1. Introduction
The use of plastics in everyday life has increased dramatically during the course of the 20th and 21st century which is primarily due to the characteristics that plastics offer in the development of new products to meet the challenges in modern society. Silverman et al. [
1] reported that in 2015, approximately 300 million tons of plastic material will be produced around the world, most of which will be derived from the ever depleting petroleum feedstocks and this has resulted in them attracting increasing interest owing to the growing concern with regards to both the environment and decrease in fossil resources [
2].
As a direct result, this has led to a substantial interest in switching to suitable eco-friendly alternatives of already existing polymers as well as developing new low-cost polymers which have competitive performance properties from feedstocks that are available from renewable stock resources [
3]. Over the last several decades, the study of both resorbable and degradable polymers has been investigated to a great extent [
4], with the vast majority, e.g., poly(ε-caprolactone) (PCL), polyvinyl alcohol (PVA) and polylactide acid (PLA), being produced from renewable sources [
5].
Poly(ε-caprolactone) is a linear synthetic biodegradable aliphatic polyester and was one of the earliest polymers synthesized by the Carothers group in the early 1930s [
6,
7]. PCL is a hydrophobic, semi-crystalline polymer exhibiting a melting point in the range of 59–64 °C [
8]. The degradation time of the homopolymer is approximately two years [
9] which is attributed to the five hydrophobic methylene (CH
2) moieties which are present in its repeating unit [
10] with higher molecular weight structures taking much longer to degrade. It is this degradation characteristic in vivo compared to other biodegradable polymers which makes PCL suitable for the development of controlled release drug delivery devices and suture materials [
11]. Further to this, PCL and PCL-based materials and devices have found applications in the areas of artificial implants, tissue engineering and three dimensional (3D) printing [
6] which has the potential to revolutionize the way in which patients’ needs may be met with regards to personalized medicine and has been reported elsewhere [
12,
13].
Fuenmayor and researchers [
13] utilized a variety of polymers, including poly(ε-caprolactone) and poly(ethylene oxide), and conducted a study to investigate drug release profiles from two distinctively different manufacturing techniques: direct compaction and fused filament fabrication (FFF), the former being a conventional method and the latter one of emerging interest. The results from their study found that the presence of PCL retarded the release of the drug particularly the FFF dosage form as only 38.5% of drug released after a duration of six hours in comparison to a 78.3% release in the direct compaction dosage form (PCL content 55%) [
13]. This illustrates that PCL has the potential to be utilized in the production of controlled drug release formulations by FFF and particularly in tailoring the release profile specific to a patient’s requirements by modification of PCL content.
Furthermore, PCL has also found applications in the areas of tissue engineering (TE) with research carried out by Patrício and researchers [
14,
15,
16] demonstrating how it may be used in the fabrication of scaffolds for tissue engineering. In the vast research, they published they fabricated both PCL and PCL/PLA scaffolds utilizing a novel additive biomanufacturing technique of BioCell Printing with the researchers concluding that it was possible to produce scaffolds which were both accurate and reproducible in addition to improved biological and mechanical properties [
14,
15,
16]. Further research conducted by Borzacchiello [
17] also evaluated PCL and the potential of producing porous scaffolds using the BioCell Printing technique combining both natural and synthetic polymers for tissue engineering applications. The authors established that the combination of PCL and a derivative of hyaluronic acid demonstrated the potential for fibrocartilage tissue engineering applications due to the characteristics with regard to the structural, mechanical and biological properties the scaffolds displayed.
Poly(ethylene oxide) (PEO) is a non-ionic, highly hydrophilic, water-soluble synthetic polyether that may be branched or linear. PEO like PCL is a semi-crystalline polymer and has a melting point in the range of 57–73 °C [
18] with Spietelun et al. [
19] indicating that the melting point of PEO increases with increasing molecular weight. PEO is highly biocompatible with a low toxicity rendering it particularly suited to drug delivery applications [
20] with a vast amount of literature published with regard to PEO being utilized in the preparation of various dosage forms.
