Screen-Printed f-EM Sensors Based on Two Chelating-Polymers and a Metal Oxide for the Continuous Detection of Cu Ions in Surface Water †
Abstract
:1. Introduction
1.1. Cu Pollution
1.2. Microwave Spectroscopy and f-EM Sensors
2. Materials and Method
2.1. Coating Development and Characterisation

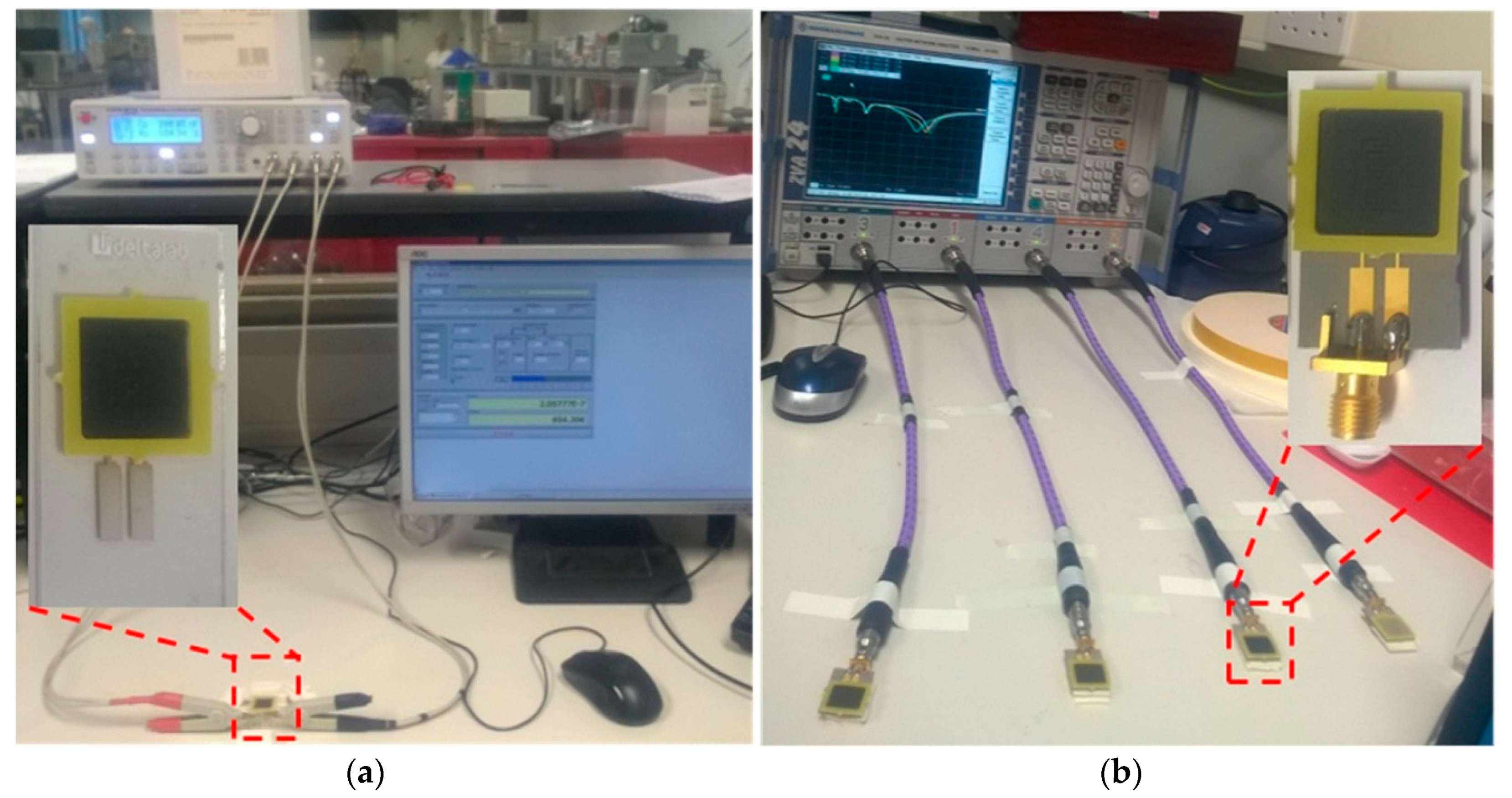
2.2. Optical, Microwave and Electrical Measurements
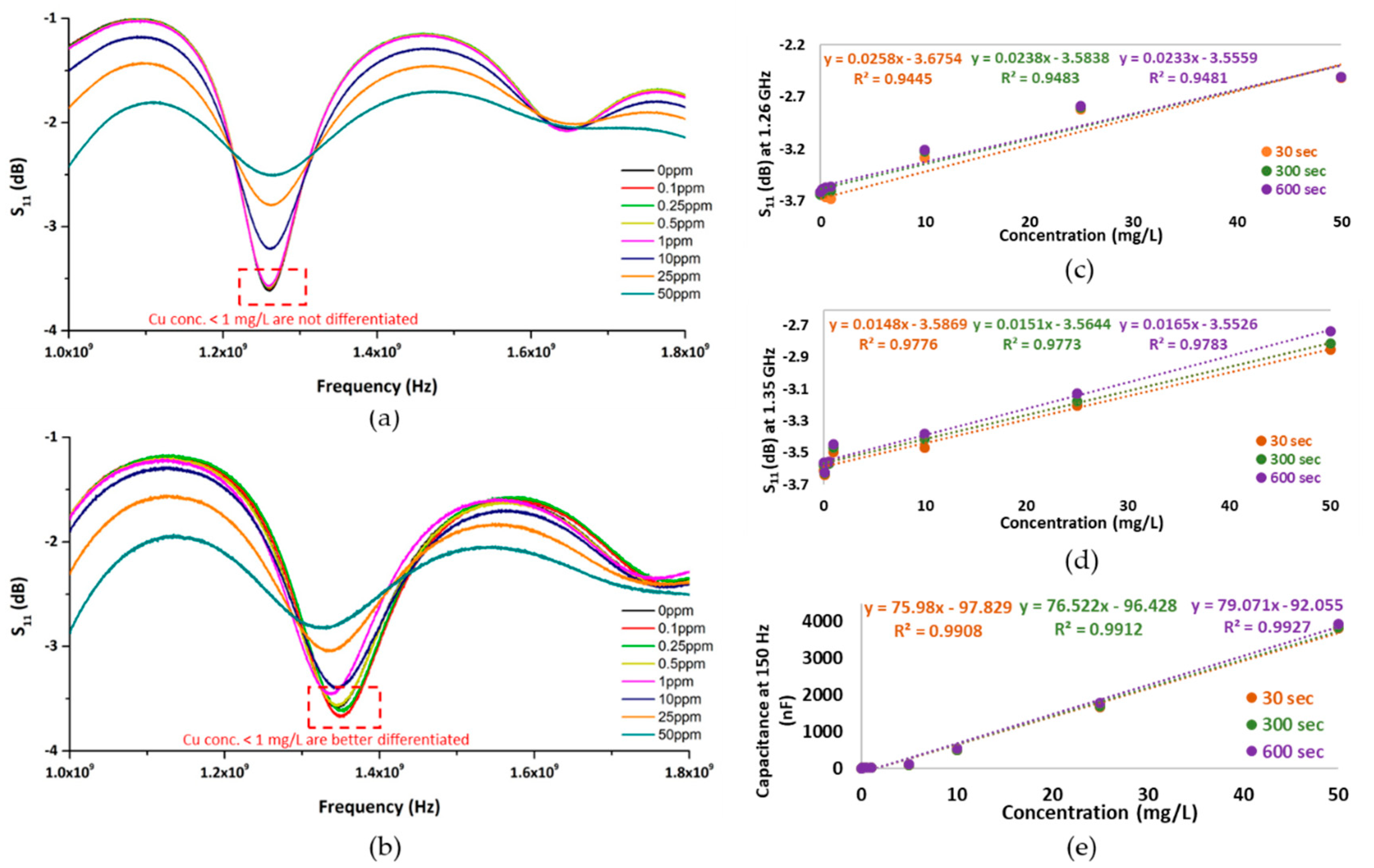
3. Results and Discussion
4. Conclusions
Acknowledgements
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Copper; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2004.
- Aragay, G.; Pons, J.; Merkoci, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.; Abdullah, B.; Muradov, M.; Korostynska, O.; Al-Shamma’a, A.; Bjarnadottir, S.G.; Lunde, K.; Alvseike, O. Theoretical basis and application for measuring pork loin drip loss using microwave spectroscopy. Sensors 2016, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Zarifi, M.H.; Farsinezhad, S.; Abdolrazzaghi, M.; Daneshmand, M.; Shankar, K. Selective microwave sensors exploiting the interaction of analytes with trap states in tio2 nanotube arrays. Nanoscale 2016, 8, 7466–7473. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Sun, H. Metamaterials application in sensing. Sensors 2012, 12, 2742. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.S.W.; Fatinathan, S. Adsorption of Cu(ii) ions in aqueous solution using chitosan beads, chitosan—Gla beads and chitosan–alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Koneswaran, M.; Narayanaswamy, R. L-cysteine-capped zns quantum dots based fluorescence sensor for cu2+ ion. Sens. Actuators B Chem. 2009, 139, 104–109. [Google Scholar] [CrossRef]
