Recent Applications of Ionic Liquids in the Sol-Gel Process for Polymer–Silica Nanocomposites with Ionic Interfaces
Abstract
:1. Introduction
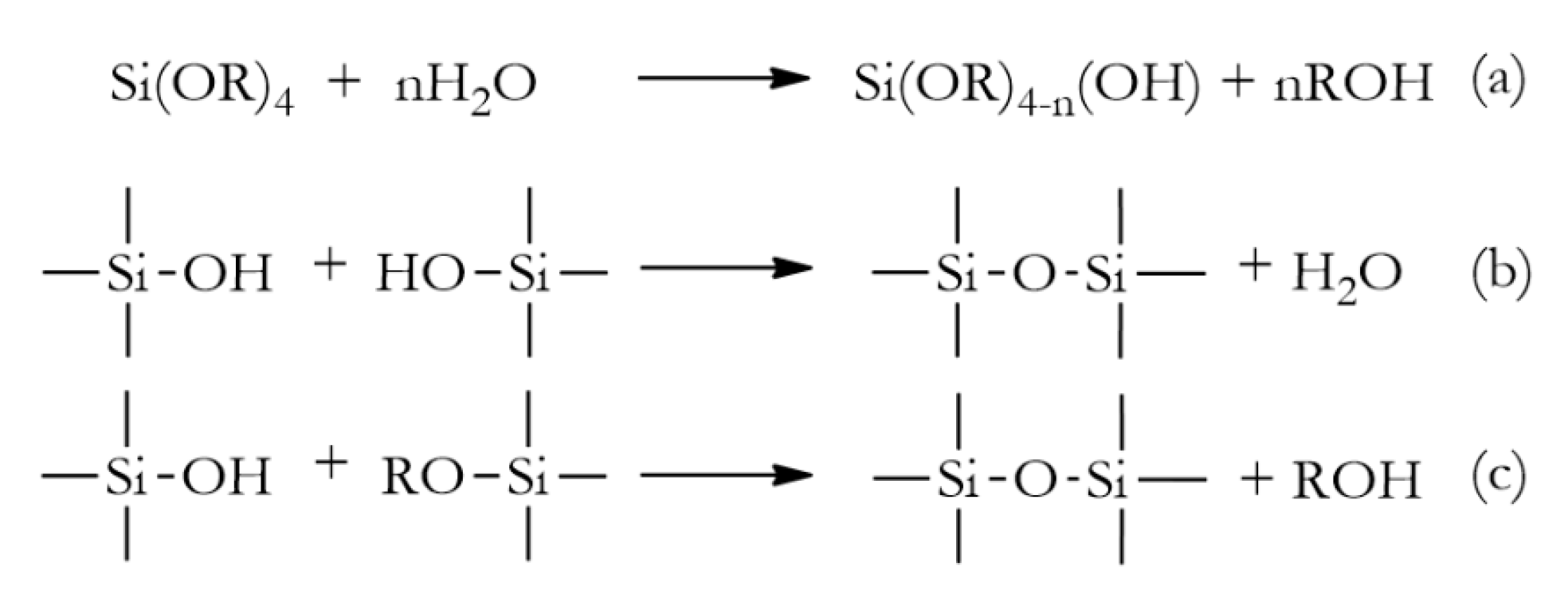
2. Hybrid Silica Materials via Sol-Gel Process
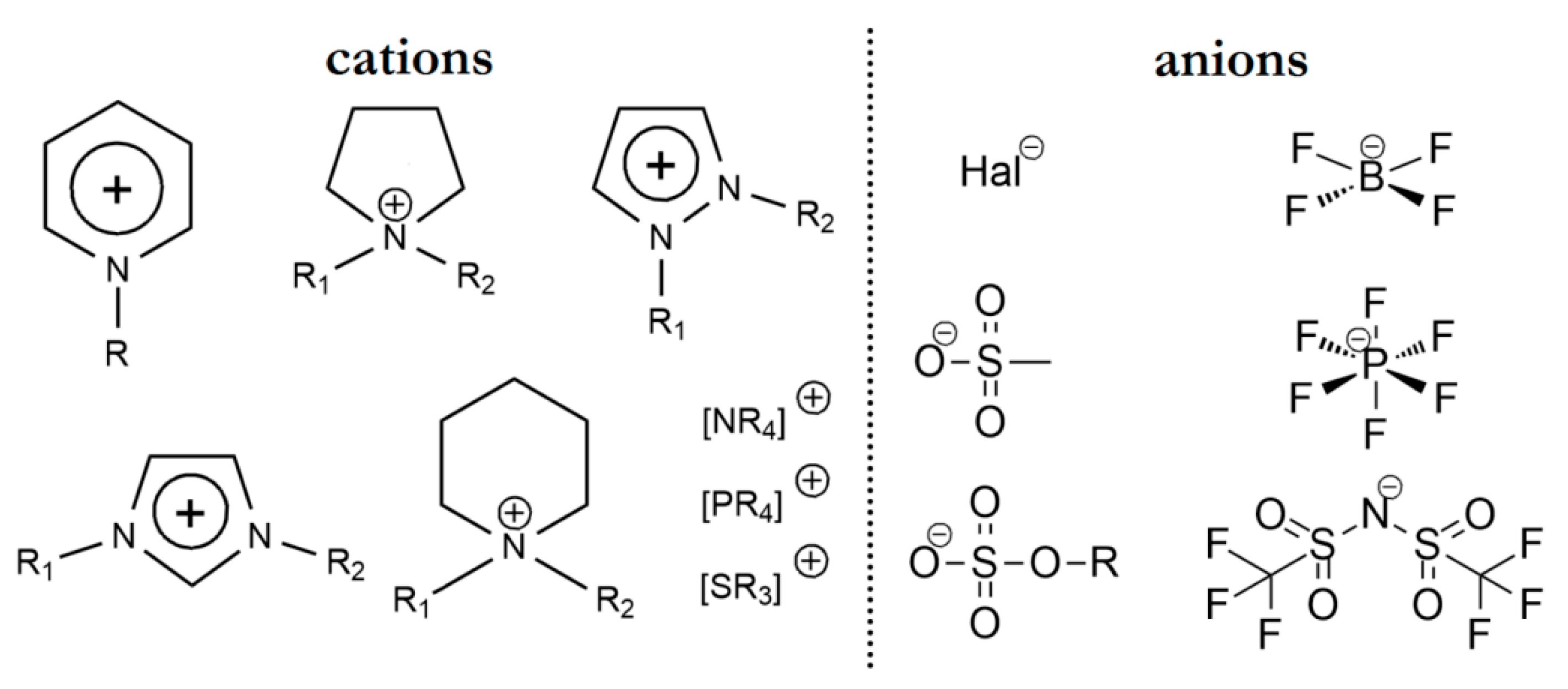
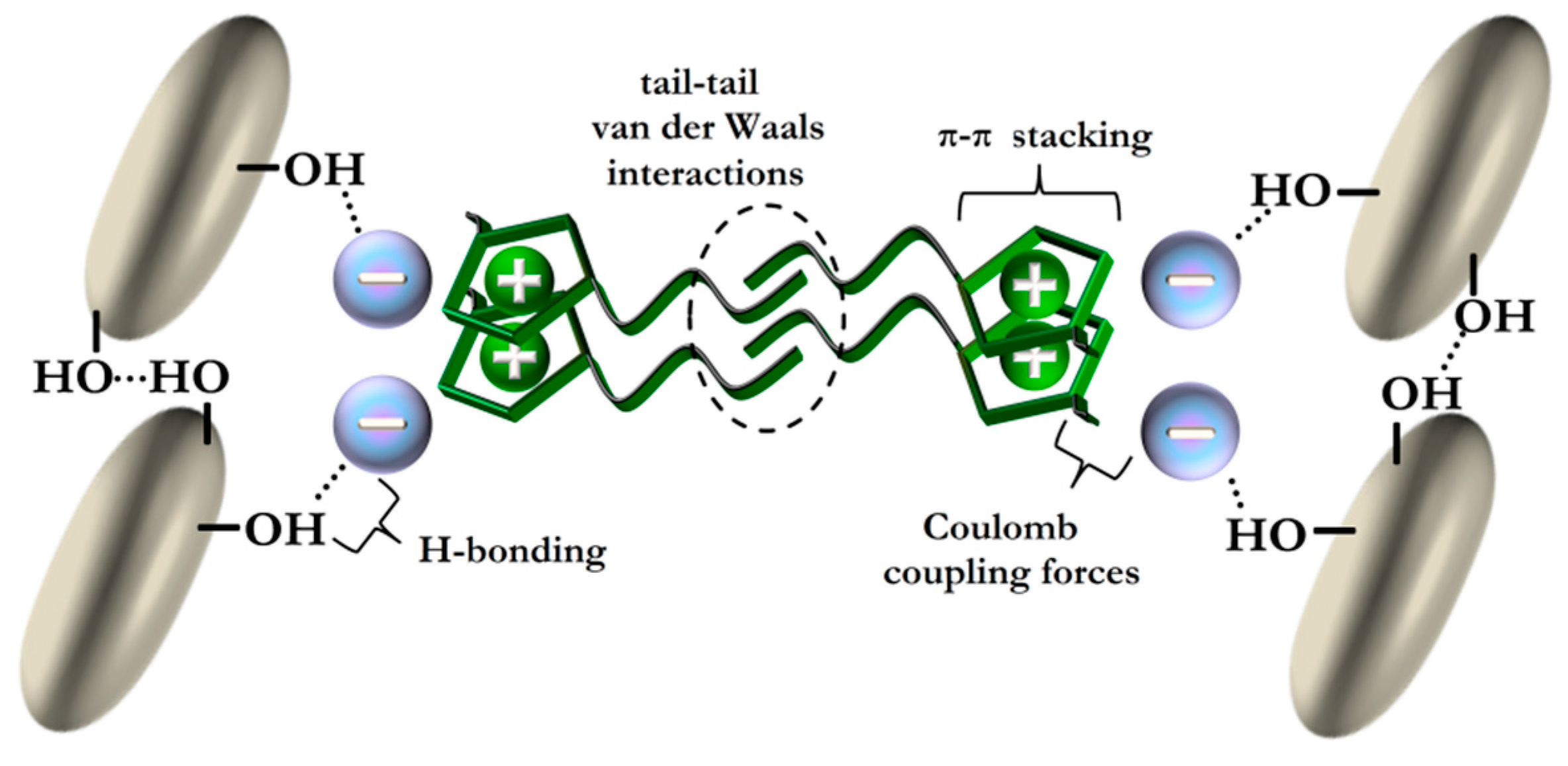
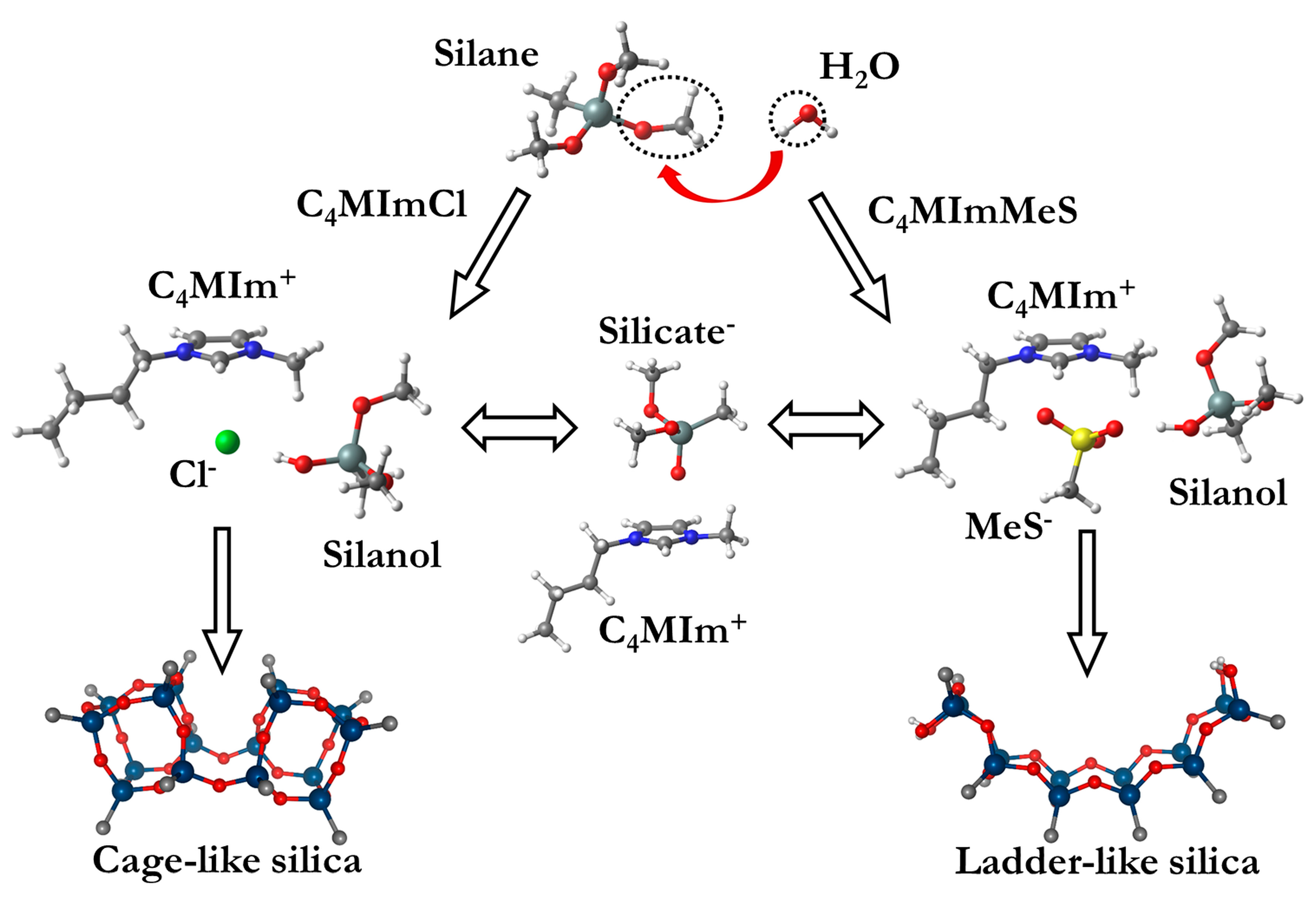
3. Ionic Liquids
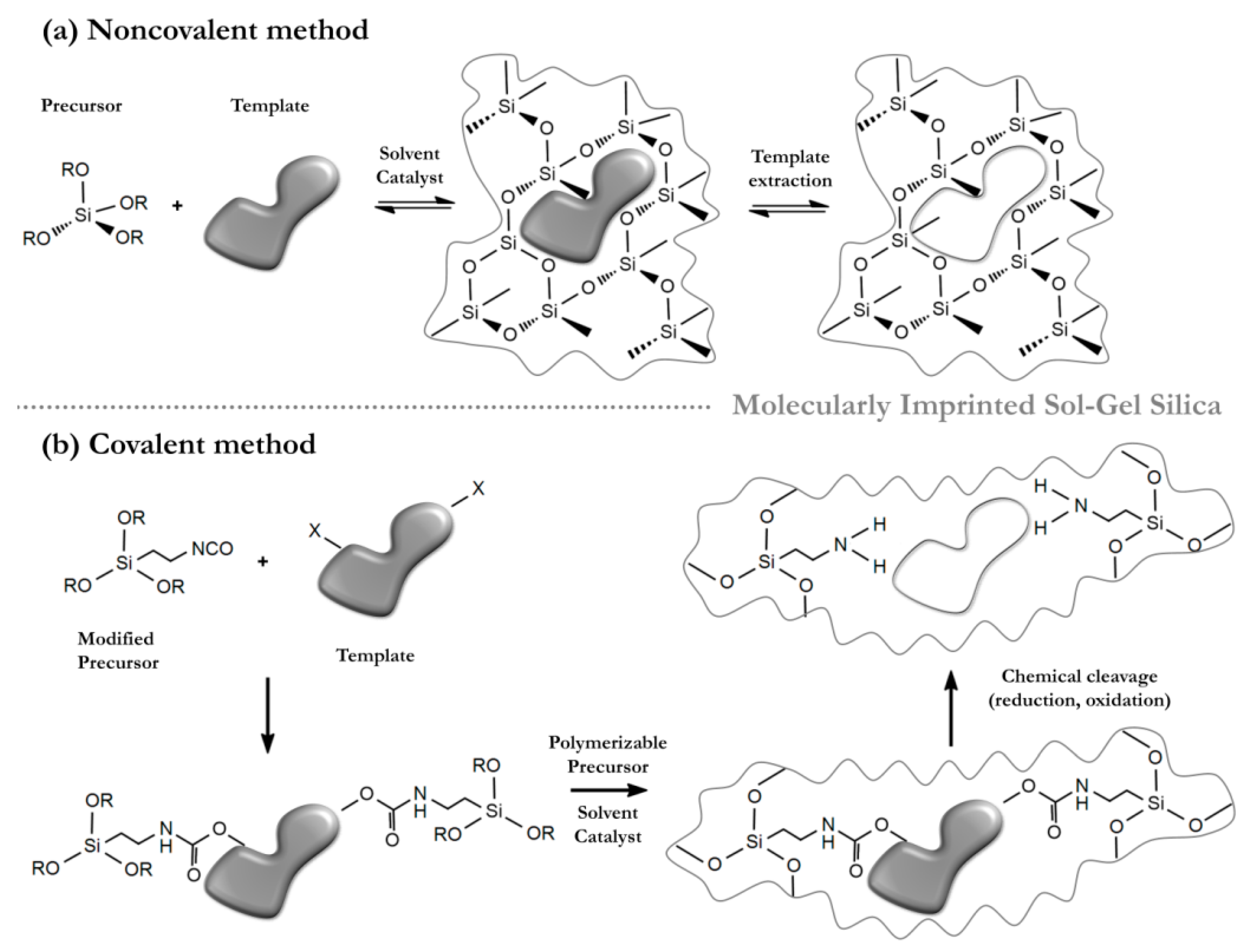
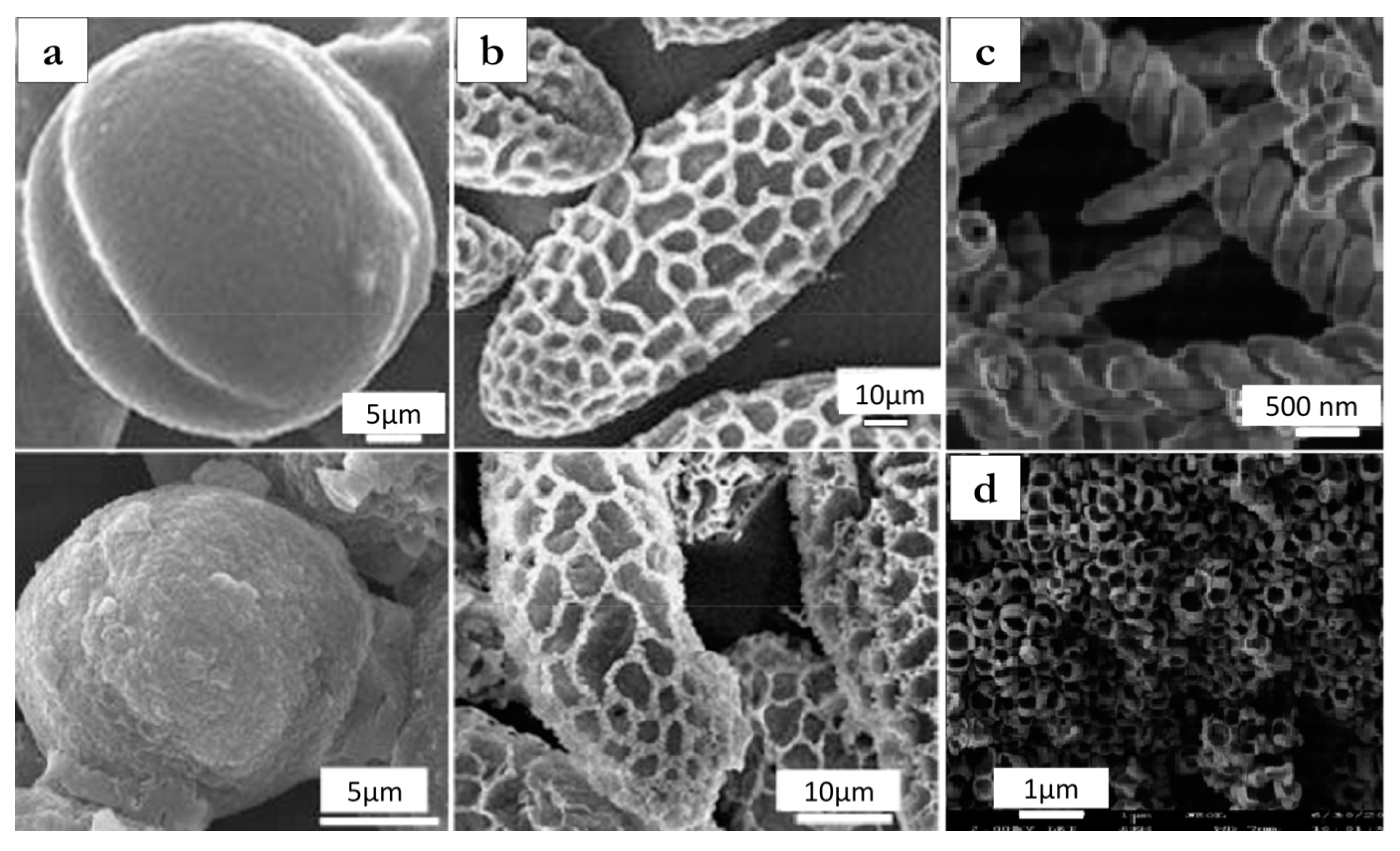
4. Molecular Imprinting in the Sol-Gel Process Using Ionic Liquids
5. Polymer-Sol-Gel Silica Hybrid Nanocomposites
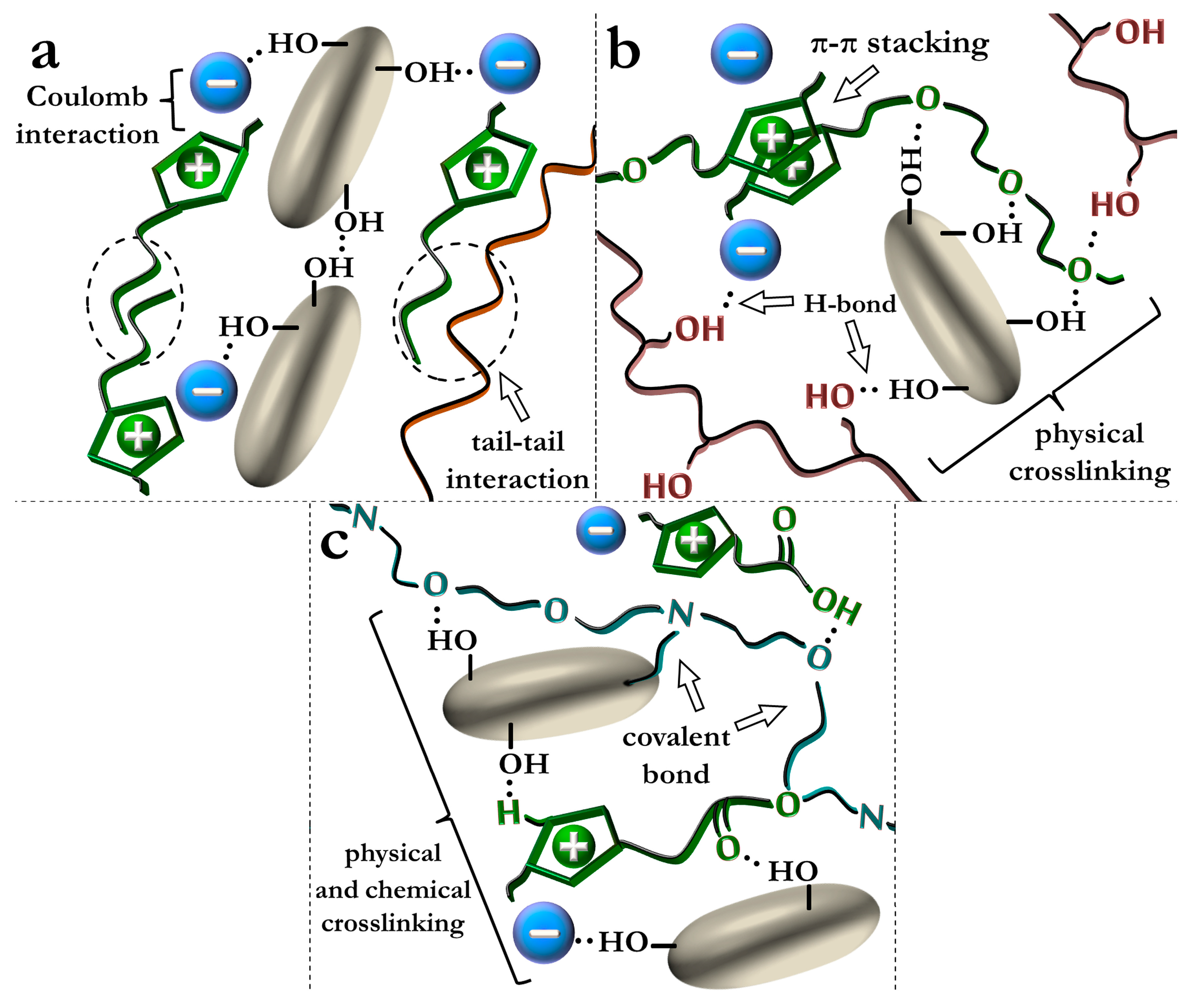
5.1. Ionic Liquids as Multifunctional Additives in Polymer–Silica Nanocomposites
5.2. Applications of Polymer–Silica-IL Nanocomposites
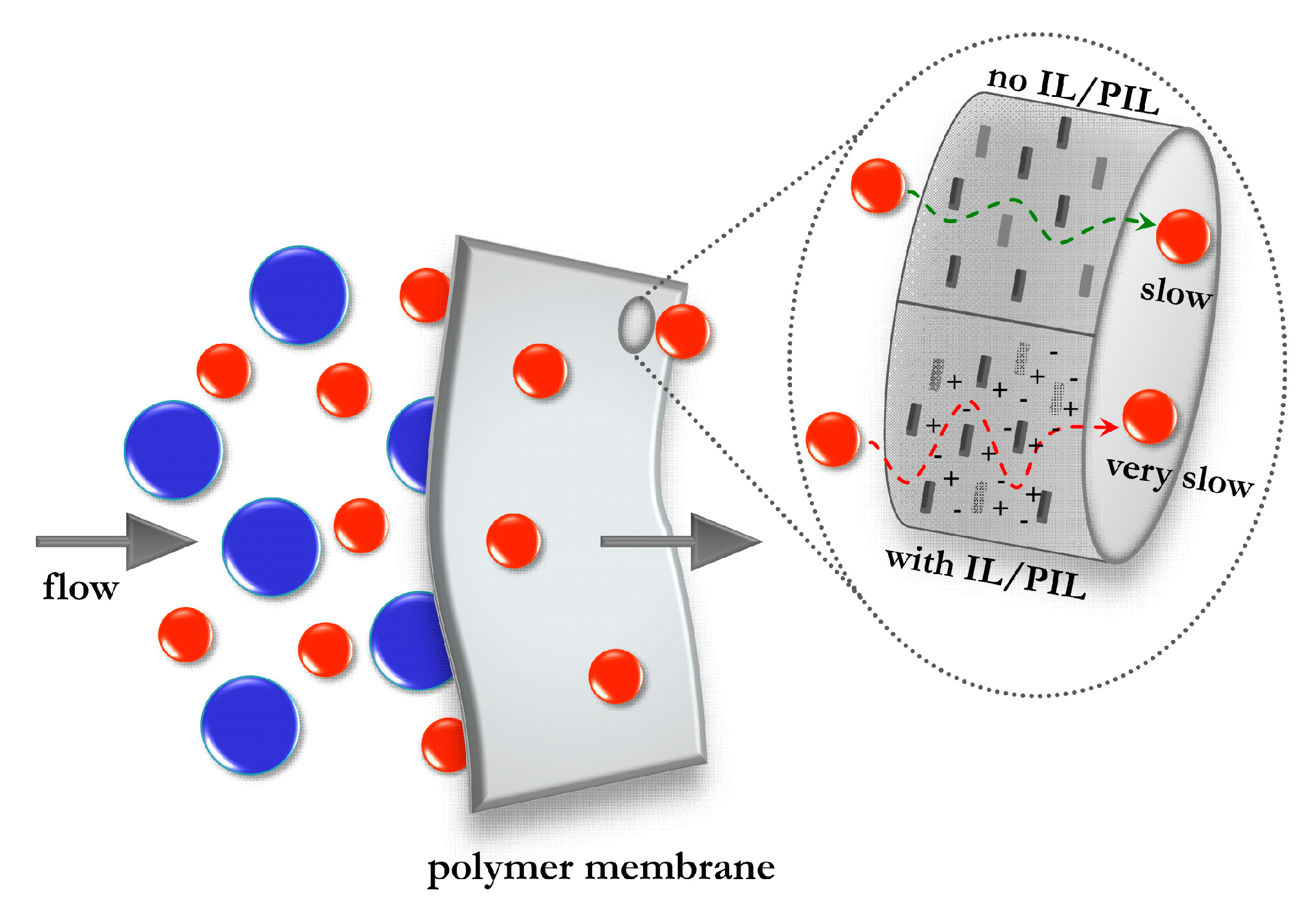
5.2.1. IL-Based Nanocomposites with Barrier Properties
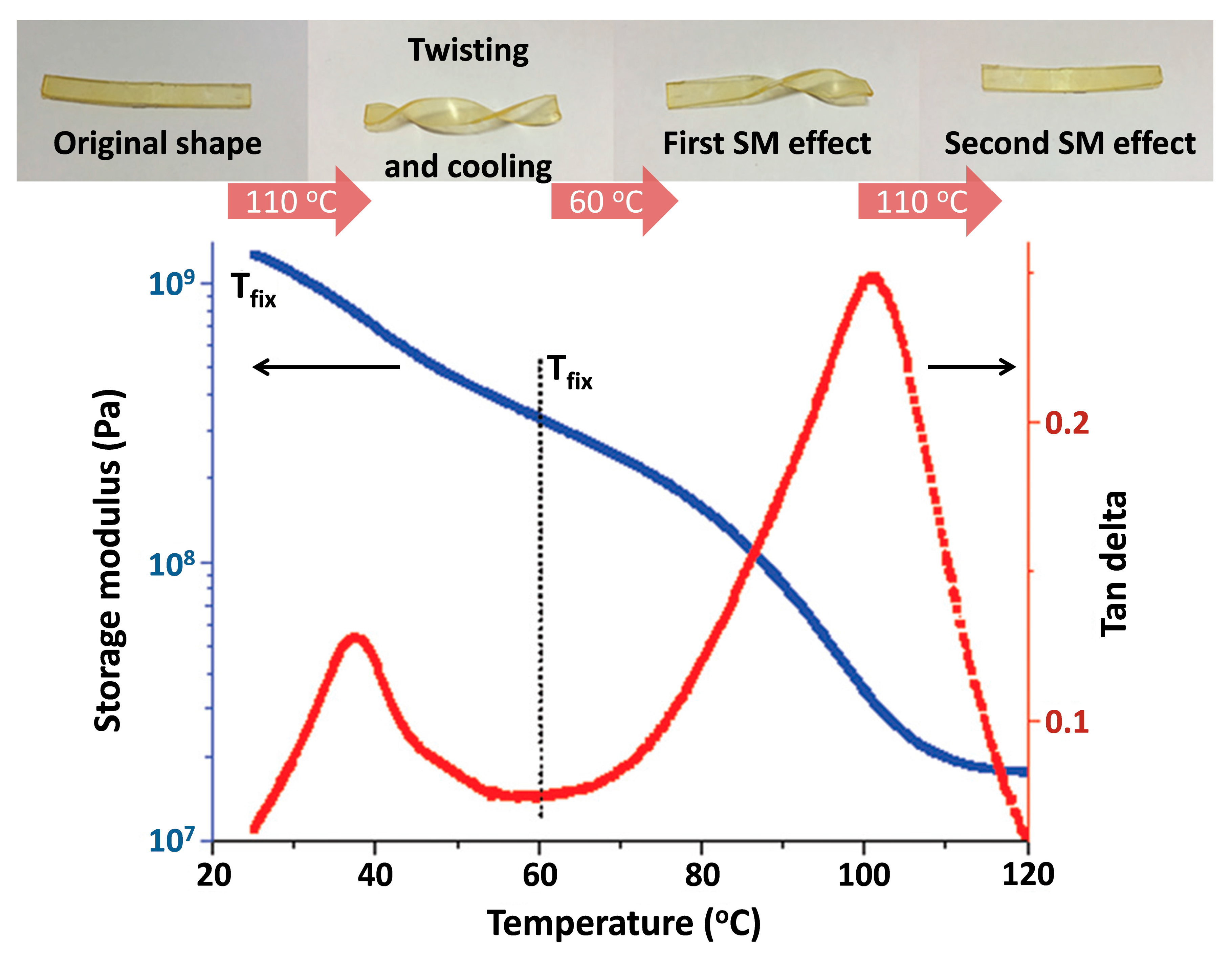
5.2.2. IL-Based Shape–Memory Materials
5.2.3. IL-Based Self-Healing Materials
6. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Elsevier: Amsterdam, The Netherlands, 1990; ISBN 9780121349707. [Google Scholar]
- Donato, R.K.; Lavorgna, M.; Musto, P.; Donato, K.Z.; Jager, A.; Štěpánek, P.; Schrekker, H.S.; Matějka, L. The role of ether-functionalized ionic liquids in the sol-gel process: Effects on the initial alkoxide hydrolysis steps. J. Colloid Interface Sci. 2015, 447, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.K.; Matějka, L.; Schrekker, H.S.; Pleštil, J.; Jigounov, A.; Brus, J.; Šlouf, M. The multifunctional role of ionic liquids in the formation of epoxy-silica nanocomposites. J. Mater. Chem. 2011, 21, 13801. [Google Scholar] [CrossRef]
- Donato, K.Z.; Donato, R.K.; Lavorgna, M.; Ambrosio, L.; Matějka, L.; Mauler, R.S.; Schrekker, H.S. Ionic liquids as dynamic templating agents for sol-gel silica systems: Synergistic anion and cation effect on the silica structured growth. J. Sol-Gel Sci. Technol. 2015, 76, 414–427. [Google Scholar] [CrossRef]
- Donato, R.K.; Benvegnú, M.A.; Furlan, L.G.; Mauler, R.S.; Schrekker, H.S. Imidazolium salts as liquid coupling agents for the preparation of polypropylene-silica composites. J. Appl. Polym. Sci. 2010, 116, 304–307. [Google Scholar] [CrossRef]
- Donato, R.K.; Donato, K.Z.; Schrekker, H.S.; Matějka, L. Tunable reinforcement of epoxy-silica nanocomposites with ionic liquids. J. Mater. Chem. 2012, 22, 9939. [Google Scholar] [CrossRef]
- Donato, R.K.; Perchacz, M.; Ponyrko, S.; Donato, K.Z.; Schrekker, H.S.; Beneš, H.; Matějka, L. Epoxy-silica nanocomposite interphase control using task-specific ionic liquids via hydrolytic and non-hydrolytic sol-gel processes. R. Soc. Chem. Adv. 2015, 5, 91330–91339. [Google Scholar] [CrossRef]
- Ponyrko, S.; Donato, R.K.; Matějka, L. Tailored high performance shape memory epoxy-silica nanocomposites. Structure design. Polym. Chem. 2016, 7, 560–572. [Google Scholar] [CrossRef]
- Perchacz, M.; Donato, R.K.; Seixas, L.; Zhigunov, A.; Serkis-rodzen, M.; Benes, H. Ionic Liquid-Silica Precursors via Solvent-Free Sol—Gel Process and Their Application in Epoxy-Amine Network: A Theoretical/Experimental Study. ACS Appl. Mater. Interfaces 2017, 9, 16474–16487. [Google Scholar] [CrossRef] [PubMed]
- Donato, K.Z.; Lavorgna, M.; Donato, R.K.; Raucci, M.G.; Buonocore, G.G.; Ambrosio, L.; Schrekker, H.S.; Mauler, R.S. High Amorphous Vinyl Alcohol-Silica Bionanocomposites: Tuning Interface Interactions with Ionic Liquids. ACS Sustain. Chem. Eng. 2017, 5, 1094–1105. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of “sol-gel” chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Palmisano, G. Silica-based hybrid coatings. J. Mater. Chem. 2009, 19, 3116. [Google Scholar] [CrossRef]
- Nassif, N.; Livage, J. From diatoms to silica-based biohybrids. Chem. Soc. Rev. 2011, 40, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; González, B. Medical applications of organic–inorganic hybrid materials within the field of silica-based bioceramics. Chem. Soc. Rev. 2011, 40, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Letailleur, A.A.; Ribot, F.; Boissière, C.; Teisseire, J.; Barthel, E.; Desmazières, B.; Chemin, N.; Sanchez, C. Sol-gel derived hybrid thin films: The chemistry behind processing. Chem. Mater. 2011, 23, 5082–5089. [Google Scholar] [CrossRef]