Park et al. [
21] conducted a study to characterize the interaction between PEO/PCL in the release of the drug, erythromycin, from microcapsules and found that the increase of PEO content increased drug release from the microcapsules demonstrating the potential PEO offers for controlled release technologies. Furthermore, a team of researchers led by Lyons in 2006 [
22] investigated the use of nanoclay particles as a novel filler material for the development of oral dosage forms with PEO present in the polymeric matrix as PEO has demonstrated in a study by Zhang and McGinity [
23] useful for modulating the release of drug from matrix tablets fabricated by hot melt extrusion technology. Additionally, PEO has also found applications in the areas of TE with Kenny and researchers [
24] investigating its suitability in tissue regenerating applications. From the study, it was concluded that altering the ratio of PEO to PCL has the potential to tailor the degradation rate of the polymeric matrix over a specified time period.
Further to this, Grehan et al. [
25] evaluated the potential of melt blending novel fillers, chitosan and k-carrageenan, into a polymeric matrix composed of PEO and PCL and studied the release rate of 4-acetaminophenol (Acetaminophen) from matrix tablets produced by hot melt extrusion. The researchers demonstrated that the addition of the fillers altered the rate at which the drug was released when compared to the matrix that contained no fillers illustrating that inclusion of these fillers could be utilized in the production of controlled release polymer composite materials for drug delivery systems.
It has been reported by Bordes and co-authors [
2] that nanocomposites are novel materials which have the ability to dramatically improve the properties of polymers such as increasing mechanical strength and thermal resistance [
26,
27,
28,
29]. Halloysite nanotubes (HNTs) are one such nanomaterial that has been utilized with polymers in the development of nanocomposites. Tzounis and researchers [
29] conducted a study in which they modified HNTs and prepared polystyrene/halloysite (PS/HNT) nanocomposites. From their study, the authors concluded that all nanocomposites illustrated improved thermal degradation with increasing HNT content. Furthermore, the PS/HNT nanocomposites retained their optical properties with the increased loading demonstrating excellent ultraviolet–visible (UV–VIS) absorbing capabilities.
Halloysite is a naturally available and low-cost aluminosilicate clay exhibiting a tube-like morphology [
25,
28,
29,
30]. Halloysite has a molecular formula of Al
2Si
2O
5(OH)
4·nH
2O ranging in the length from 0.2–2.0 µm. The inner and outer diameters of the halloysite nanotubes (HNTs) range from 15–50 nm and 30–50 nm respectively [
31] with the difference in the inner and outer diameter imparting significant lumen space ranging from 10.7–39.0% [
26]. In a recent publication by Liu and researchers [
26], it has been stated that extensive research into the use of HNTs began in the 1940s and more recently it appears to be at the forefront of research partly due to the growing interest in tube-like nanoparticles.
Hot melt extrusion (HME) is a process that involves the conversion of a raw material into a product which has a uniform shape and density by forcing it through a die of specified geometry under controlled conditions [
25]. Furthermore, HME does not require the use of either solvents or water, due to the molten polymer having the ability to function as a thermal binder which therefore means that fewer processing steps are required as well as the time-consuming drying steps being eradicated [
30]. The industrial application of the HME process dates back to the 1930s when it was first utilized in both the plastics and food industry [
32,
33]. Therefore, it can be considered that HME is a well-established manufacturing process and this is highlighted in a publication by Kenny et al. [
24] in which the authors explain that HME meets the FDA’s process analytical technology (PAT) scheme for designing, analyzing and controlling the manufacturing process via quality control measurements during active extrusion processes.
In this present research study, the main objective was to investigate the possibility of developing a degradable nanocomposite, incorporating the aliphatic polymers PEO and PCL and the nanoclay halloysite. Furthermore, the research study aims to develop a suitable polymeric filament that would be suitable to be utilized as a feedstock in fused filament fabrication a form of 3D printing.
4. Conclusions
The work which has been described in this research paper outlines the possibility of developing a degradable nanocomposite which could further be developed into a polymeric filament, suitable for use as a feedstock in the FFF process. All blends were prepared via HME and characterized by performing an array of commonly utilized polymeric testing procedures.