| Measured Parameter | R 2 | RSD | Sensitivity 1 |
|---|---|---|---|
| Absorbance (at 217 nm) | 0.98 | 1–2% | 0.10 |
| Capacitance using Ag f-EM sensors (at 150 Hz) 2 | 0.99 | 1–3% | 7.91 nF |
| S11 using uncoated Au EM sensors (at 1.26 GHz) 2 | 0.95 | 1–3% | 0.005 dB |
| S11 using coated Au f-EM sensors (at 1.35 GHz) 2 | 0.98 | 2–3% | 0.018 dB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frau, I.; Wylie, S.; Byrne, P.; Cullen, J.; Korostynska, O.; Mason, A. Screen-Printed f-EM Sensors Based on Two Chelating-Polymers and a Metal Oxide for the Continuous Detection of Cu Ions in Surface Water. Proceedings 2018, 2, 828. https://doi.org/10.3390/proceedings2130828
Frau I, Wylie S, Byrne P, Cullen J, Korostynska O, Mason A. Screen-Printed f-EM Sensors Based on Two Chelating-Polymers and a Metal Oxide for the Continuous Detection of Cu Ions in Surface Water. Proceedings. 2018; 2(13):828. https://doi.org/10.3390/proceedings2130828
Chicago/Turabian StyleFrau, Ilaria, Stephen Wylie, Patrick Byrne, Jeff Cullen, Olga Korostynska, and Alex Mason. 2018. "Screen-Printed f-EM Sensors Based on Two Chelating-Polymers and a Metal Oxide for the Continuous Detection of Cu Ions in Surface Water" Proceedings 2, no. 13: 828. https://doi.org/10.3390/proceedings2130828





