Use of Intermittent Preventive Treatment among Pregnant Women in Sub-Saharan Africa: Evidence from Malaria Indicator Surveys
Abstract
:1. Introduction
2. Methods
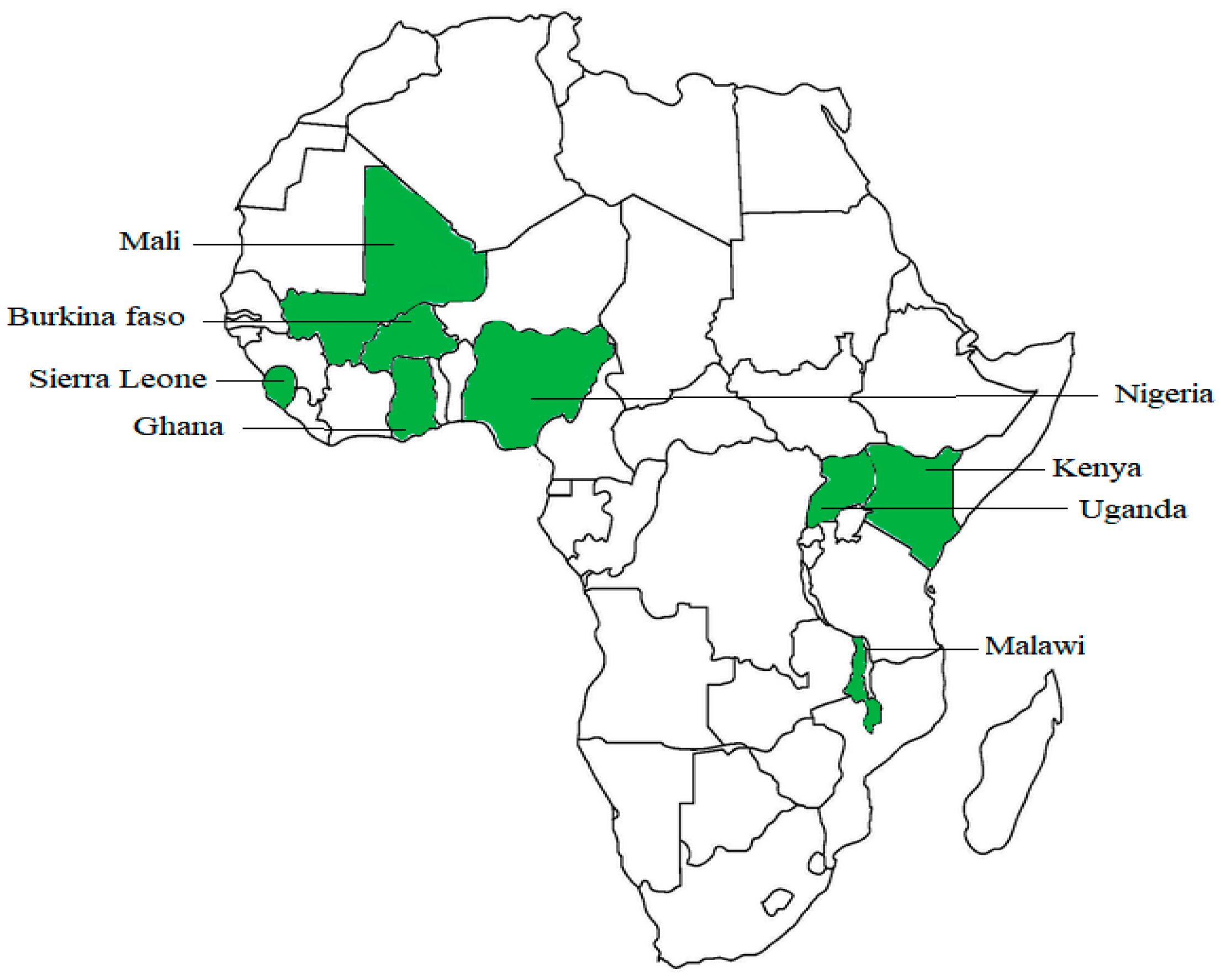
2.1. Countries Included in the Study
2.2. Survey Design and Objectives
2.3. Variables
2.4. Data Analysis
2.5. Ethical Approval
3. Results
3.1. Descriptive Statistics
3.2. Regression Analysis on the Association between Educational Level and Wealth Status with the Uptake of IPTp-SP
4. Discussion and Policy Recommendation
Strengths and Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bardají, A.; Sigauque, B.; Sanz, S.; Maixenchs, M.; Ordi, J.; Aponte, J.J.; Mabunda, S.; Alonso, P.L.; Menéndez, C. Impact of malaria at the end of pregnancy on infant mortality and morbidity. J. Infect. Dis. 2011, 203, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Moya-Alvarez, V.; Abellana, R.; Cot, M. Pregnancy-associated malaria and malaria in infants: An old problem with present consequences. Malar. J. 2014, 13, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uneke, C.J. Impact of placental Plasmodium falciparum malaria on pregnancy and perinatal outcome in sub-Saharan Africa. Yale J. Biol. Med. 2007, 80, 95–103. [Google Scholar] [PubMed]
- Agarwal, K.; Alonso, P.; Chico, R.M.; Coleman, J.; Dellicour, S.; Hill, J.; Majeres-Lugand, M.; Mangiaterra, V.; Menendez, C.; Mitchell, K.; et al. Global Call to Action to scale-up coverage of intermittent preventive treatment of malaria in pregnancy: Seminar report. Malar. J. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Steketee, R.W.; Campbell, C.C. Impact of national malaria control scale-up programmes in Africa: Magnitude and attribution of effects. Malar. J. 2010, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Salomão, C.; Sacarlal, J.; Gudo, E.S. Assessment of coverage of preventive treatment and insecticide-treated mosquito nets in pregnant women attending antenatal care services in 11 districts in Mozambique in 2011: The critical role of supply chain. Malar. J. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, C.; D’Alessandro, U.; ter Kuile, F.O. Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infect. Dis. 2007, 7, 126–135. [Google Scholar] [CrossRef]
