Exploration of Mechanical and Thermal Properties of CTAB-Modified MoS2/LLDPE Composites Prepared by Melt Mixing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
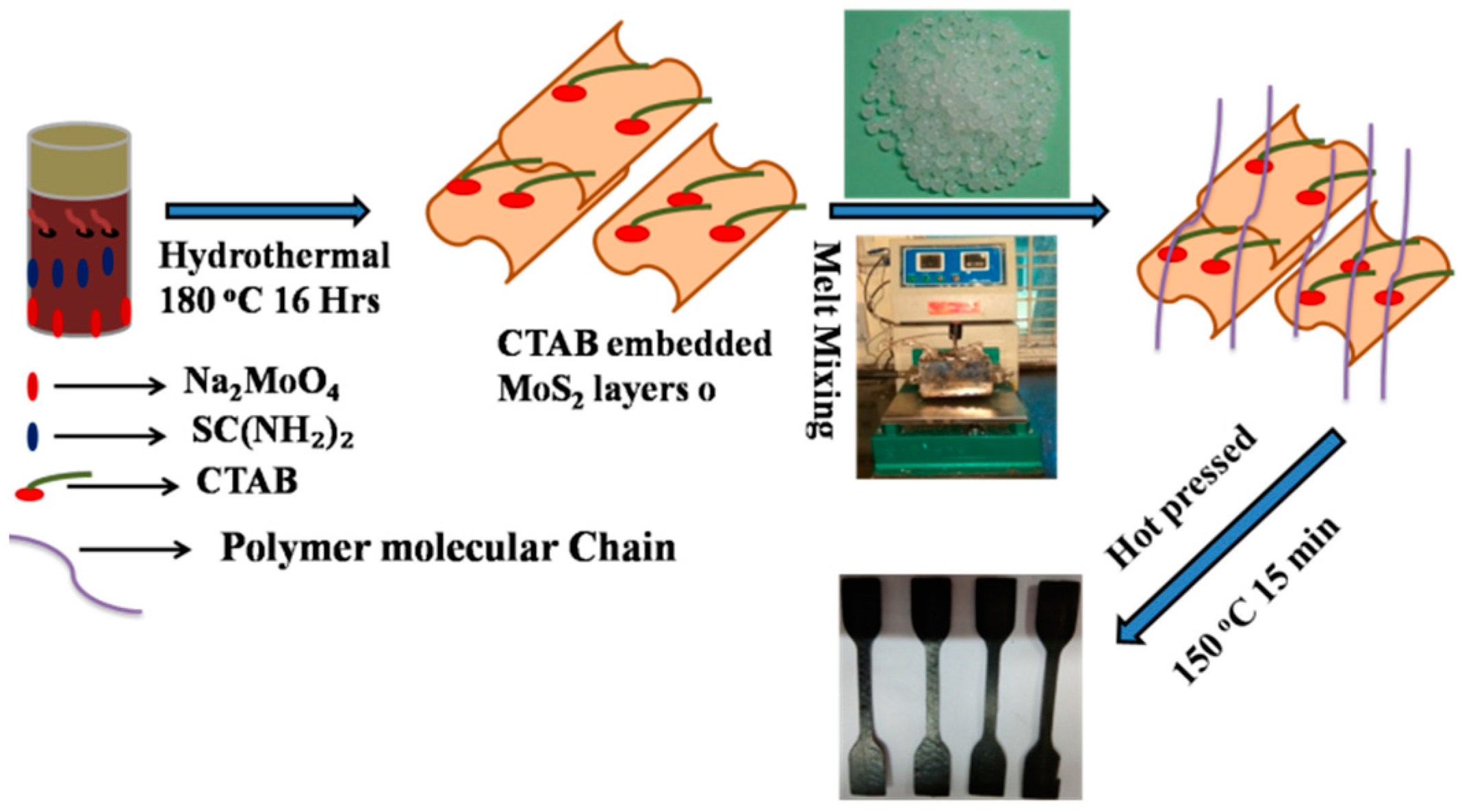
2.2. CTAB Assisted Synthesis of MoS2
2.3. Preparation of CTAB–MoS2/LLDPE Composites
2.4. Characterization
3. Results and Discussion
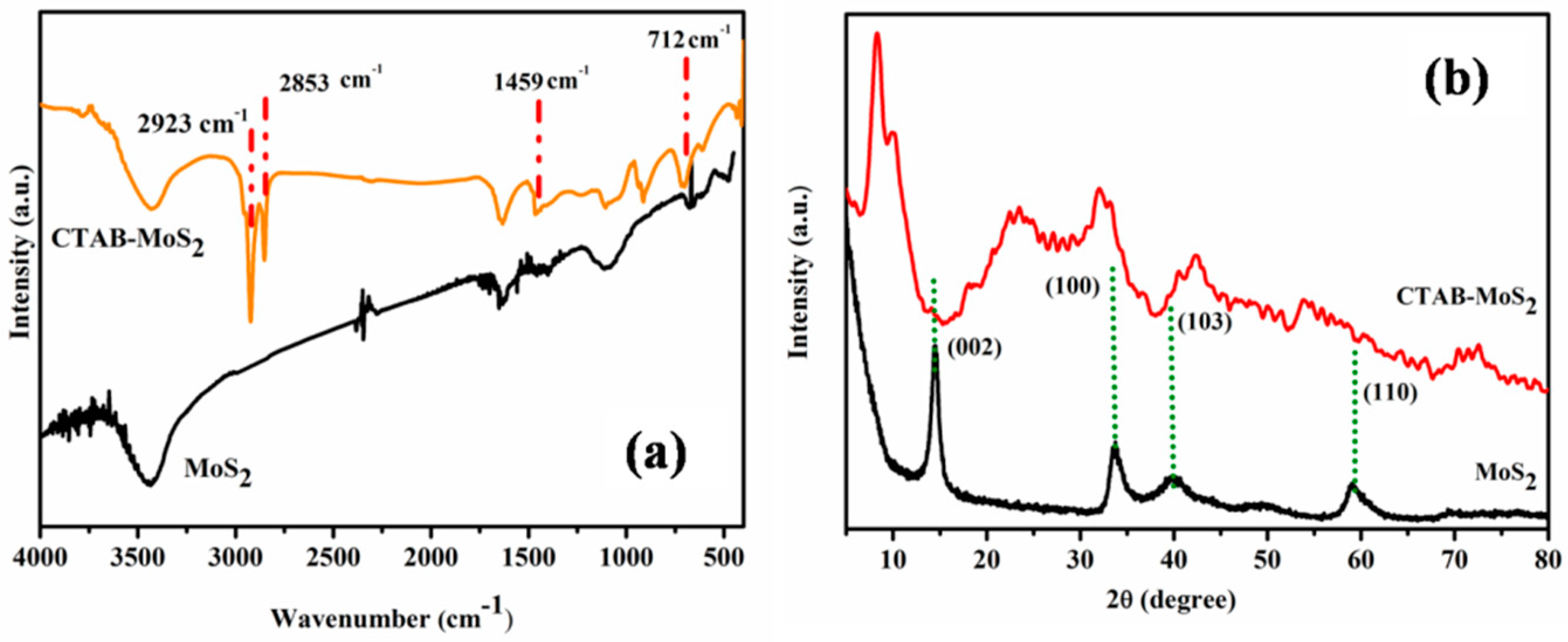
3.1. Characterization of MoS2and CTAB–MoS2
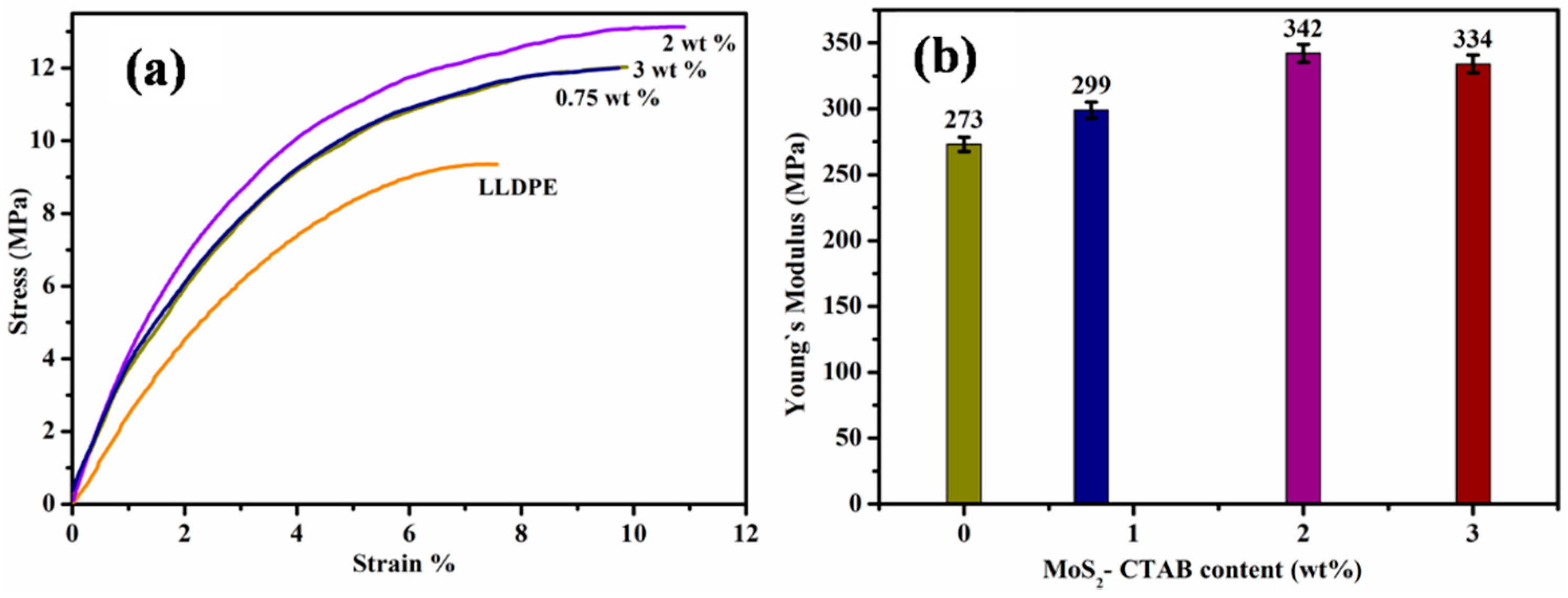
3.2. Stress–Strain Behaviour Analysis
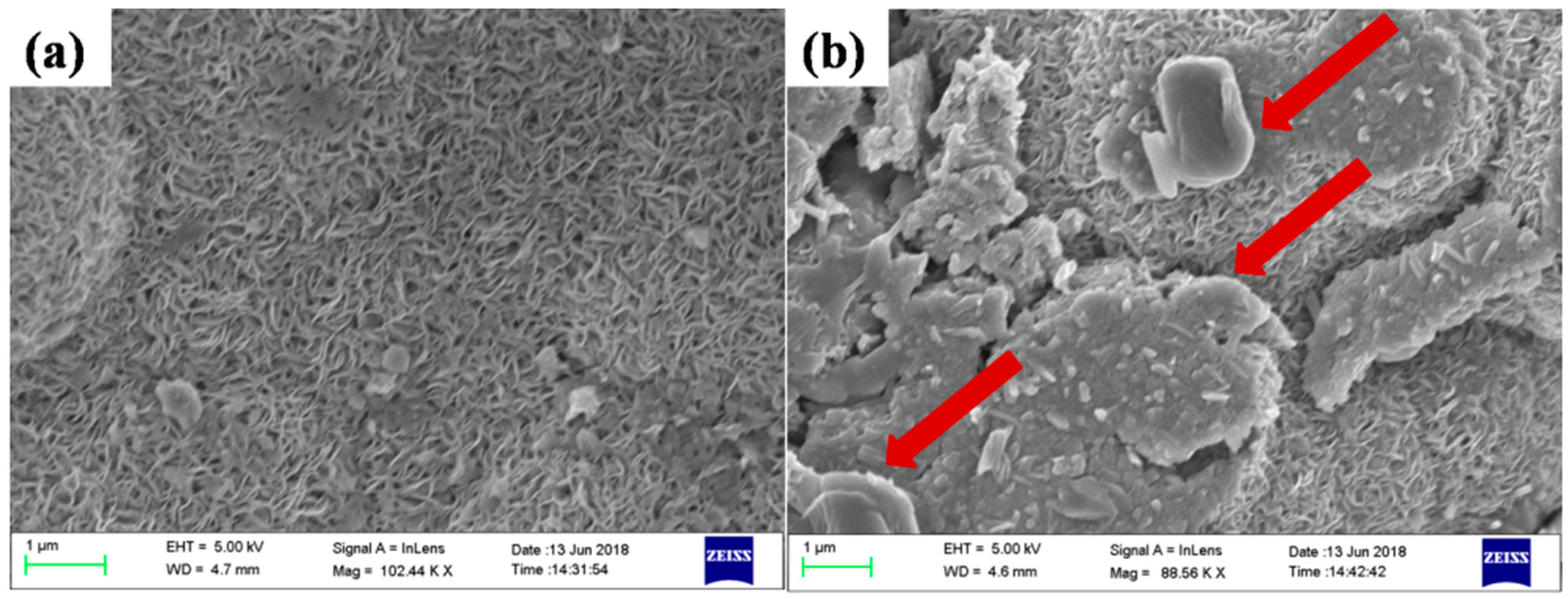
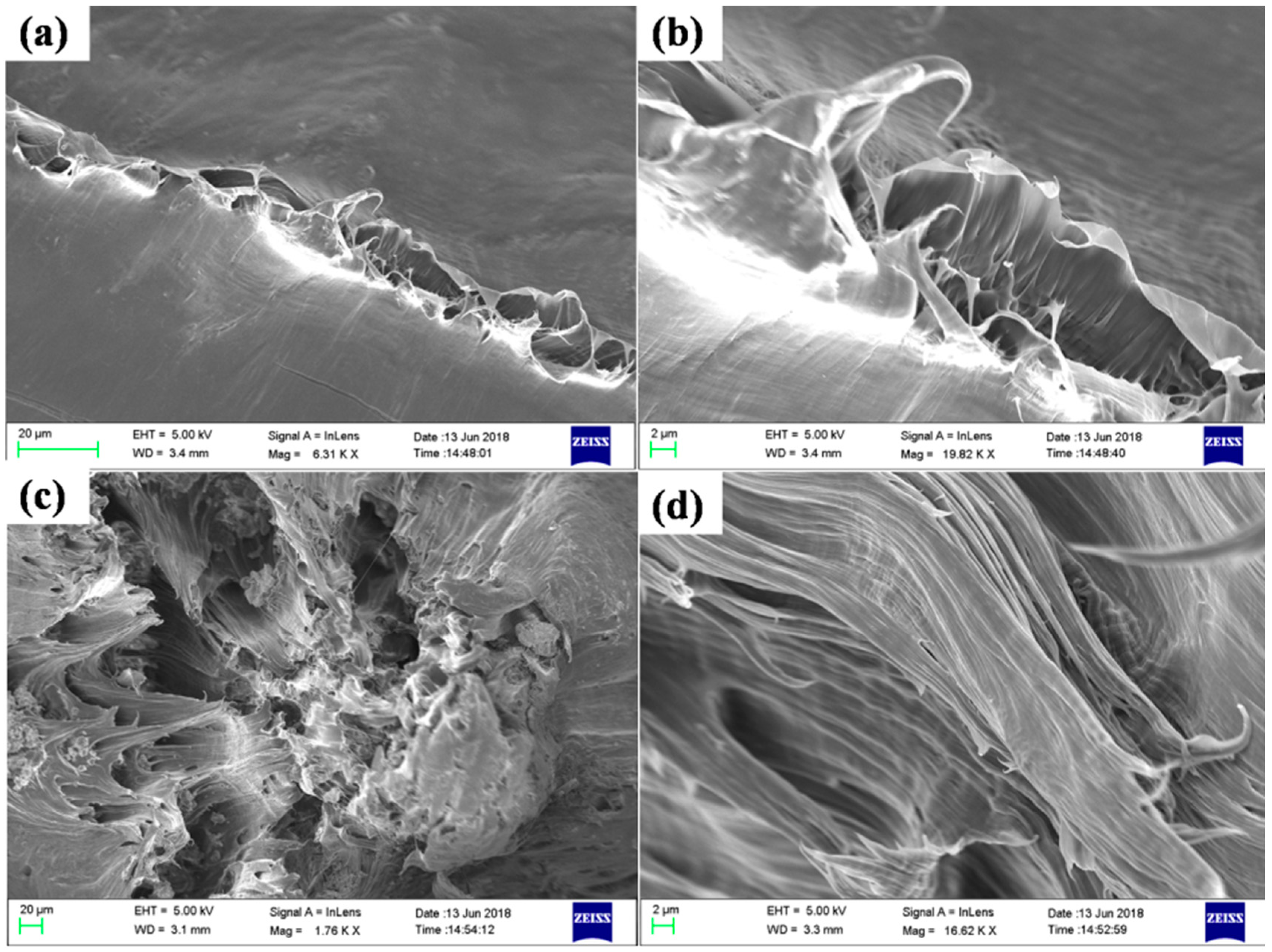
3.3. Morphological and Structural Investigation
3.4. Dynamic Mechanical Analysis
3.5. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, C.N.R.; Maitra, U.; Waghmare, U.V. Extraordinary attributes of 2-dimensional MoS2 nanosheets. Chem. Phys. Lett. 2014, 609, 172–183. [Google Scholar] [CrossRef]
- Benck, J.D.; Chen, Z.; Kuritzky, L.Y.; Forman, A.J.; Jaramillo, T.F. Amorphous molybdenum sulfide catalysts for electrochemical hydrogen production: insights into the origin of their catalytic activity. ACS Catal. 2012, 2, 1916–1923. [Google Scholar] [CrossRef]
- Merki, D.; Hu, X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 2011, 4, 3878–3888. [Google Scholar] [CrossRef]
- Ma, G.; Peng, H.; Mu, J.; Huang, H.; Zhou, X.; Lei, Z. In situ intercalative polymerization of pyrrole in graphene analogue of MoS2 as advanced electrode material in supercapacitor. J. Power Sources 2013, 229, 72–78. [Google Scholar] [CrossRef]