- Sanchez, C.; Shea, K.J.; Kitagawa, S. Recent progress in hybrid materials science. Chem. Soc. Rev. 2011, 40, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Avnir, D.; Levy, D.; Reisfeld, R. The nature of the silica cage as reflected by spectral changes and enhanced photostability of trapped Rhodamine 6G. J. Phys. Chem. 1984, 88, 5956–5959. [Google Scholar] [CrossRef]
- Kaufman, V.R.; Levy, D.; Avnir, D. A photophysical study of the sol/gel transition in silica: Structural dynamics and oscillations, room-temperature phosphorescence and photochromic gel glasses. J. Non-Cryst. Solids 1986, 82, 103–109. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol-gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Tang, S.; Liu, S.; Ren, H.; Liang, X.; Qiu, H.; Guo, Y.; Liu, X.; Jiang, S. A novel imidazolium-based organic–silica hybrid monolith for per aqueous capillary electrochromatography. R. Soc. Chem. Adv. 2014, 4, 25819. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical Properties of Ionic Liquids: Database and Evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Gabriel, S.; Weiner, J. Ueber einige Abkömmlinge des Propylamins. Eur. J. Inorg. Chem. 1888, 21, 2669–2679. [Google Scholar] [CrossRef]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 8, 405–422. [Google Scholar]
- Hurley, F.H.; WIer, T.P. The Electrodeposition of Aluminum from Nonaqueous Solutions at Room Temperature. J. Electrochem. Soc. 1951, 98, 207. [Google Scholar] [CrossRef]
- Işık, M.; Sardon, H.; Mecerreyes, D. Ionic Liquid and Cellulose Technologies: Dissolution, Modification and Composite Preparation. In Applications of Ionic Liquids in Polymer Science and Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 135–152. [Google Scholar]
- Dupont, J.; Suarez, P.A.Z. Physico-chemical processes in imidazolium ionic liquids. Phys. Chem. Chem. Phys. 2006, 8, 2441. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Baker, G.A.; Zhao, H. Ether- and alcohol-functionalized task-specific ionic liquids: Attractive properties and applications. Chem. Soc. Rev. 2012, 41, 4030–4066. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Xue, T.; Lee, J.-M. What causes the low viscosity of ether-functionalized ionic liquids? Its dependence on the increase of free volume. R. Soc. Chem. Adv. 2012, 2, 10564–10574. [Google Scholar] [CrossRef]
- Hunt, P.A.; Ashworth, C.R.; Matthews, R.P. Hydrogen bonding in ionic liquids. Chem. Soc. Rev. 2015, 44, 1257–1288. [Google Scholar] [CrossRef] [PubMed]
- Schrekker, H.S.; Silva, D.O.; Gelesky, M.A.; Stracke, M.P.; Schrekker, C.M.L.; Gonçalves, R.S.; Dupont, J. Preparation, cation-anion interactions and physicochemical properties of ether-functionalized imidazolium ionic liquids. J. Braz. Chem. Soc. 2008, 19, 426–433. [Google Scholar] [CrossRef]
- Zhou, Z.-B.; Matsumoto, H.; Tatsumi, K. Low-Melting, Low-Viscous, Hydrophobic Ionic Liquids: Aliphatic Quaternary Ammonium Salts with Perfluoroalkyltrifluoroborates. Chem. A Eur. J. 2005, 11, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Borodin, O.; Li, L.; Kim, H.; Liu, Q.; Bara, J.E.; Gin, D.L.; Nobel, R. A comparison of ether- and alkyl-derivatized imidazolium-based room-temperature ionic liquids: A molecular dynamics simulation study. Phys. Chem. Chem. Phys. 2008, 10, 6301–6312. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Ang, W.H.; Zhao, D.; Scopelliti, R.; Zvereva, E.E.; Katsyuba, S.A.; Dyson, P.J. Revisiting ether-derivatized imidazolium-based ionic liquids. J. Phys. Chem. B 2007, 111, 10095–10108. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, V.Z.; Donato, R.K.; Dalla Lana, D.F.; Donato, K.J.Z.; Ortega, G.G.; Schrekker, H.S.; Fuentefria, A.M. Imidazolium salts as antifungal agents: Strong antibiofilm activity against multidrug-resistant Candida tropicalis isolates. Lett. Appl. Microbiol. 