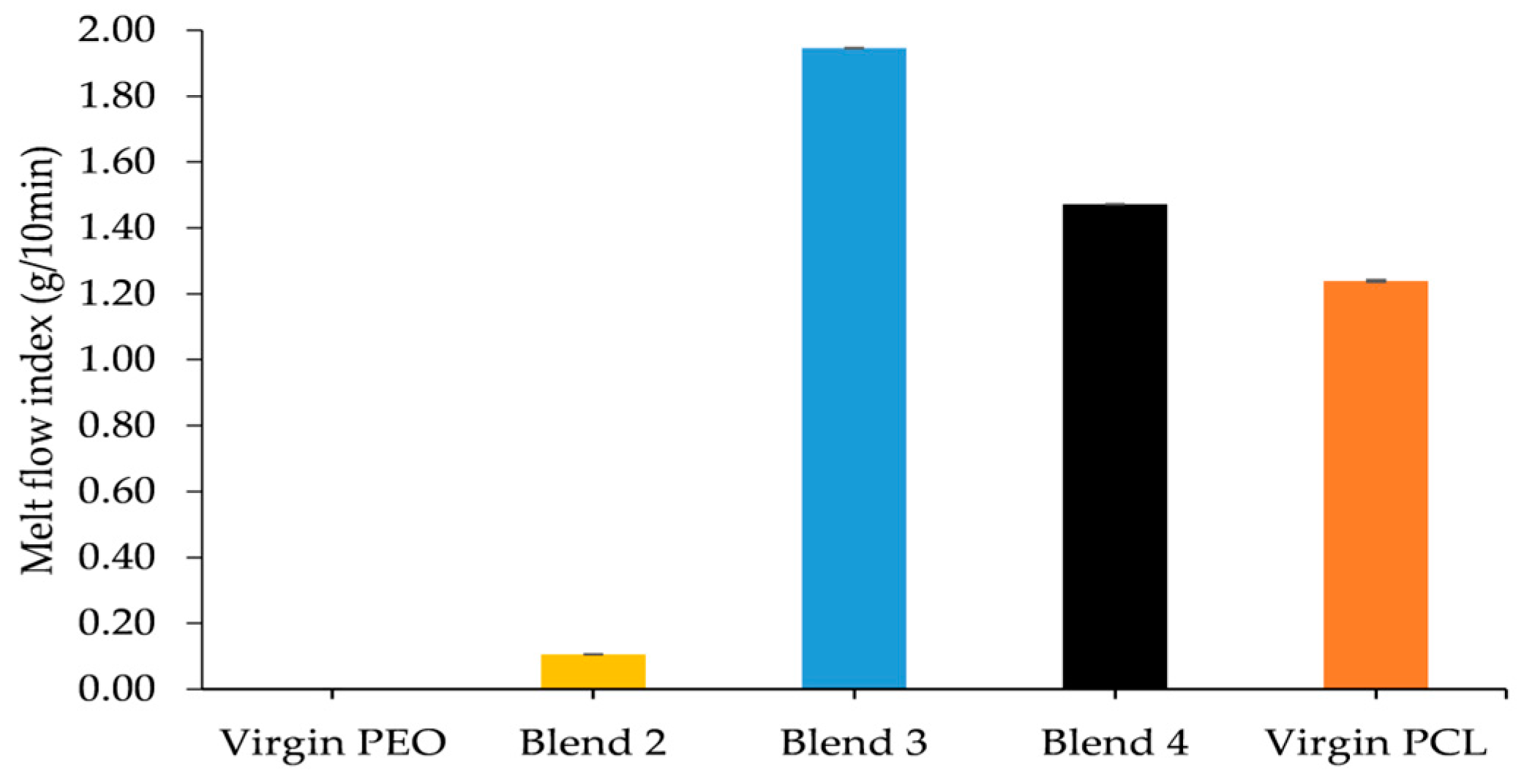
It was evident that the incorporation of PCL into the PEO matrix provided a plasticizing effect as rheological studies showed that the MFI of the monolithic batches increased with increasing PCL content. From conducting DSC it was apparent that increasing the content of PCL within the polymeric matrix caused a reduction in the melt temperature of the blend. Therefore, subsequently, Blend 4 was chosen as the matrix for the HNT reinforced nanocomposite material.
