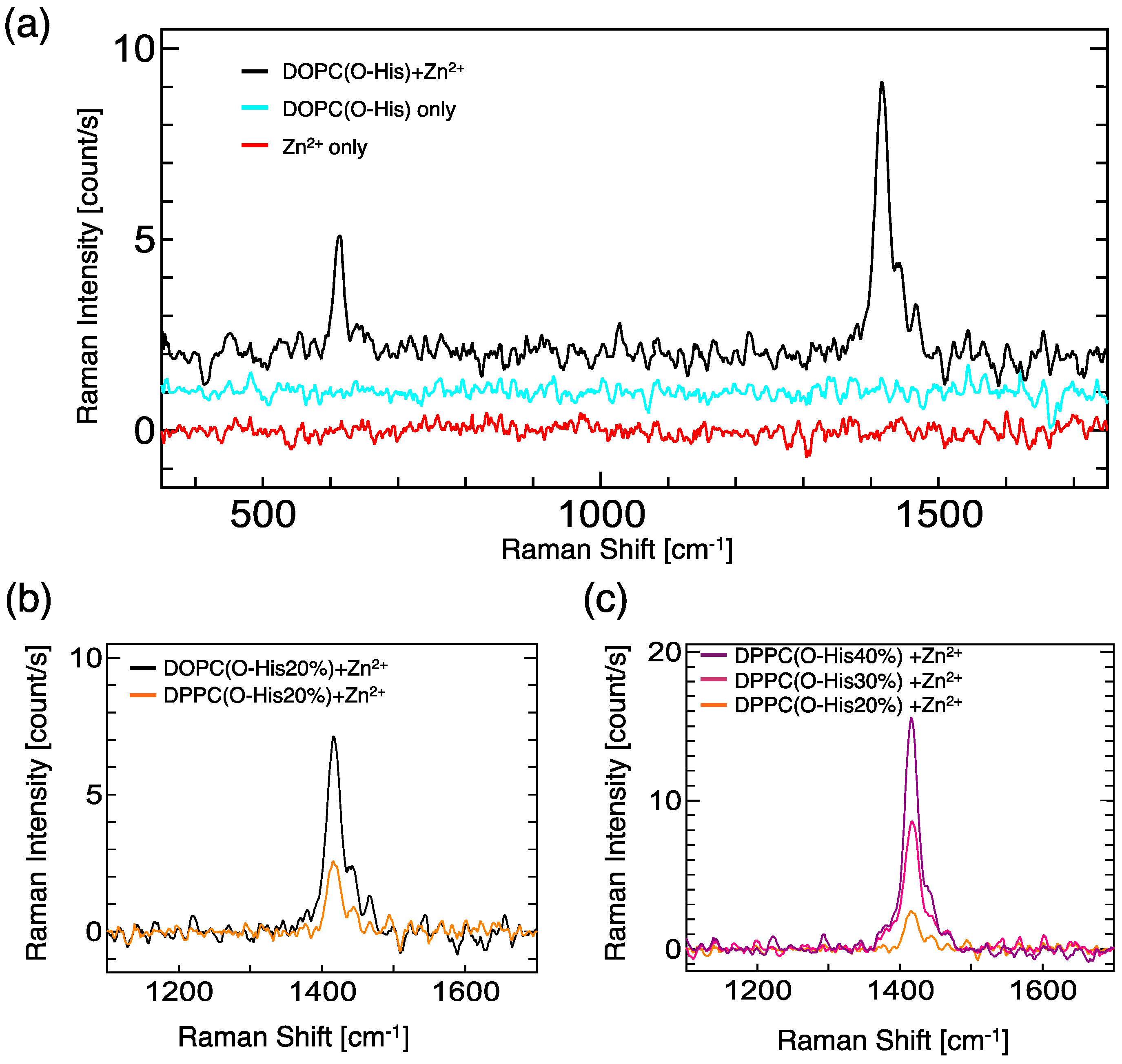
2.2. Evaluation of Complex Creation
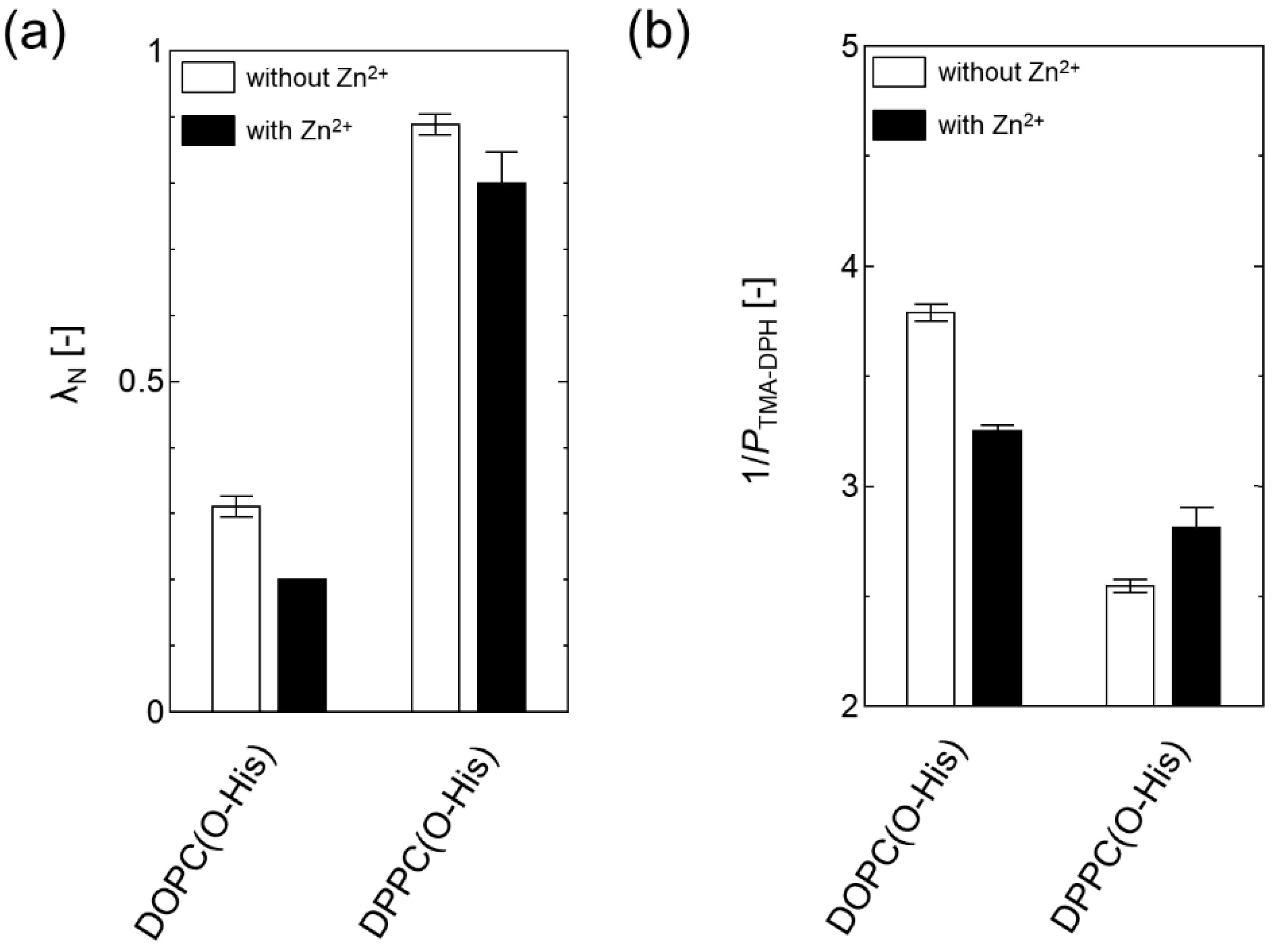
2.3. Evaluation of the Membrane Property of O-His Modified Liposomes in the Presence of Zn2+
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of O-His
4.3. Liposome Preparation
4.4. Evaluation of Hydrolysis Activity
4.5. Evaluation of Zn-Imidazole Complex by Raman Spectroscopy
4.6. Evaluation of Membrane Fluidity and Polarity
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | carbonic anhydrase |
| O-His | oleoyl-histidine |
| p-NPA | p-nitrophenylacetate |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| Dansyl-DHPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(5-dimethylamino-1-naphthalenesulfonyl) |
| Laurdan | 6-lauroyl-2-dimethylamino-naphthalene |
| DPH | 1,6-diphenyl-1,3,5-hexatriene (DPH), |
| TMA-DPH | 1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene |
| p-NP | p-nitrophenol |
References
- Savile, C.K.; Lalonde, J.J. Biotechnology for the acceleration of carbon dioxide capture and sequestration. Curr. Opin. Biotechnol. 2011, 22, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.M.; Kaufman, G.K.; Urbach, A.R.; Gitlin, I.; Gudiksen, K.L.; Weibel, D.B.; Whitesides, G.M. Carbonic Anhydrase as a Model for Biophysical and Physical-Organic Studies of Proteins and Protein−Ligand Binding. Chem. Rev. 2008, 108, 946–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, P.C.; Jang, Y.-N.; Lee, S.-W. Immobilization of carbonic anhydrase and an artificial Zn (II) complex on a magnetic support for biomimetic carbon dioxide sequestration. J. Mol. Catal. B Enzym. 2012, 82, 37–45. [Google Scholar] [CrossRef]
- Sahoo, P.C.; Jang, Y.-N.; Suh, Y.-J.; Lee, S.-W. Bioinspired design of mesoporous silica complex based on active site of carbonic anhydrase. J. Mol. Catal. A Chem. 2014, 390, 105–113. [Google Scholar] [CrossRef]