2015, 60, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Schrekker, H.S.; Donato, R.K.; Fuentefria, A.M.; Bergamo, V.; Oliveira, L.F.; Machado, M.M. Imidazolium salts as antifungal agents: Activity against emerging yeast pathogens, without human leukocyte toxicity. Medchemcomm 2013, 4, 1457. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gilmore, B.F. The antimicrobial potential of ionic liquids: A source of chemical diversity for infection and biofilm control. Int. J. Antimicrob. Agents 2015, 46, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Riduan, S.N.; Zhang, Y. Imidazolium salts and their polymeric materials for biological applications. Chem. Soc. Rev. 2013, 42, 9055–9070. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Zheng, B.L.; He, K.; Zheng, Q.Y. Imidazole alkaloids from Lepidium meyenii. J. Nat. Prod. 2003, 66, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Gathergood, N. Biodegradation of ionic liquids—A critical review. Chem. Soc. Rev. 2015, 44, 8200–8237. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Inamuddin, D. (Eds.) Green Solvents II: Properties and Applications of Ionic Liquids; Springer: Dordrecht, The Netherlands, 2012; ISBN 9789400728912. [Google Scholar]
- Vijayaraghavan, R.; MacFarlane, D.R. Living cationic polymerisation of styrene in an ionic liquid. Chem. Commun. 2004, 34, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S.; Davis, T.P.; Carmichael, A.J.; Haddleton, D.M. First report of reversible addition–fragmentation chain transfer (RAFT) polymerisation in room temperature ionic liquids. Chem. Commun. 2002, 101, 2226–2227. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Ding, S. Catalyst separation in atom transfer radical polymerization. Prog. Polym. Sci. 2004, 29, 1053–1078. [Google Scholar] [CrossRef]
- Sarbu, T.; Matyjaszewski, K. ATRP of methyl methacrylate in the presence of ionic liquids with ferrous and cuprous anions. Macromol. Chem. Phys. 2001, 202, 3379–3391. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, K.; Mays, J.W. Synthesis of block copolymers of styrene and methyl methacrylate by conventional free radical polymerization in room temperature ionic liquids. Macromolecules 2002, 35, 5738–5741. [Google Scholar] [CrossRef]
- Hanyu, Y.; Honma, I. Rechargeable quasi-solid state lithium battery with organic crystalline cathode. Sci. Rep. 2012, 2, 453. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Unemoto, A.; Ogawa, H.; Tomai, T.; Honma, I. Application of quasi-solid-state silica nanoparticles–ionic liquid composite electrolytes to all-solid-state lithium secondary battery. J. Power Sources 2012, 208, 271–275. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Comte, P.; Exnar, I.; Grätzel, M. Gelation of ionic liquid-based electrolytes with silica nanoparticles for quasi-solid-state dye-sensitized solar cells. J. Am. Chem. Soc. 2003, 125, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Kubo, W.; Kitamura, T.; Hanabusa, K.; Wada, Y.; Yanagida, S. Quasi-solid-state dye-sensitized solar cells using room temperature molten salts and a low molecular weight gelator. Chem. Commun. 2002, 115, 374–375. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef] [PubMed]
- Lodge, T.P. A Unique Platform for Materials Design. Science 2008, 321, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Texter, J. Ionic liquids and polymeric ionic liquids as stimuli-responsive functional materials. In Applications of Ionic Liquids in Polymer Science and Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 103–134. ISBN 9783662449035. [Google Scholar]
- Klingshirn, M.A.; Spear, S.K.; Subramanian, R.; Holbrey, J.D.; Huddleston, J.G.; Rogers, R.D. Gelation of Ionic Liquids Using a Cross-Linked Poly(Ethylene Glycol) Gel Matrix. Chem. Mater. 2004, 16, 3091–3097. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s as ionic liquid-based innovative polyelectrolytes. In Applications of Ionic Liquids in Polymer Science and Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 47–67. ISBN 9783662449035. [Google Scholar]