- Ramadoss, A.; Kim, T.; Kim, G.S.; Kim, S.J. Enhanced activity of a hydrothermally synthesized mesoporous MoS2 nanostructure for high performance supercapacitor applications. New J. Chem. 2014, 38, 2379–2385. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Chen, W.; Chang, K.; Ma, L.; Huang, G.; Chen, D.; Lee, J.Y. CTAB-assisted synthesis of single-layer MoS2–graphene composites as anode materials of Li-ion batteries. J. Mater. Chem. A 2013, 1, 2202–2210. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Fu, H.; Guo, B.; Lei, X.; Zhu, Z. MoS2/graphene hybrid nano flowers with enhanced electrochemical performances as anode for lithium-ion batteries. J. Phys. Chem. C 2015, 119, 7959–7968. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wena, P.; Cheng, Y.; Liua, L.; Tai, Q.; Hu, Y.; Liew, K.M. Defect-free MoS2 nanosheets: Advanced nanofillers for polymer nanocomposites. Compos. Part A: Appl. Sci. Manuf. 2016, 81, 61–68. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Poot, M.; Steele, G.A.; van der Zant, H.S.J.; Agrait, N.; Rubio-Bollinger, G. Elastic properties of freely suspended MoS2 nanosheets. Adv. Mater. 