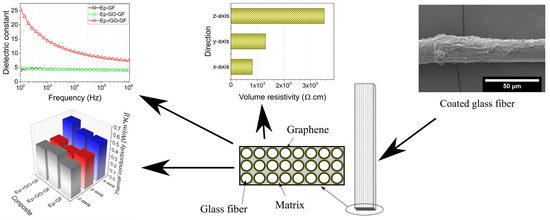
Tuning Electrical and Thermal Properties in Epoxy/Glass Composites by Graphene-Based Interphase
Abstract
:1. Introduction
2. Materials and Methods
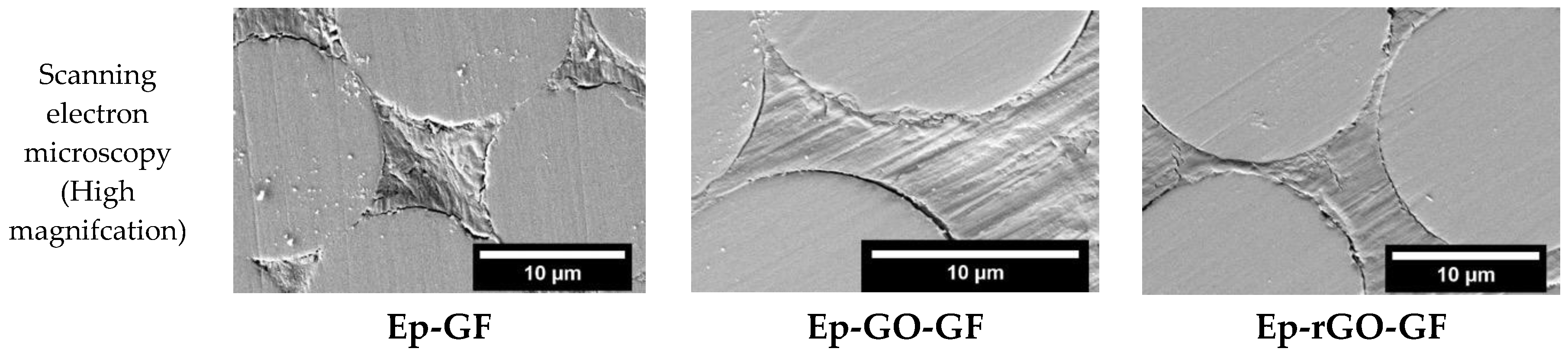
3. Results and Discussion
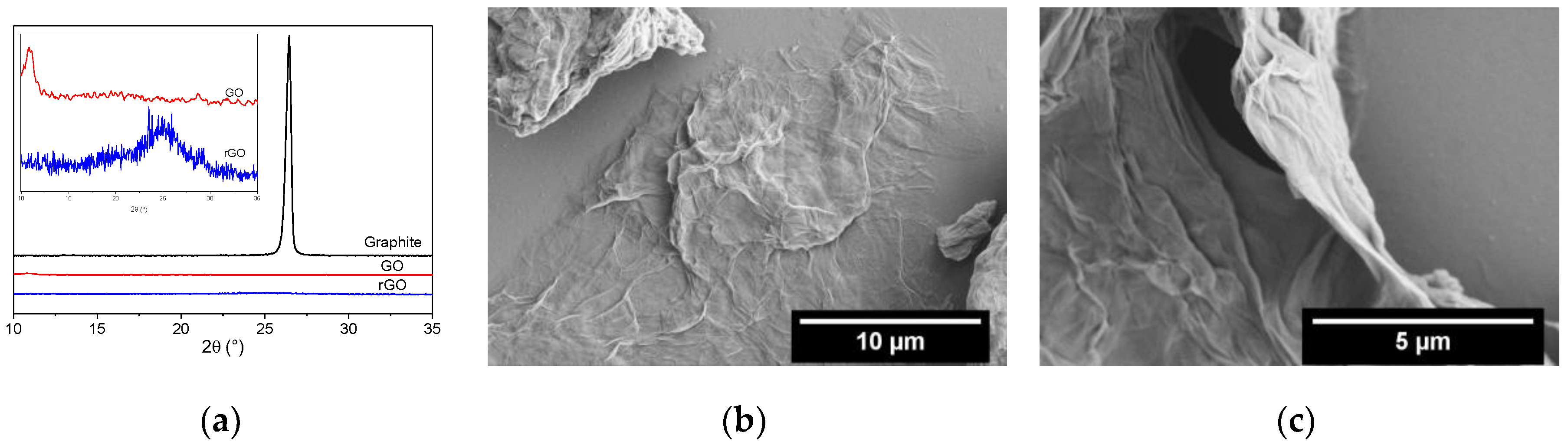
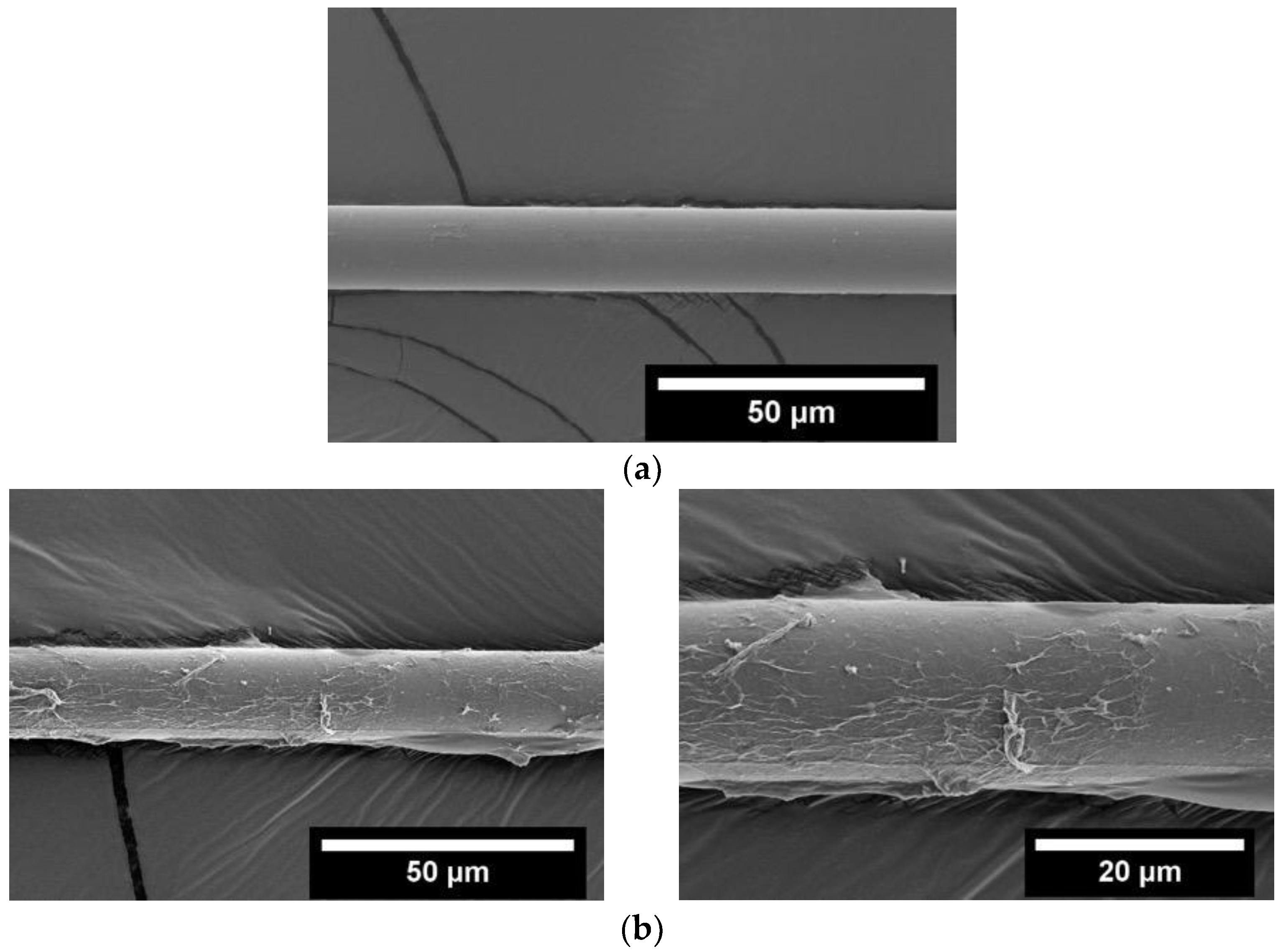
3.1. Graphene Oxide Synthesis and Its Reduction
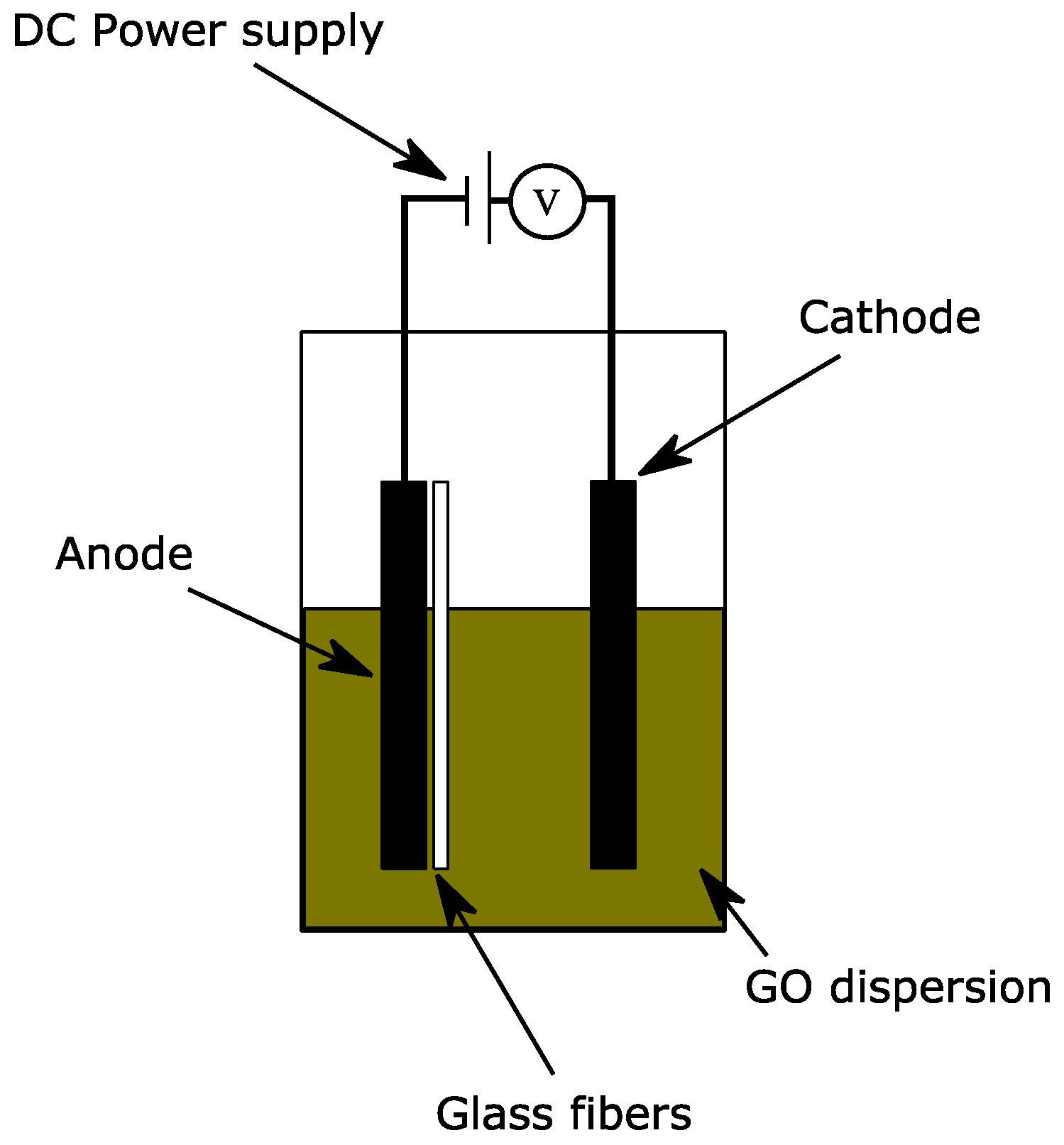
3.2. Electrophoretic Deposition of GO and Composite Fabrication
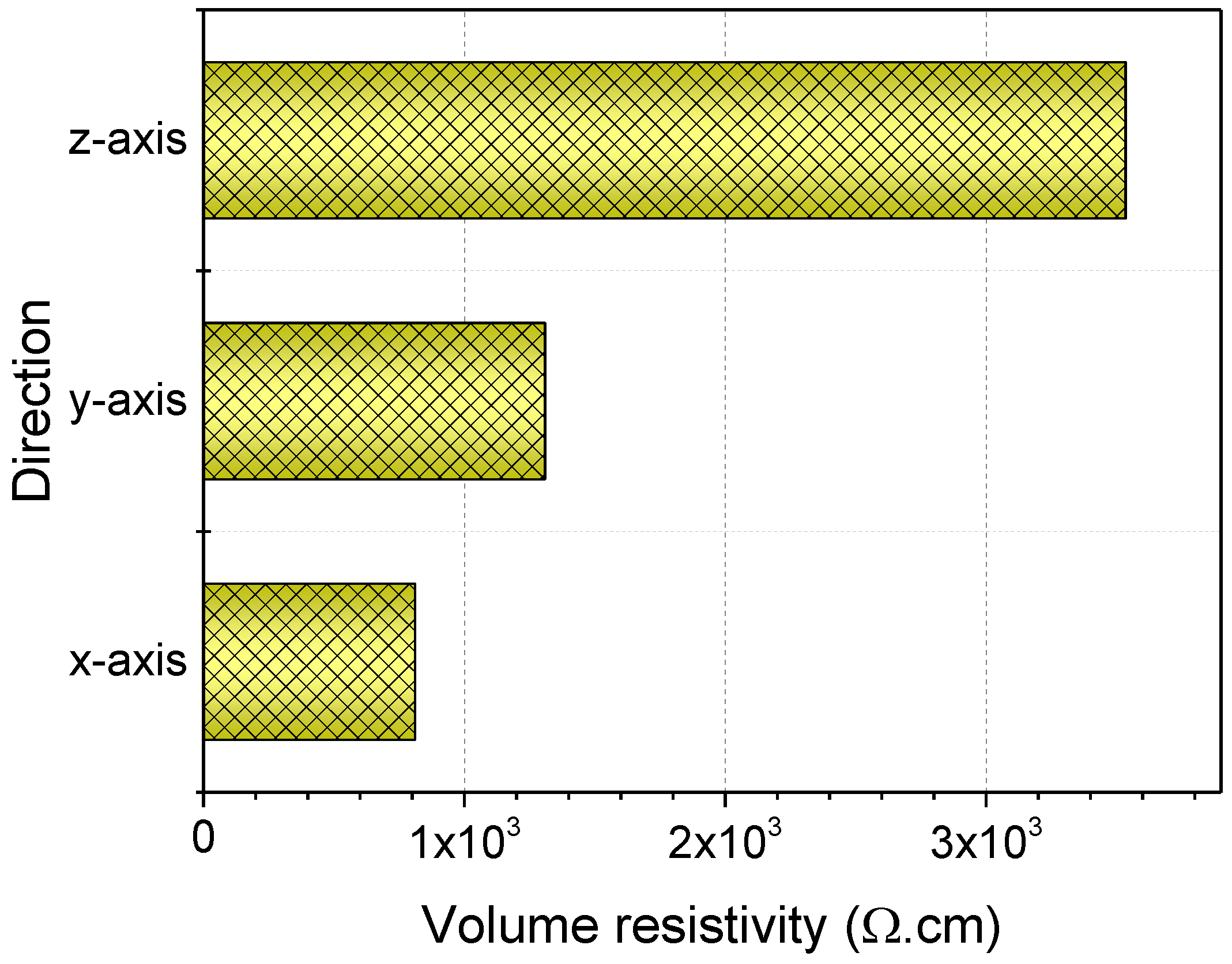
3.3. Electrical Resistivity
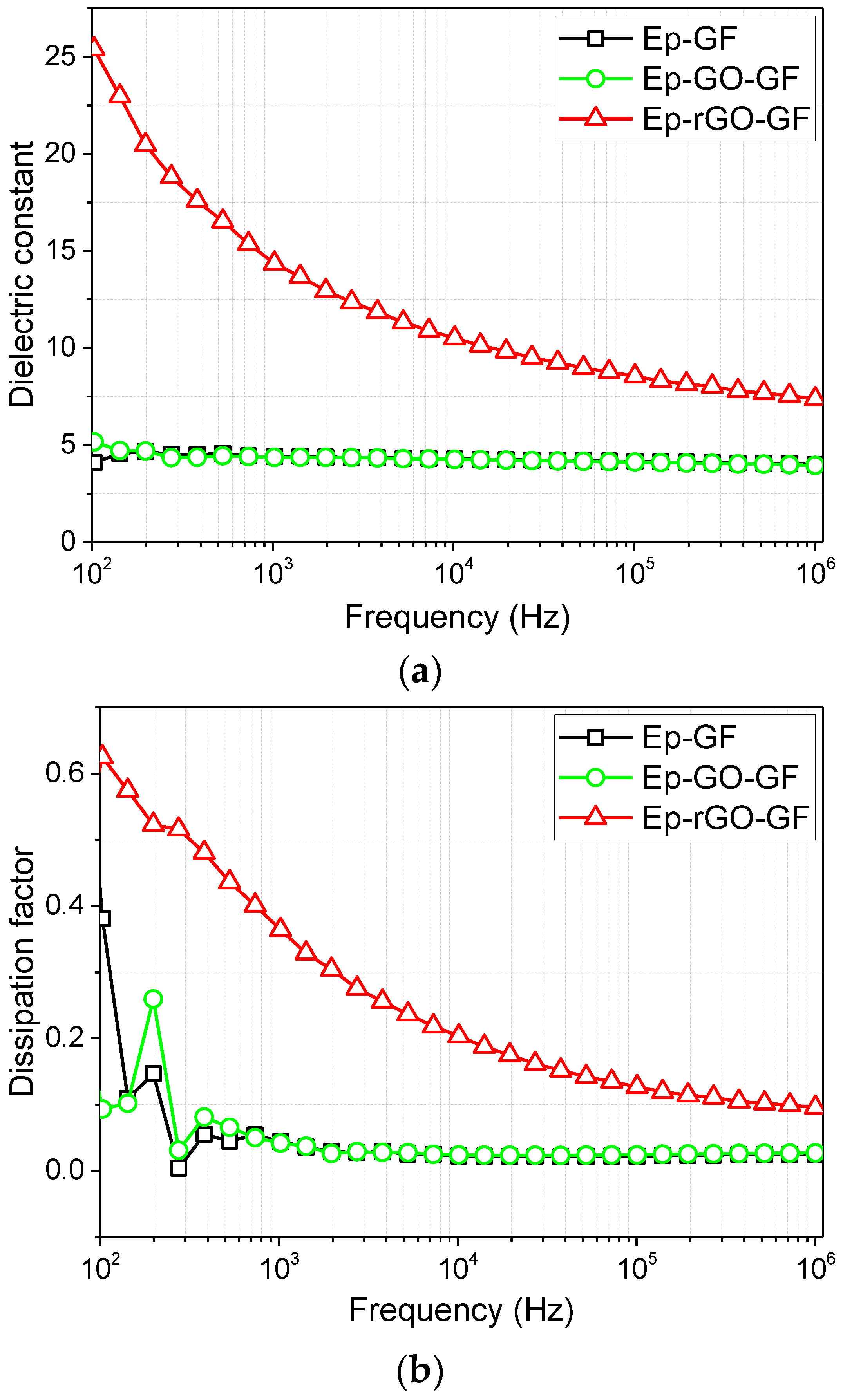
3.4. Dielectric Properties
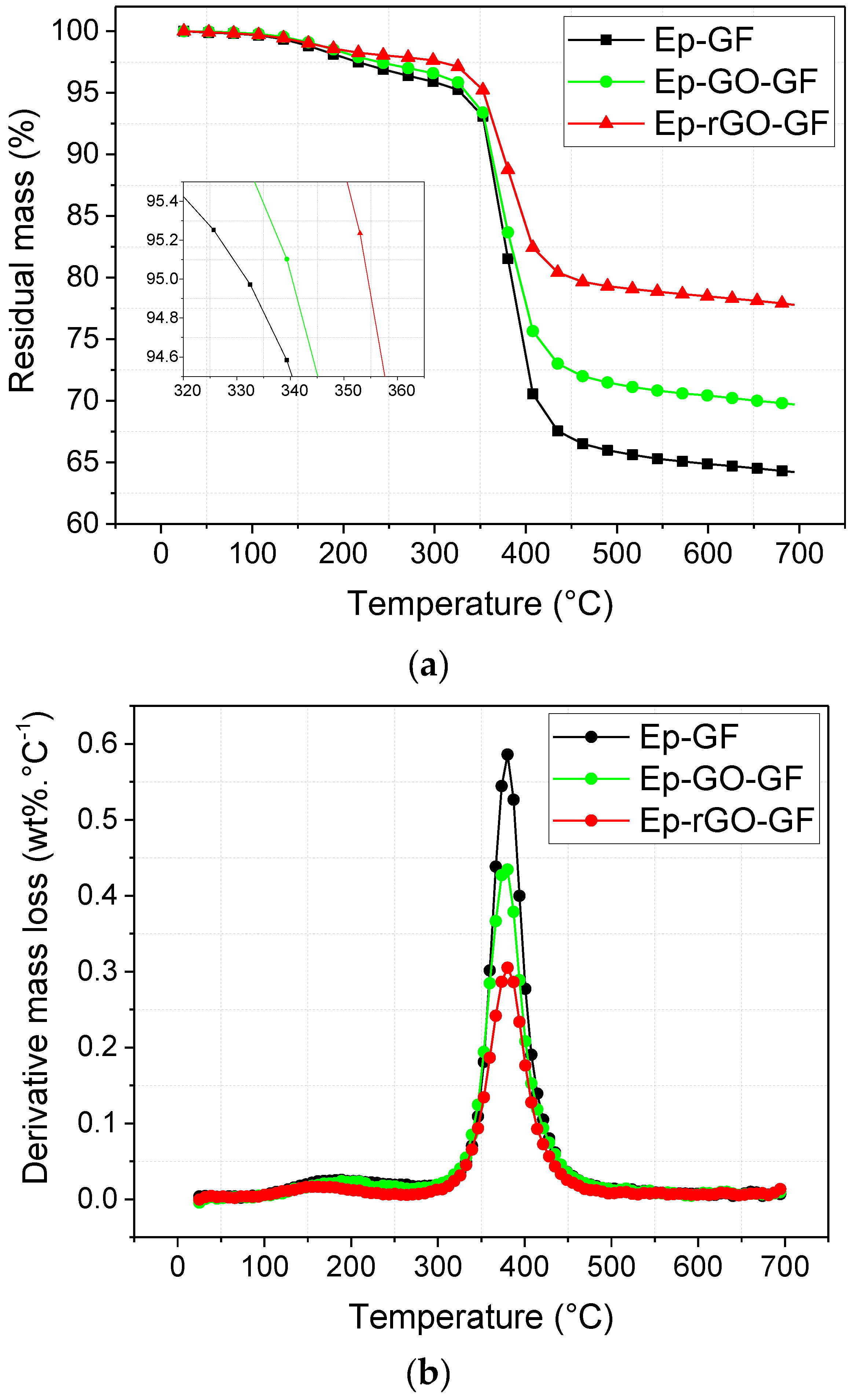
3.5. Thermal Stability
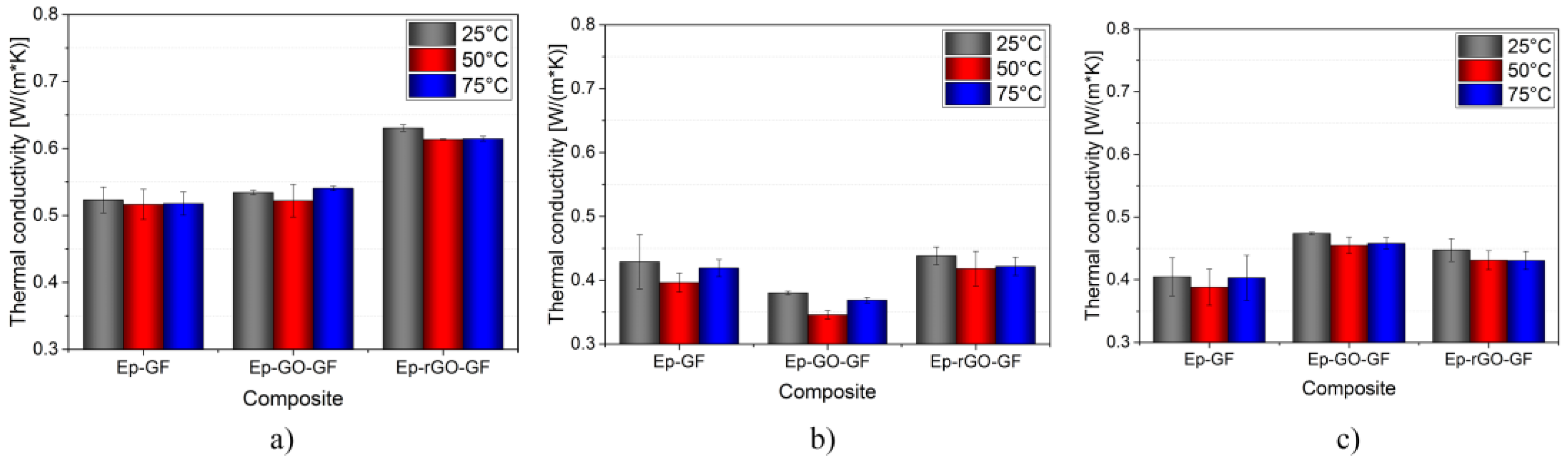
3.6. Thermal Conductivity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kornmann, X.; Rees, M.; Thomann, Y.; Necola, A.; Barbezat, M.; Thomann, R. Epoxy-layered silicate nanocomposites as matrix in glass fibre-reinforced composites. Compos. Sci. Technol. 2005, 65, 2259–2268. [Google Scholar] [CrossRef]