- Cioci, F.; Lavecchia, R.; Marrelli, L. Effect of surface tension on the conformational stability of erythrocyte carbonic anhydrase. Fluid Phase Equilib. 1996, 116, 118–125. [Google Scholar] [CrossRef]
- Juan, D.; Bingying, J.; Xingming, K.; Xiancheng, Z.; Qingxiang, X. Enhanced Hydrolysis of Carboxylic Acid Esters Catalyzed by Metallomicelles Made of Cu(II) and Zn(II) Complexes. J. Colloid Interface Sci. 2002, 256, 428–434. [Google Scholar] [CrossRef]
- Poznik, M.; König, B. Cooperative hydrolysis of aryl esters on functionalized membrane surfaces and in micellar solutions. Org. Biomol. Chem. 2014, 12, 3175–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Rio, L.; Mejuto, J.C.; Perez-Lorenzo, M. Modification of reactivity by changing microemulsion composition. Basic hydrolysis of nitrophenyl acetate in AOT/isooctane/water systems. New. J. Chem. 2004, 28, 988–995. [Google Scholar] [CrossRef]
- Kim, M.-C.; Lee, S.-Y. Carbonic Anhydrase-Mimetic Bolaamphiphile Self-Assembly for CO2 Hydration and Sequestration. Chem. Eur. J. 2014, 20, 17019–17024. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Umakoshi, H.; Stano, P.; Mavelli, F. Emergent Properties Arising from the Assembly of Amphiphiles. Artificial vesicle membranes as reaction promoters and regulators. Chem. Commun. 2014, 50, 10177–10197. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, T.; Tauchi, A.; Suga, K.; Umakoshi, H. Effect of Boundary Edge in DOPC/DPPC/Cholesterol Liposomes on Acceleration of l-Histidine Preferential Adsorption. Langmuir 2016, 32, 6011–6019. [Google Scholar] [CrossRef] [PubMed]
- Metso, A.J.; Zhao, H.; Tuunainen, I.; Kinnunen, P.K.J. Observation of the main phase transition of dinervonoylphosphocholine giant liposomes by fluorescence microscopy. Biochim. Biophys. Acta 2005, 1713, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, J.; Heberle, F.A.; Mills, T.T.; Klawitter, P.; Huang, G.; Costanza, G.; Freigenson, G.W. Phase studies of model biomembranes: Complex behavior of DSPC/DOPC/Cholesterol. Biochim. Biophys. Acta 2007, 1768, 2764–2776. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.W.; Fierke, C.A. Carbonic Anhydrase: Evolution of the Zinc Binding Site by Nature and by Design. Acc. Chem. Res. 1996, 29, 331–339. [Google Scholar] [CrossRef]