- Livi, S.; Gérard, J.-F.; Duchet-Rumeau, J. Ionic Liquids as Polymer Additives. In Applications of Ionic Liquids in Polymer Science and Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–21. ISBN 978-3-662-44903-5. [Google Scholar]
- Cai, M.; Liang, Y.; Zhou, F.; Liu, W. Ionic Liquids as Lubricants. In Green Solvents II; Springer: Dordrecht, The Netherlands, 2012; pp. 203–233. [Google Scholar]
- Donato, R.K.; Migliorini, M.V.; Benvegnu, M.A.; Stracke, M.P.; Gelesky, M.A.; Pavan, F.A.; Schrekker, C.M.L.; Benvenutti, E.V.; Dupont, J.; Schrekker, H.S. Synthesis of silica xerogels with highly distinct morphologies in the presence of imidazolium ionic liquids. J. Sol-Gel Sci. Technol. 2009, 49, 71–77. [Google Scholar] [CrossRef]
- Zhou, Y.; Schattka, J.H.; Antonietti, M. Room-temperature ionic liquids as template to monolithic mesoporous silica with wormlike pores via a sol-gel nanocasting technique. Nano Lett. 2004, 4, 477–481. [Google Scholar] [CrossRef]
- Zhou, Y.; Antonietti, M. Preparation of highly ordered monolithic super-microporous lamellar silica with a room-temperature ionic liquid as template via the nanocasting technique. Adv. Mater. 2003, 15, 1452–1455. [Google Scholar] [CrossRef]
- Zhou, Y.; Antonietti, M. A novel tailored bimodal porous silica with well-defined inverse opal microstructure and super-microporous lamellar nanostructure. Chem. Commun. 2003, 206, 2564–2565. [Google Scholar] [CrossRef]
- Zhou, Y.; Antonietti, M. A Series of Highly Ordered, Super-Microporous, Lamellar Silicas Prepared by Nanocasting with Ionic Liquids. Chem. Mater. 2004, 16, 544–550. [Google Scholar] [CrossRef]
- Adams, C.J.; Bradley, A.E.; Seddon, K.R. The Synthesis of Mesoporous Materials Using Novel Ionic Liquid Templates in Water. Aust. J. Chem. 2002, 54, 679–681. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.R.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Schüth, F. Nanocasting: A versatile strategy for creating nanostructured porous materials. Adv. Mater. 2006, 18, 1793–1805. [Google Scholar] [CrossRef]
- Jiang, P.; Bertone, J.F.; Colvin, V.L. A Lost-Wax Approach to Monodisperse Colloids and Their Crystals. Science 2001, 291, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, M.E.; Laíñ, R.B. Molecular Imprinting in Sol-Gel Materials: Recent Developments and Applications. Microchim. Acta 2005, 149, 19–36. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Dhainaut, J.; Dacquin, J.P.; Lee, A.F.; Wilson, K. Hierarchical macroporous-mesoporous SBA-15 sulfonic acid catalysts for biodiesel synthesis. Green Chem. 2010, 12, 296–303. [Google Scholar] [CrossRef]
- Guan, Z.S.; Lu, C.H.; Zhang, Y.; Zi, X.Z. Morphology-controlled Synthesis of SiO2 Hierarchical Structures Using Pollen Grains as Templates. Chin. J. Chem. 2008, 26, 467–470. [Google Scholar] [CrossRef]
- Marx, S.; Avnir, D. The induction of chirality in sol-gel materials. Acc. Chem. Res. 2007, 40, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Fireman-Shoresh, S.; Avnir, D.; Marx, S. General method for chiral imprinting of sol-gel thin films exhibiting enantioselectivity. Chem. Mater. 2003, 15, 3607–3613. [Google Scholar] [CrossRef]
- Huo, H.; Wang, S.; Lin, S.; Li, Y.; Li, B.; Yang, Y. Chiral zirconia nanotubes prepared through a sol-gel transcription approach. J. Mater. Chem. A 2014, 2, 333–338. [Google Scholar] [CrossRef]
- Dupont, J. On the solid, liquid and solution structural organization of imidazolium ionic liquids. J. Braz. Chem. Soc. 2004, 15, 341–350. [Google Scholar] [CrossRef]
- Dai, S.; Ju, Y.H.; Gao, H.J.; Lin, J.S.; Pennycook, S.J.; Barnes, C.E. Preparation of silica aerogel using ionic liquids as solvents. Chem. Commun. 2000, 37, 243–244. [Google Scholar] [CrossRef]
- Xu, C.; Tang, R.; Hua, Y.; Zhang, P. Mesoporous Silica Materials Synthesized via Sol-Gel Methods Modified with Ionic Liquid and Surfactant Molecules Mesoporous Silica Materials Synthesized via Sol-Gel Methods Modified with Ionic Liquid and Surfactant Molecules. Chin. J. Chem. Phys. 2008, 21, 596–600. [Google Scholar] [CrossRef]
- Karout, A.; Pierre, A.C. Silica xerogels and aerogels synthesized with ionic liquids. J. Non-Cryst. Solids 2007, 353, 2900–2909. [Google Scholar] [CrossRef]