2012, 24, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Eksik, O.; Gao, J.; Shojaee, S.A.; Thomas, A.; Chow, P.; Bartolucci, S.F.; Lucca, D.A.; Koratkar, N. Epoxy nanocomposites with two-dimensional transition metal dichalcogenide additives. ACS Nano 2014, 8, 5282–5289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Jiang, S.; Bao, C.; Song, L.; Wang, B.; Tang, G.; Hu, Y.; Gui, Z. Preparation of poly(vinyl alcohol) nanocomposites with molybdenum disulfide (MoS2): Structural characteristics and markedly enhanced properties. RSC Adv. 2012, 2, 11695–11703. [Google Scholar] [CrossRef]
- Zhou, K.; Jiang, S.; Shi, Y.; Liu, J.; Wang, B.; Hu, Y.; Gui, Z. Multigram-scale fabrication of organic modified MoS2 nanosheets dispersed in polystyrene with improved thermal stability, fire resistance, and smoke suppression properties. RSC Adv. 2014, 4, 40170–40180. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, J.; Wang, B.; Zhang, Q.; Shi, Y.; Jiang, S.; Hu, Y.; Gui, Z. Facile preparation of poly (methylmethacrylate)/MoS2 nanocomposites via in situ emulsion polymerization. Mater. Lett. 2014, 126, 159–161. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Yeoh, G.H.; Hu, Y. Enhanced mechanical and barrier properties of polyurethane nanocomposite films with randomly distributed molybdenum disulfide nanosheets. Compos. Sci. Technol. 2016, 127, 142–148. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, J.; Zeng, W.; Hu, Y.; Gui, Z. In situ synthesis, morphology, and fundamental properties of polymer/MoS2 nanocomposites. Compos. Sci. Technol. 2015, 107, 120–128. [Google Scholar] [CrossRef]