- Vlasveld, D.; Parlevliet, P.; Bersee, H.; Picken, S. Fibre-matrix adhesion in glass-fibre reinforced polyamide-6 silicate nanocomposites. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1–11. [Google Scholar] [CrossRef]
- Wichmann, M.H.G.; Sumfleth, J.; Gojny, F.H.; Quaresimin, M.; Fiedler, B.; Schulte, K. Glass-fibre-reinforced composites with enhanced mechanical and electrical properties–benefits and limitations of a nanoparticle modified matrix. Eng. Fract. Mech. 2006, 73, 2346–2359. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Boggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Atif, R.; Shyha, I.; Inam, F. Modeling and experimentation of multi-layered nanostructured graphene-epoxy nanocomposites for enhanced thermal and mechanical properties. J. Compos. Mater. 2017, 51, 209–220. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, J. Electrical conductivity and percolation behavior of polymer nanocomposites. In Polymer Nanocomposites: Electrical and Thermal Properties; Huang, X., Zhi, C., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 51–82. [Google Scholar]
- Cheng, X.; Yokozeki, T.; Wu, L.; Wang, H.; Zhang, J.; Koyanagi, J.; Weng, Z.; Sun, Q. Electrical conductivity and interlaminar shear strength enhancement of carbon fiber reinforced polymers through synergetic effect between graphene oxide and polyaniline. Compos. Part A Appl. Sci. Manuf. 2016, 90, 243–249. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Chen, C.; Gu, Y.; Wang, S.; Zhang, Z.; Li, M.; Zhang, Z. Fabrication and characterization of structural/dielectric three-phase composite: Continuous basalt fiber reinforced epoxy resin modified with graphene nanoplates. Compos. Part A Appl. Sci. Manuf. 2017, 94, 199–208. [Google Scholar] [CrossRef]
- Jan, R.; Habib, A.; Gul, I.H. Stiff, strong, yet tough free-standing dielectric films of graphene nanosheets-polyurethane nanocomposites with very high dielectric constant and loss. Electron. Mater. Lett. 2016, 12, 91–99. [Google Scholar] [CrossRef]
- Yousefi, N.; Sun, X.; Lin, X.; Shen, X.; Jia, J.; Zhang, B.; Tang, B.; Chan, M.; Kim, J.-K. Highly aligned graphene/polymer nanocomposites with excellent dielectric properties for high-performance electromagnetic interference shielding. Adv. Mater. 