- Pocker, Y.; Stone, T. The Catalytic Versatility of Erythrocyte Carbonic Anhydrase. III. Kinetic Studies of the Enzyme-Catalyzed Hydrolysis of p-Nitrophenyl Acetate. Bichemistry 1967, 6, 668–678. [Google Scholar] [CrossRef]
- Suga, K.; Hamasaki, A.; Chinzaka, J.; Umakoshi, H. Liposomes modified with cardiolipin can act as a platform to regulate the potential flux of NADP+-dependent isocitrate dehydrogenase. Metab. Eng. 2016, 3, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Mesu, J.G.; Visser, T.; Soulimani, F.; Weckhuysen, B.M. Infrared and Raman spectroscopic study of pH-induced structural changes of l-histidine in aqueous environment. Vib. Spectrpsc. 2005, 39, 114–125. [Google Scholar] [CrossRef]
- Salama, S.; Spiro, T.G. Resonance Raman Spectra of Cobalt(II)-Imidazole Complexes: Analogues of the Binding Site of Cobalt-Substituted Zinc Proteins. J. Am. Chem. Soc. 1978, 100, 1105–1111. [Google Scholar] [CrossRef]
- Lentz, B.R. Membrane “fluidity” as detected by dipheylhexatriene probes. Chem. Phys. Lipids 1989, 50, 171–190. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimanouchi, T.; Kato, K.; Miyazaki, T.; Nakamura, A.; Umakoshi, H. Investigation of Fatty Acid Ketohydrazone Modified Liposome’s Properties as a Drug Carrier. Colloids Surf. B 2011, 87, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; Krasnowska, E.-K.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as Polarity-Sensitive Fluorescent Membrane Probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Bui, T.T.; Suga, K.; Umakoshi, H. Roles of Sterol Derivatives in Regulating the Properties of Phospholipid Bilayer Systems. Langmuir 2016, 32, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.K.; Hatzakis, N.S. Insights in enzyme functional dynamics and activity regulation by single molecule studies. Biophys. Rev. Lett. 2013, 8, 137–160. [Google Scholar] [CrossRef]