- Yang, X.; Meng, N.; Zhu, Y.; Zhou, Y.; Nie, W.; Chen, P. Greatly improved mechanical and thermal properties of chitosan by carboxyl-functionalized MoS2 nanosheets. J. Mater. Sci. 2016, 51, 1344–1353. [Google Scholar] [CrossRef]
- Chhetri, S.; Adak, N.C.; Samanta, P.; Mandal, N.; Kuila, T.; Murmu, N.C. Investigation of mechanical and thermal properties of the cetyltrimethylammonium bromide functionalized molybdenum disulfide (MoS2)/epoxy composites. Polym. Bull. 2018, 75, 327–343. [Google Scholar] [CrossRef]
- Tang, G.; Huang, W.; Chang, D.; Nie, W.; Mi, W.; Yan, W. The friction and wear of aramid fiber-reinforced polyamide 6 composites filled with nano-MoS2. Polym. Plast. Technol. Eng. 2011, 50, 1537–1540. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, G.; Ma, K.; Chen, T.; Zhao, X. Study on the friction and wear behaviors of modified PA66 composites. Polym. Plast. Technol. Eng. 2009, 48, 633–638. [Google Scholar] [CrossRef]
- Kuila, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Wang, K.; Pasbakhsh, P.; De Silva, R.T.; Goh, K.L. A Comparative analysis of the reinforcing efficiency of silsesquioxane nanoparticles versus apatite nanoparticles in chitosan biocomposite fibres. J. Compos. Sci. 2017, 1, 9. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Merli, M.; Milioto, S.; Parisi, F.; Sciascia, L. Effect of the biopolymer charge and the nanoclay morphology on nanocomposite materials. Ind. Eng. Chem. Res. 2016, 55, 7373–7380. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of morphology and size of halloysite nanotubes on functional pectin bionanocomposites for food packaging applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sue, H.J. Morphology and mechanical properties of blown films of a low-density polyethylene/linear low-density polyethylene. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 507–518. [Google Scholar] [CrossRef]
- Niaounakis, M.; Kontou, E. Effect of LDPE on the thermo-mechanical properties of LLDPE-based films. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 1712–1727. [Google Scholar] [CrossRef]
- Huang, G.; Chen, T.; Chen, W.; Wang, Z.; Chang, K.; Ma, L.; Huang, F.; Chen, D.; Lee, J.Y. Graphene-like MoS2/graphene composites: Cationic surfactant-assisted hydrothermal synthesis and electrochemical reversible storage of lithium. Small 2013, 9, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lei, Z.; Wang, Z.; Huang, B. Studies on blends of LLDPE and ethylene-methacrylic acid random copolymer. Eur. Polym. J. 1999, 35, 355–360. [Google Scholar] [CrossRef]
- Tang, L.C.; Wan, Y.J.; Yan, D.; Pei, Y.B.; Zhao, L.; Li, Y.B.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Kim, S.; Do, I.; Drzal, L.T. Thermal stability and dynamic mechanical behavior of exfoliated graphite nanoplatelets-LLDPE nanocomposites. Polym. Compos. 2010, 31, 755–761. [Google Scholar] [CrossRef]