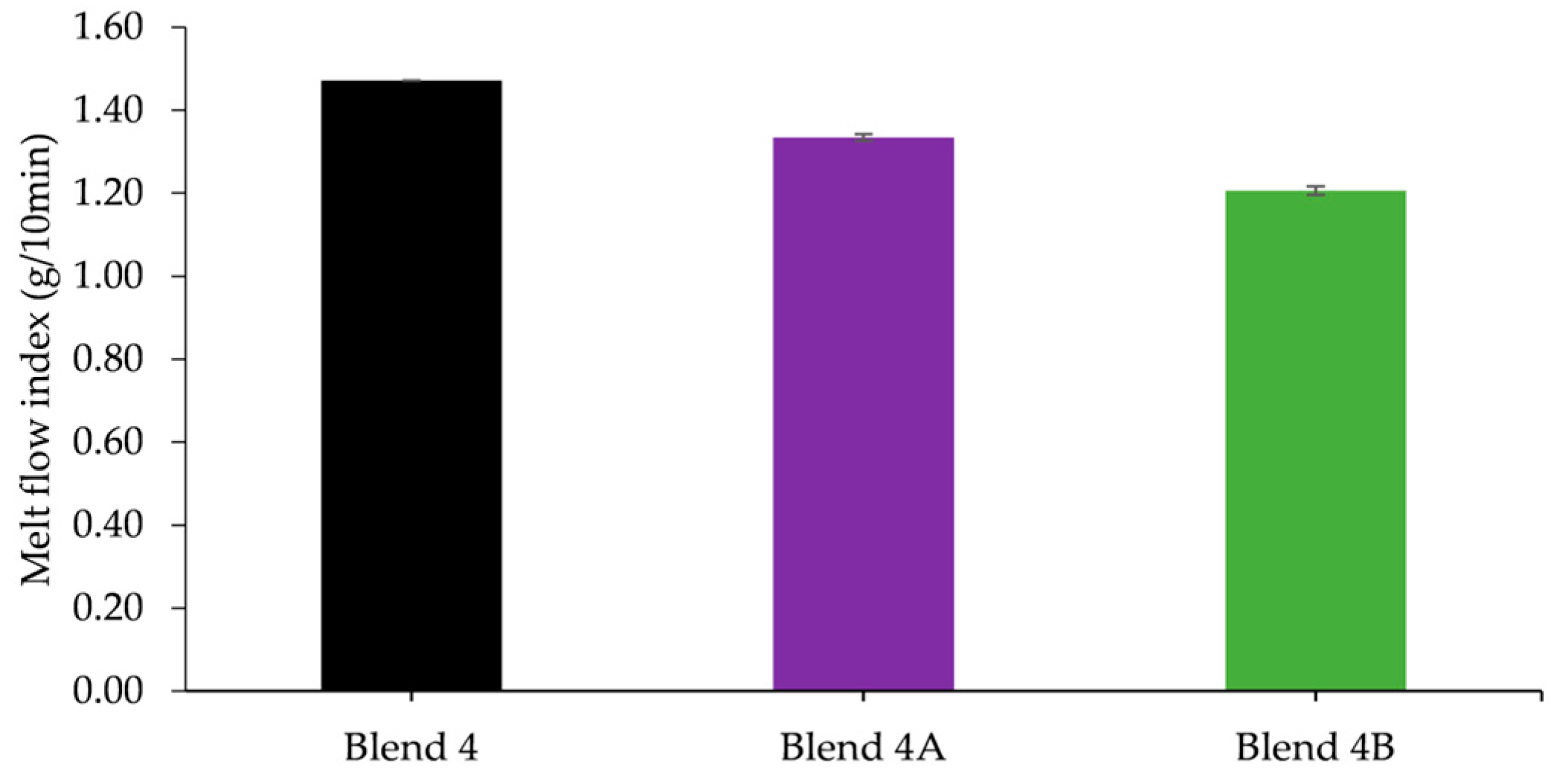
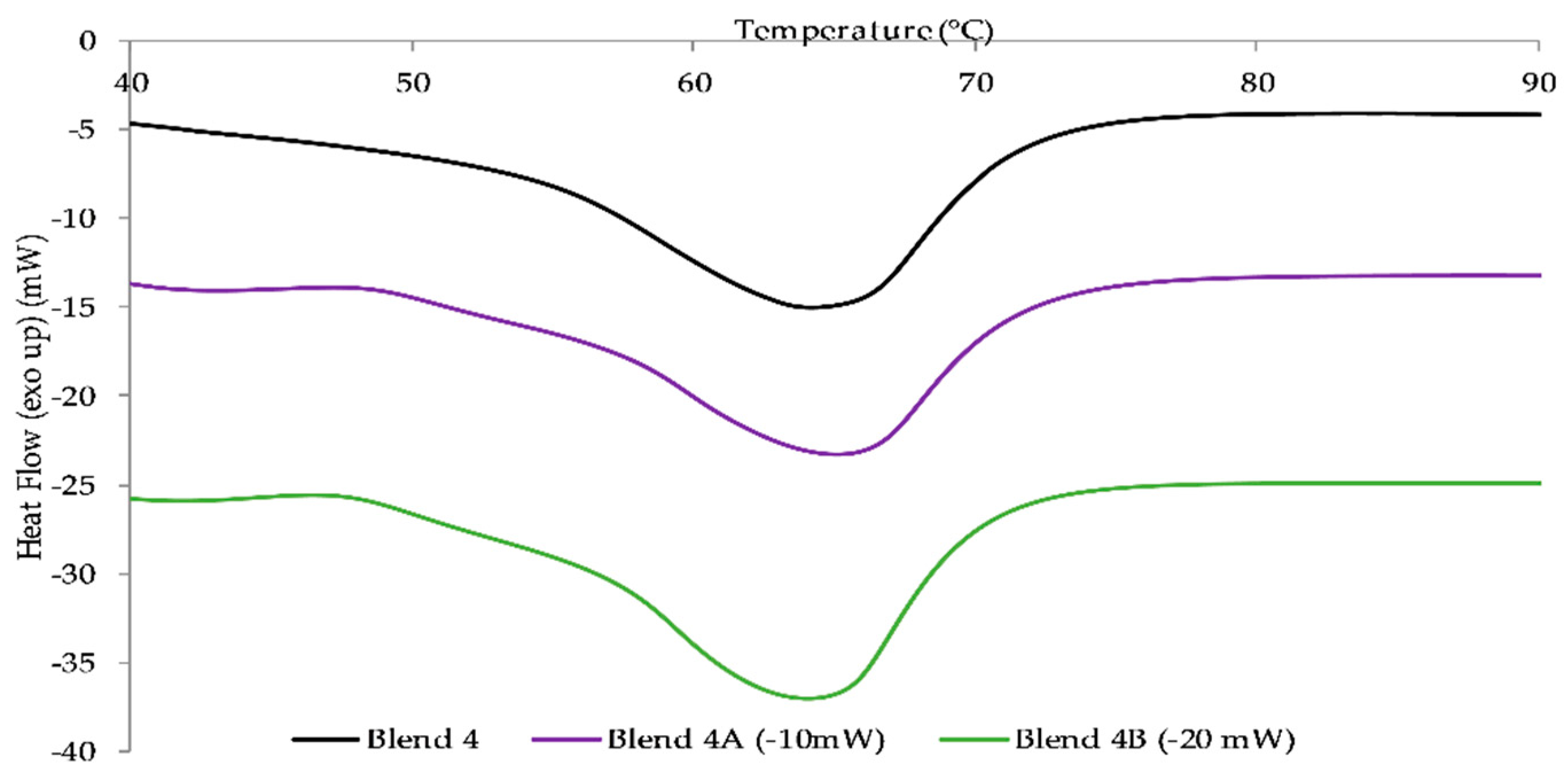
The two nanocomposite batches, Blend 4A and Blend 4B, were reinforced by the addition of two and six weight percent respectively in order to ascertain if the addition of the nanoclay would improve the mechanical strength. The rheological studies conducted indicated that the MFI of the nanocomposite batches increased with an increased loading of HNTs as did the yellowing of the batches with Blend 4B exhibiting a higher Hunter b value, 7.81, than the Hunter b value of Blend 4A, 3.94. Upon conducting tensile tests, the results obtained illustrated that the addition of HNTs significantly increased Young’s modulus of Blend 4 with an increase of 11% and 25% when the loading was two and six weight percent respectively. In addition to this it was apparent from DSC studies that the addition of HNTs had a negligible effect on the melt temperature of the polymeric matrix.
Furthermore, from conducting the study it was possible to produce a filament with a suitable diameter, 1.75 ± 0.05 mm, which could be utilized as a feedstock in the FFF process. Subsequent studies will examine the performance and characteristics of the manufactured filaments.