- Klingshirn, M.A.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Ionic liquids as solvent and solvent additives for the synthesis of sol-gel materials. J. Mater. Chem. 2005, 15, 5174–5180. [Google Scholar] [CrossRef]
- Kinoshita, K.; Yanagimoto, H.; Suzuki, T.; Minami, H. Influence of the molecular-oriented structure of ionic liquids on the crystallinity of aluminum hydroxide prepared by a sol-gel process in ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 18705–18709. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Antonietti, M. Synthesis of Very Small TiO2 Nanocrystals in a Room-Temperature Ionic Liquid and Their Self-Assembly toward Mesoporous Spherical Aggregates. J. Am. Chem. Soc. 2003, 125, 14960–14961. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yu, J.; Dai, S. Preparation of inorganic materials using ionic liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Vioux, A.; Viau, L.; Volland, S.; Le Bideau, J. Use of ionic liquids in sol-gel; ionogels and applications. Comptes Rendus Chim. 2010, 13, 242–255. [Google Scholar] [CrossRef]
- Viau, L.; Néouze, M.A.; Biolley, C.; Volland, S.; Brevet, D.; Gaveau, P.; Dieudonné, P.; Galarneau, A.; Vioux, A. Ionic liquid mediated sol-gel synthesis in the presence of water or formic acid: Which synthesis for which material? Chem. Mater. 2012, 24, 3128–3134. [Google Scholar] [CrossRef]
- Verma, Y.L.; Singh, R.K.; Oh, I.-K.; Chandra, S. Ionic liquid template assisted synthesis of porous nano-silica nails. R. Soc. Chem. Adv. 2014, 4, 39978. [Google Scholar] [CrossRef]
- Gupta, A.K.; Singh, R.K.; Chandra, S. Studies on mesoporous silica ionogels prepared by sol-gel method at different gelation temperatures. R. Soc. Chem. Adv. 2013, 3, 13869. [Google Scholar] [CrossRef]
- Martinelli, A.; Nordstierna, L. An investigation of the sol-gel process in ionic liquid–silica gels by time resolved Raman and 1H NMR spectroscopy. Phys. Chem. Chem. Phys. 2012, 14, 13216. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A. Effects of a protic ionic liquid on the reaction pathway during non-aqueous sol-gel synthesis of silica: A Raman spectroscopic investigation. Int. J. Mol. Sci. 2014, 15, 6488–6503. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Zakeeruddin, S.M.; Comte, P.; Liska, P.; Kuang, D.; Grätzel, M. Bifacial dye-sensitized solar cells based on an ionic liquid electrolyte. Nat. Photonics 2008, 2, 693–698. [Google Scholar] [CrossRef]
- Singh, M.P.; Singh, R.K.; Chandra, S. Ionic liquids confined in porous matrices: Physicochemical properties and applications. Prog. Mater. Sci. 2014, 64, 73–120. [Google Scholar] [CrossRef]
- Li, H.; Bhadury, P.S.; Song, B.; Yang, S. Immobilized functional ionic liquids: Efficient, green, and reusable catalysts. R. Soc. Chem. Adv. 2012, 2, 12525. [Google Scholar] [CrossRef]
- Safaei, S.; Mohammadpoor-baltork, I.; Khosropour, A.R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Nano-silica supported acidic ionic liquid as an efficient catalyst for the multi-component synthesis of indazolophthalazine-triones and bis-indazolophthalazine-triones. Catal. Sci. Technol. 2013, 3, 2717–2722. [Google Scholar] [CrossRef]
- Marins, J.A.; Soares, B.G.; Silva, A.A.; Livi, S. Silica prepared in the presence of alkylphosphonium-based ionic liquids and its performance in electrorheological fluids. RSC Adv. 2014, 4, 50925–50931. [Google Scholar] [CrossRef]
- Marins, J.A.; Soares, B.G. Ionic liquid-based organically modified silica for the development of new electrorheological fluids. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 311–319. [Google Scholar] [CrossRef]
- Marins, J.A.; Soares, B.G.; Silva, A.A.; Hurtado, M.G.; Livi, S. Electrorheological and dielectric behavior of new ionic liquid/silica systems. J. Colloid Interface Sci. 2013, 405, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Matĕjka, L. Epoxy-silica/silsesquioxane Polymer Nanocomposites. In Hybrid Nanocomposites for Nanotechnology; Springer: Boston, MA, USA, 2009; pp. 1–84. [Google Scholar]