2014, 26, 5480–5487. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, J.; Ge, H.; Zhao, D.; Liu, C.; Hong, X. Reduced graphene oxide deposited carbon fiber reinforced polymer composites for electromagnetic interference shielding. Compos. Part A Appl. Sci. Manuf. 2016, 82, 141–150. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Zhao, Y.; Liu, W.; Zhang, W. Effect of electrophoretic condition on the electromagnetic interference shielding performance of reduced graphene oxide-carbon fiber/epoxy resin composites. Compos. Part B Eng. 2016, 105, 167–175. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Pegoretti, A. Expanded graphite nanoplatelets as coupling agents in glass fiber reinforced polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2014, 66, 25–34. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Pegoretti, A.; Kalaitzidou, K. Synergistic effect of exfoliated graphite nanoplatelets and short glass fiber on the mechanical and interfacial properties of epoxy composites. Compos. Sci. Technol. 2014, 98, 15–21. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Pegoretti, A. Hybridization of short glass fiber polypropylene composites with nanosilica and graphite nanoplatelets. J. Reinf. Plast. Compos. 2014, 33, 1682–1695. [Google Scholar] [CrossRef]
- Kumar, S.K.; Benicewicz, B.C.; Vaia, R.A.; Winey, K.I. 50th anniversary perspective: Are polymer nanocomposites practical for applications? Macromolecules 2017, 50, 714–731. [Google Scholar] [CrossRef]
- Park, J.G.; Cheng, Q.; Lu, J.; Bao, J.; Li, S.; Tian, Y.; Liang, Z.; Zhang, C.; Wang, B. Thermal conductivity of mwcnt/epoxy composites: The effects of length, alignment and functionalization. Carbon 2012, 50, 2083–2090. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Ma, C.; Zhang, W.; Zhang, R.-P.; Koratkar, N.; Liang, J. Alignment of multiwalled carbon nanotubes in bulk epoxy composites via electric field. J. Appl. Phys. 2009, 105, 054319. [Google Scholar] [CrossRef]
- Schwarz, M.-K.; Bauhofer, W.; Schulte, K. Alternating electric field induced agglomeration of carbon black filled resins. Polymer 2002, 43, 3079–3082. [Google Scholar] [CrossRef]