- Haghdoost, A.A.; Alexander, N.; Smith, T. Maternal malaria during pregnancy and infant mortality rate: Critical literature review and a new analytical approach. J. Vector Borne Dis. 2007, 44, 98–104. [Google Scholar] [PubMed]
- Brahmbhatt, H.; Sullivan, D.; Kigozi, G.; Askin, F.; Wabwire-Mangenm, F.; Serwadda, D.; Sewankambo, N.; Wawer, M.; Gray, R. Association of HIV and malaria with mother-to-child transmission, birth outcomes, and child mortality. J. Acquir. Immune Defic. Syndr. 2008, 47, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F. Social implications of malaria and their relationships with poverty. Mediterr. J. Hematol. Infect. Dis. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, E.O.; Iferikigwe, E.S.; Obi, S.N.; Ugwu, A.O.; Agu, P.U.; Okezie, O.A. Anti-malaria prescription in pregnancy among general practitioners in Enugu state, southeast Nigeria. Niger. Med. J. 2013, 54, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Takem, E.N.; D’Alessandro, U. Malaria in pregnancy. Mediterr. J. Hematol. Infect. Dis. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Mulumba, P.M.; Kabongo, M.J.; Woto, E.E. Chloroquine drug prophylaxis no longer prevents Plasmodium falciparum-induced fetal hypotrophy in Kinshasa. Med. Trop. Rev. Corps Sante Colon. 2003, 63, 168–170. [Google Scholar]
- Rogerson, S.J.; Mwapasa, V.; Meshnick, S.R. Malaria in pregnancy: Linking immunity and pathogenesis to prevention. Am. J. Trop. Med. Hyg. 2007, 77, 14–22. [Google Scholar] [PubMed]
- Fievet, N.; Cot, M.; Ringwald, P.; Bickii, J.; Dubois, B.; Le Hesran, J.Y.; Migot, F.; Deloron, P. Immune response to Plasmodium falciparum antigens in Cameroonian primigravidae: Evolution after delivery and during second pregnancy. Clin. Exp. Immunol. 1997, 107, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Alecrim, W.D.; Espinosa, F.E.; Alecrim, M.G. Plasmodium falciparum infection in the pregnant patient. Infect. Dis. Clin. N. Am. 2000, 14, 83–95. [Google Scholar] [CrossRef]
- Kayentao, K.; Garner, P.; van Eijk, A.M.; Naidoo, I.; Roper, C.; Mulokozi, A.; MacArthur, J.R.; Luntamo, M.; Ashorn, P.; Doumbo, O.K.; et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs. 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: Systematic review and meta-analysis. JAMA 2013, 309, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Peeters Grietens, K.; Gies, S.; Coulibaly, S.O.; Ky, C.; Somda, J.; Toomer, E.; Muela Ribera, J.; D’Alessandro, U. Bottlenecks for high coverage of intermittent preventive treatment in pregnancy: The case of adolescent pregnancies in rural Burkina Faso. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.A.; Harvey, S.A.; Rao, N.; Diarra, N.H.; Klein, M.C.; Diop, S.I.; Doumbia, S.O. Underreporting and missed opportunities for uptake of intermittent preventative treatment of malaria in pregnancy (IPTp) in Mali. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Kanté, A.M.; Nathan, R.; Helleringer, S.; Sigilbert, M.; Levira, F.; Masanja, H.; de Savigny, D.; Abdulla, S.; Phillips, J.F. The contribution of reduction in malaria as a cause of rapid decline of under-five mortality: Evidence from the Rufiji Health and Demographic Surveillance System (HDSS) in rural Tanzania. Malar. J. 2014, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Protas, J.; Tarimo, D.; Moshiro, C. Determinants of timely uptake of ITN and SP (IPT) and pregnancy time protected against malaria in Bukoba, Tanzania. BMC Res. Notes 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Steketee, R.W.; Eisele, T.P. Is the scale-up of malaria intervention coverage also achieving equity? PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Ameh, S.; Owoaje, E.; Oyo-Ita, A.; Kabiru, C.W.; Akpet, O.E.O.; Etokidem, A.; Enembe, O.; Ekpenyong, N. Barriers to and determinants of the use of intermittent preventive treatment of malaria in pregnancy in Cross River State, Nigeria: A cross-sectional study. BMC Pregnancy Childbirth 2016, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Iliyasu, Z.; Gajida, A.U.; Galadanci, H.S.; Abubakar, I.S.; Baba, A.S.; Jibo, A.M.; Aliyu, M.H. Adherence to intermittent preventive treatment for malaria in pregnancy in urban Kano, northern Nigeria. Pathog. Glob. Health 2012, 106, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zelman, B.; Melgar, M.; Larson, E.; Phillips, A.; Shretta, R. Global fund financing to the 34 malaria-eliminating countries under the new funding model 2014–2017: An analysis of national allocations and regional grants. Malar. J. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Malaria Indicator Surveys—Access to Malaria Indicator Surveys, Datasets. Available online: http://www.malariasurveys.org/ (accessed on 28 December 2017).