- Mark, J.E.; Jiang, C.Y.; Tang, M.Y. Simultaneous curing and filling of elastomers. Macromolecules 1984, 17, 2613–2616. [Google Scholar] [CrossRef]
- Landry, C.J.; Coltrain, B.K.; Brady, B.K. In situ polymerization of tetraethoxysilane in poly(methyl methacrylate): Morphology and dynamic mechanical properties. Polymer 1992, 33, 1486–1495. [Google Scholar] [CrossRef]
- Bauer, B.J.; Liu, D.-W.; Jackson, C.L.; Barnes, J.D. Epoxy/SiO2 Interpenetrating Polymer Networks. Polym. Adv. Technol. 1996, 7, 333–339. [Google Scholar] [CrossRef]
- Matějka, L.; Dušek, K.; Pleštil, J.; Kříž, J.; Lednický, F. Formation and structure of the epoxy-silica hybrids. Polymer 1999, 40, 171–181. [Google Scholar] [CrossRef]
- Matějka, L.; Dukh, O.; Meissner, B.; Hlavata, D.; Brus, J.; Strachota, A. Block Copolymer Organic-Inorganic Networks. Formation and Structure Ordering. Macromolecules 2003, 36, 7977–7985. [Google Scholar] [CrossRef]
- Huang, H.H.; Orler, B.; Wilkes, G.L. Ceramers: Hybrid materials incorporating polymeric/oligomeric species with inorganic glasses by a sol-gel process. Polym. Bull. 1985, 14, 557–564. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S., II; Choe, C.R.; Park, M.; Rim, S.; Kim, J. Preparation and characterization of epoxy composites filled with functionalized nanosilica particles obtained via sol-gel process. Polymer 2001, 42, 879–887. [Google Scholar] [CrossRef]
- Palza, H.; Vergara, R.; Zapata, P. Composites of polypropylene melt blended with synthesized silica nanoparticles. Compos. Sci. Technol. 2011, 71, 535–540. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of Silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites: A review. J. Nanomater. 2012, 2012, 132424. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review article: Polymer-matrix Nanocomposites, Processing, Manufacturing, and Application: An Overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.S.; Barry, C.M.F.; Mead, J.L. Effect of fill factor and validation of characterizing the degree of mixing in polymer nanocomposites. Polym. Eng. Sci. 2007, 47, 2049–2056. [Google Scholar] [CrossRef]
- Matĕjka, L.; Dukh, O.; Kolařík, J. Reinforcement of crosslinked rubbery epoxies by in-situ formed silica. Polymer 2000, 41, 1449–1459. [Google Scholar] [CrossRef]
- Ponyrko, S.; Kobera, L.; Brus, J.; Matějka, L. Epoxy-silica hybrids by nonaqueous sol-gel process. Polymer 2013, 54, 6271–6282. [Google Scholar] [CrossRef]
- Jain, S.; Goossens, H.; Picchioni, F.; Magusin, P.; Mezari, B.; Van Duin, M. Synthetic aspects and characterization of polypropylene-silica nanocomposites prepared via solid-state modification and sol-gel reactions. Polymer 2005, 46, 6666–6681. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, M.Q.; Rong, M.Z.; Friedrich, K. Silica nanoparticles filled polypropylene: Effects of particle surface treatment, matrix ductility and particle species on mechanical performance of the composites. Compos. Sci. Technol. 2005, 65, 635–645. [Google Scholar] [CrossRef]
- Zhu, X.; Melian, C.; Dou, Q.; Peter, K.; Demco, D.E.; Möller, M.; Anokhin, D.V.; Le Meins, J.M.; Ivanov, D.A. Morphology of injection-molded isotactic polypropylene/silica composites prepared via in-situ sol-gel technology. Macromolecules 2010, 43, 6067–6074. [Google Scholar] [CrossRef]
- Landry, C.; Coltrain, B.; Landry, M.; Fitzgerald, J.; Long, V. Poly (viny1 acetate)/Silica Filled Materials: Material Properties of in Situ vs Fumed Silica Particles. Macromolecules 1993, 26, 3702–3712. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Kittur, A.A.; Aralaguppi, M.I.; Kariduraganavar, M.Y. Synthesis and characterization of hybrid membranes using poly(vinyl alcohol) and tetraethylorthosilicate for the pervaporation separation of water-isopropanol mixtures. J. Appl. Polym. Sci. 2004, 94, 1304–1315. [Google Scholar] [CrossRef]