- Martin, C.A.; Sandler, J.K.W.; Windle, A.H.; Schwarz, M.K.; Bauhofer, W.; Schulte, K.; Shaffer, M.S.P. Electric field-induced aligned multi-wall carbon nanotube networks in epoxy composites. Polymer 2005, 46, 877–886. [Google Scholar] [CrossRef]
- Abdalla, M.; Dean, D.; Theodore, M.; Fielding, J.; Nyairo, E.; Price, G. Magnetically processed carbon nanotube/epoxy nanocomposites: Morphology, thermal, and mechanical properties. Polymer 2010, 51, 1614–1620. [Google Scholar] [CrossRef]
- Mahfuz, H.; Zainuddin, S.; Parker, M.R.; Al-Saadi, T.; Rangari, V.K.; Jeelani, S. Reinforcement of SC-15 epoxy with CNT/CNF under high magnetic field: An investigation of mechanical and thermal response. J. Mater. Sci. 2009, 44, 1113–1120. [Google Scholar] [CrossRef]
- Fragouli, D.; Das, A.; Innocenti, C.; Guttikonda, Y.; Rahman, S.; Liu, L.; Caramia, V.; Megaridis, C.M.; Athanassiou, A. Polymeric films with electric and magnetic anisotropy due to magnetically assembled functional nanofibers. ACS Appl. Mater. Interfaces 2014, 6, 4535–4541. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M. Polymer nanocomposites—A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.; Tripathi, M.; Pugno, N.; Pegoretti, A. Enhancement of interfacial adhesion in glass fiber/epoxy composites by electrophoretic deposition of graphene oxide on glass fibers. Compos. Sci. Technol. 2016, 126, 149–157. [Google Scholar] [CrossRef]
- Mahmood, H.; Vanzetti, L.; Bersani, M.; Pegoretti, A. Mechanical properties and strain monitoring of glass-epoxy composites with graphene-coated fibers. Compos. Part A Appl. Sci. Manuf. 2017. Submitted. [Google Scholar]
- Parker, W.J.; Jenkins, R.J.; Butler, C.P.; Abbott, G.L. Flash method of determining thermal diffusivity, heat capacity, and thermal conductivity. J. Appl. Phys. 1961, 32, 1679–1684. [Google Scholar] [CrossRef]
- Buchsteiner, A.; Lerf, A.; Pieper, J. Water dynamics in graphite oxide investigated with neutron scattering. J. Phys. Chem. B 2006, 110, 22328–22338. [Google Scholar] [CrossRef] [PubMed]
- Nan, C.-W.; Shen, Y.; Ma, J. Physical properties of composites near percolation. Annu. Rev. Mater. Res. 2010, 40, 131–151. [Google Scholar] [CrossRef]