- Rabe, M.; Tabaei, S.R.; Zetterberg, H.; Zhdanov, V.P.; Höök, F. Hydrolysis of a lipid membrane by single enzyme molecules: Accurate determination of kinetic parameters. Angew. Chem. Int. Ed. 2015, 54, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, A.; Snijder, A.; Hicks, J.; Gunnarsson, J.; Höök, F.; Geschwindner, S. Drug discovery at the single molecule level: Inhibition-in-solution assay of membrane-reconstituted β-secretase using single-molecule imaging. Anal. Chem. 2015, 87, 4100–4103. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, Y.; Rappoport, S.; Wolman, Y. Use of esters of N-hydroxysuccinimide in the synthesis of N-acylamino acids. J. Lipid Res. 1967, 8, 142–145. [Google Scholar] [PubMed]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.H.M.; Takeshita, K.; Subbarao, N.K.; Hu, L.-R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. BBA Biomembr. 1991, 30, 297–303. [Google Scholar] [CrossRef]
- Kim, M.-C.; Lee, S.-Y. Comparative Study on the Catalytic Hydration of Carbon Dioxide by Catalysts that Mimic Carbonic Anhydrase Prepared with Zinc Salts. ChemCatChem 2015, 7, 698–704. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauchi, A.; Suga, K.; Umakoshi, H. Hydrolase-Like Activity Provided by Zinc(II) and Oleoyl-Histidine at Liposome Membrane Surface. Colloids Interfaces 2018, 2, 24. https://doi.org/10.3390/colloids2020024
Tauchi A, Suga K, Umakoshi H. Hydrolase-Like Activity Provided by Zinc(II) and Oleoyl-Histidine at Liposome Membrane Surface. Colloids and Interfaces. 2018; 2(2):24. https://doi.org/10.3390/colloids2020024
Chicago/Turabian StyleTauchi, Atsushi, Keishi Suga, and Hiroshi Umakoshi. 2018. "Hydrolase-Like Activity Provided by Zinc(II) and Oleoyl-Histidine at Liposome Membrane Surface" Colloids and Interfaces 2, no. 2: 24. https://doi.org/10.3390/colloids2020024