- Uragami, T.; Okazaki, K.; Matsugi, H.; Miyata, T. Structure and permeation characteristics of an aqueous ethanol solution of organic—Inorganic hybrid membranes composed of poly(vinyl alcohol) and tetraethoxysilane. Macromolecules 2002, 35, 9156–9163. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Liu, Q.L.; Huang, S.P.; Hu, W.W.; Zhu, A.M. Microstructure-related performances of poly(vinyl alcohol)-silica hybrid membranes: A molecular dynamics simulation study. J. Mater. Chem. 2012, 22, 10860. [Google Scholar] [CrossRef]
- Silveira, K.F.; Yoshida, I.V.P.; Nunes, S.P. Phase separation in PMMA/silica sol-gel systems. Polymer 1995, 36, 1425–1434. [Google Scholar] [CrossRef]
- Bokobza, L.; Diop, A.L. Reinforcement of Silicone Rubbers by Sol-Gel In Situ Generated Filler Particles. In Rubber Nanocomposites: Preparation, Properties, and Applications; John Wiley & Sons Ltd.: New York, NY, USA, 2010; pp. 63–85. ISBN 9780470823453. [Google Scholar]
- Roy, N.; Bhowmick, A.K. Novel in situ Silica/Polydimethylsiloxane Nanocomposites: Facile One-Pot Synthesis and Characterization. Rubber Chem. Technol. 2012, 85, 92–107. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, L.; Ye, H.; Liu, H.; Li, W. Modifying a waterborne polyacrylate coating with a silica sol for enhancing anti-fogging performance. R. Soc. Chem. Adv. 2016, 6, 92252–92258. [Google Scholar] [CrossRef]
- Gao, J.G.; Lv, H.Q.; Zhang, X.F. Preparation and Properties of Emulsion Coating of Polyacrylate Modified by In-situ Nano-hybrid Silica. Adv. Mater. Res. 2011, 239, 3221–3224. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Schadler, L.S.; Braun, P.V. Nanocomposite Science and Technology; John Wiley & Sons: New York, NY, USA, 2003; Volume 230. [Google Scholar]
- Ponyrko, S.; Kovářová, J.; Kobera, L.; Matějka, L. High-Tg, heat resistant epoxy-silica hybrids with a low content of silica generated by nonaqueous sol-gel process. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Carvalho, A.P.A.; Soares, B.G.; Livi, S. Organically modified silica (ORMOSIL) bearing imidazolium—Based ionic liquid prepared by hydrolysis/co-condensation of silane precursors: Synthesis, characterization and use in epoxy networks. Eur. Polym. J. 2016, 83, 311–322. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Barber, P.S.; Kelley, S.P.; Berton, P.; Rogers, R.D. Double Salt Ionic Liquids Based on 1-Ethyl-3-Methylimidazolium Acetate and Hydroxyl-Functionalized Ammonium Acetates: Strong Effects of Weak Interactions. Phys. Chem. Chem. Phys. 2017, 19, 26934–26943. [Google Scholar] [CrossRef] [PubMed]
- Lozano, L.J.; Godínez, C.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Sánchez-Segado, S.; Alguacil, F.J. Recent advances in supported ionic liquid membrane technology. J. Membr. Sci. 2011, 376, 1–14. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Hirota, Y.; Maeda, Y.; Yamamoto, Y.; Miyamoto, M.; Nishiyama, N. Organosilica membrane with ionic liquid properties for separation of toluene/H2 mixture. Materials 2017, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Tomé, L.C.; Mecerreyes, D.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Polymeric ionic liquid membranes containing IL–Ag+ for ethylene/ethane separation via olefin-facilitated transport. J. Mater. Chem. A 2014, 2, 5631. [Google Scholar] [CrossRef]
- Luza, L.; Rambor, C.P.; Gual, A.; Bernardi, F.; Domingos, J.B.; Grehl, T.; Brüner, P.; Dupont, J. Catalytically Active Membranelike Devices: Ionic Liquid Hybrid Organosilicas Decorated with Palladium Nanoparticles. Am. Chem. Soc. Catal. 2016, 6, 6478–6486. [Google Scholar] [CrossRef]
- Sun, J.-K.; Lin, H.-J.; Zhang, W.-Y.; Gao, M.-R.; Antonietti, M.; Yuan, J. A tale of two membranes: From poly (ionic liquid) to metal-organic framework hybrid nanoporous membranes via pseudomorphic replacement. Mater. Horiz. 2017, 4, 681–687. [Google Scholar] [CrossRef]
- Tomé, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A. (Ed.) Advances in Polymer Science. In Shape-Memory Polymers; Springer: Berlin/Heidelberg, Germany, 2010; Volume 226, ISBN 978-3-642-12358-0. [Google Scholar]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543. [Google Scholar] [CrossRef]
- Yuan, C.; Guo, J.; Yan, F. Shape memory poly(ionic liquid) gels controlled by host-guest interaction with β-cyclodextrin. Polymer 2014, 55, 3431–3435. [Google Scholar] [CrossRef]