| Property | Ep-GF | Ep-GO-GF | Ep-rGO-GF |
|---|---|---|---|
| Volume resistivity (Ω·cm) | 3.7 × 1014 | 6.9 × 1013 | 4.5 × 102 |
| Characteristic | Ep-GF | Ep-GO-GF | Ep-rGO-GF |
|---|---|---|---|
| Onset temperature (°C) | 331.8 | 340.3 | 354.4 |
| Residual mass at 700 °C (%) | 64.2 | 71.6 | 77.8 |
| Peak temperature (°C) | 380.3 | 380.3 | 380.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, H.; Unterberger, S.H.; Pegoretti, A. Tuning Electrical and Thermal Properties in Epoxy/Glass Composites by Graphene-Based Interphase. J. Compos. Sci. 2017, 1, 12. https://doi.org/10.3390/jcs1020012
Mahmood H, Unterberger SH, Pegoretti A. Tuning Electrical and Thermal Properties in Epoxy/Glass Composites by Graphene-Based Interphase. Journal of Composites Science. 2017; 1(2):12. https://doi.org/10.3390/jcs1020012
Chicago/Turabian StyleMahmood, Haroon, Seraphin H. Unterberger, and Alessandro Pegoretti. 2017. "Tuning Electrical and Thermal Properties in Epoxy/Glass Composites by Graphene-Based Interphase" Journal of Composites Science 1, no. 2: 12. https://doi.org/10.3390/jcs1020012