- Cai, W.; Zhan, J.; Feng, X.; Yuan, B.; Liu, J.; Hu, W.; Hu, Y. Facile construction of flame-retardant-wrapped molybdenum disulfide nanosheets for properties enhancement of thermoplastic polyurethane. Ind. Eng. Chem. Res. 2017, 56, 7229–7238. [Google Scholar] [CrossRef]




| Sample Code | E’ (MPa) −78 °C | E’ (MPa) −50 °C | E’ (MPa) 5 °C | E’ (MPa) 60 °C |
|---|---|---|---|---|
| Pure LLDPE | 1560 | 1291 | 632 | 250 |
| 0.75 wt % | 2444 | 2117 | 1059 | 401 |
| 2 wt % | 2797 | 2362 | 1167 | 451 |
| 3 wt % | 1345 | 2025 | 979 | 371 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chhetri, S.; Adak, N.C.; Samanta, P.; Murmu, N.C.; Kuila, T. Exploration of Mechanical and Thermal Properties of CTAB-Modified MoS2/LLDPE Composites Prepared by Melt Mixing. J. Compos. Sci. 2018, 2, 37. https://doi.org/10.3390/jcs2030037
Chhetri S, Adak NC, Samanta P, Murmu NC, Kuila T. Exploration of Mechanical and Thermal Properties of CTAB-Modified MoS2/LLDPE Composites Prepared by Melt Mixing. Journal of Composites Science. 2018; 2(3):37. https://doi.org/10.3390/jcs2030037
Chicago/Turabian StyleChhetri, Suman, Nitai Chandra Adak, Pranab Samanta, Naresh Chandra Murmu, and Tapas Kuila. 2018. "Exploration of Mechanical and Thermal Properties of CTAB-Modified MoS2/LLDPE Composites Prepared by Melt Mixing" Journal of Composites Science 2, no. 3: 37. https://doi.org/10.3390/jcs2030037