- Du, H.; Liu, X.; Yu, Y.; Xu, Y.; Wang, Y.; Liang, Z. Microwave-Induced Poly(ionic liquid)/Poly(vinyl alcohol) Shape Memory Composites. Macromol. Chem. Phys. 2016, 217, 2626–2634. [Google Scholar] [CrossRef]
- Rossiter, J.; Takashima, K.; Mukai, T. Shape memory properties of ionic polymer-metal composites. Smart Mater. Struct. 2012, 21, 112002. [Google Scholar] [CrossRef]
- Odent, J.; Raquez, J.M.; Samuel, C.; Barrau, S.; Enotiadis, A.; Dubois, P.; Giannelis, E.P. Shape-Memory Behavior of Polylactide/Silica Ionic Hybrids. Macromolecules 2017, 50, 2896–2905. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.A.; Swait, T.J.; Lafferty, A.D. Recent Advances in Smart Self-Healing Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9781782422808. [Google Scholar]
- Döhler, D.; Peterlik, H.; Binder, W.H. A dual crosslinked self-healing system: Supramolecular and covalent network formation of four-arm star polymers. Polymer 2014, 69, 264–273. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Luo, X.; Mather, P.T. Shape memory assisted self-healing coating. Am. Chem. Soc. Macro Lett. 2013, 2, 152–156. [Google Scholar] [CrossRef]
- Nji, J.; Li, G. A biomimic shape memory polymer based self-healing particulate composite. Polymer 2010, 51, 6021–6029. [Google Scholar] [CrossRef]
- Grigoriev, D.; Shchukina, E.; Shchukin, D.G. Nanocontainers for Self-Healing Coatings. Adv. Mater. Interfaces 2017, 4, 1600318. [Google Scholar] [CrossRef]
- Kalista, S.J.; Pflug, J.R.; Varley, R.J. Effect of ionic content on ballistic self-healing in EMAA copolymers and ionomers. Polym. Chem. 2013, 4, 4910. [Google Scholar] [CrossRef]
- Ueki, T.; Usui, R.; Kitazawa, Y.; Lodge, T.P.; Watanabe, M. Thermally Reversible Ion Gels with Photohealing Properties Based on Triblock Copolymer Self-Assembly. Macromolecules 2015, 48, 5928–5933. [Google Scholar] [CrossRef]
- Saurín, N.; Sanes, J.; Carrión, F.J.; Bermúdez, M.D. Self-healing of abrasion damage on epoxy resin controlled by ionic liquid. R. Soc. Chem. Adv. 2016, 6, 37258–37264. [Google Scholar] [CrossRef]
- Cao, Y.; Morrissey, T.G.; Acome, E.; Allec, S.I.; Wong, B.M.; Keplinger, C.; Wang, C. A Transparent, Self-Healing, Highly Stretchable Ionic Conductor. Adv. Mater. 2017, 29, 1605099. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, T.J.; Jadischke, J.J.; De Bruyn, J.R.; Ragogna, P.J.; Gillies, E.R. Self-Healing Polyphosphonium Ionic Networks. Macromolecules 2017, 50, 5253–5260. [Google Scholar] [CrossRef]
- Das, A.; Sallat, A.; Böhme, F.; Suckow, M.; Basu, D.; Wießner, S.; Stöckelhuber, K.W.; Voit, B.; Heinrich, G. Ionic Modification Turns Commercial Rubber into a Self-Healing Material. Am. Chem. Soc. Appl. Mater. Interfaces 2015, 7, 20623–20630. [Google Scholar] [CrossRef] [PubMed]
- Odent, J.; Raquez, J.-M.; Dubois, P.; Giannelis, E.P. Ultra-stretchable ionic nanocomposites: From dynamic bonding to multi-responsive behavior. J. Mater. Chem. A 2017, 5, 13357–13363. [Google Scholar] [CrossRef]


| Tm (°C) | ||
|---|---|---|
| Inorganic Salts | NaCl | 803 |
| KCl | 772 | |
| Ionic Liquids | [C4MIm][Cl] | 68 * |
| [C4MIm][NTf2] | −4 * | |
| [C4MIm][PF6] | 10 * | |
| [C10MIm][PF6] | 32 * | |
| [C16MIm][PF6] | 75 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donato, K.Z.; Matějka, L.; Mauler, R.S.; Donato, R.K. Recent Applications of Ionic Liquids in the Sol-Gel Process for Polymer–Silica Nanocomposites with Ionic Interfaces. Colloids Interfaces 2017, 1, 5. https://doi.org/10.3390/colloids1010005
Donato KZ, Matějka L, Mauler RS, Donato RK. Recent Applications of Ionic Liquids in the Sol-Gel Process for Polymer–Silica Nanocomposites with Ionic Interfaces. Colloids and Interfaces. 2017; 1(1):5. https://doi.org/10.3390/colloids1010005
Chicago/Turabian StyleDonato, Katarzyna Z., Libor Matějka, Raquel S. Mauler, and Ricardo K. Donato. 2017. "Recent Applications of Ionic Liquids in the Sol-Gel Process for Polymer–Silica Nanocomposites with Ionic Interfaces" Colloids and Interfaces 1, no. 1: 5. https://doi.org/10.3390/colloids1010005