- Ter Kuile, F.O.; van Eijk, A.M.; Filler, S.J. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: A systematic review. JAMA 2007, 297, 2603–2616. [Google Scholar] [CrossRef] [PubMed]
- Ghose, B. Frequency of TV viewing and prevalence of overweight and obesity among adult women in Bangladesh: A cross-sectional study. BMJ Open 2017, 7, e014399. [Google Scholar] [CrossRef] [PubMed]
- Thiam, S.; Kimotho, V.; Gatonga, P. Why are IPTp coverage targets so elusive in sub-Saharan Africa? A systematic review of health system barriers. Malar. J. 2013, 12, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaya, S.; Bishwajit, G.; Ekholuenetale, M.; Shah, V.; Kadio, B.; Udenigwe, O. Timing and adequate attendance of antenatal care visits among women in Ethiopia. PLoS ONE 2017, 12, e0184934. [Google Scholar] [CrossRef] [PubMed]
- Holtz, T.H.; Kachur, S.P.; Roberts, J.M.; Marum, L.H.; Mkandala, C.; Chizani, N.; Macheso, A.; Parise, M.E. Use of antenatal care services and intermittent preventive treatment for malaria among pregnant women in Blantyre District, Malawi. Trop. Med. Int. Health 2004, 9, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Dionne-Odom, J.; Westfall, A.O.; Apinjoh, T.O.; Anchang-Kimbi, J.; Achidi, E.A.; Tita, A.T.N. Predictors of the use of interventions to prevent malaria in pregnancy in Cameroon. Malar. J. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Nsibu, C.N.; Manianga, C.; Kapanga, S.; Mona, E.; Pululu, P.; Aloni, M.N. Determinants of antenatal care attendance among pregnant women living in endemic malaria settings: Experience from the Democratic Republic of Congo. Obstet. Gynecol. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Anchang-Kimbi, J.K.; Achidi, E.A.; Apinjoh, T.O.; Mugri, R.N.; Chi, H.F.; Tata, R.B.; Nkegoum, B.; Mendimi, J.-M.N.; Sverremark-Ekström, E.; Troye-Blomberg, M. Antenatal care visit attendance, intermittent preventive treatment during pregnancy (IPTp) and malaria parasitaemia at delivery. Malar. J. 2014, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Bouyou-Akotet, M.K.; Mawili-Mboumba, D.P.; Kombila, M. Antenatal care visit attendance, intermittent preventive treatment and bednet use during pregnancy in Gabon. BMC Pregnancy Childbirth 2013, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Sirima, S.B.; Cotte, A.H.; Konaté, A.; Moran, A.C.; Asamoa, K.; Bougouma, E.C.; Diarra, A.; Ouédraogo, A.; Parise, M.E.; Newman, R.D. Malaria prevention during pregnancy: Assessing the disease burden one year after implementing a program of intermittent preventive treatment in Koupela District, Burkina Faso. Am. J. Trop. Med. Hyg. 2006, 75, 205–211. [Google Scholar] [PubMed]
- D’Almeida, T.C.D.A.; Agboton-Zoumenou, M.-A.; Garcia, A.; Massougbodji, A.; Briand, V.; Imorou, Y.; Cottrell, G. Field evaluation of the intermittent preventive treatment of malaria during pregnancy (IPTp) in Benin: Evolution of the coverage rate since its implementation. Parasites Vectors 2011, 4, 108. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, A.M.; Hill, J.; Alegana, V.A.; Kirui, V.; Gething, P.W.; ter Kuile, F.O.; Snow, R.W. Coverage of malaria protection in pregnant women in sub-Saharan Africa: A synthesis and analysis of national survey data. Lancet Infect. Dis. 2011, 11, 190–207. [Google Scholar] [CrossRef]
- Gikandi, P.W.; Noor, A.M.; Gitonga, C.W.; Ajanga, A.A.; Snow, R.W. Access and barriers to measures targeted to prevent malaria in pregnancy in rural Kenya. Trop. Med. Int. Health 2008, 13, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Sangaré, L.R.; Stergachis, A.; Brentlinger, P.E.; Richardson, B.A.; Staedke, S.G.; Kiwuwa, M.S.; Weiss, N.S. Determinants of use of intermittent preventive treatment of malaria in pregnancy: Jinja, Uganda. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Kemble, S.K.; Davis, J.C.; Nalugwa, T.; Njama-Meya, D.; Hopkins, H.; Dorsey, G.; Staedke, S.G. Prevention and treatment strategies used for the community management of childhood fever in Kampala, Uganda. Am. J. Trop. Med. Hyg. 2006, 74, 999–1007. [Google Scholar] [PubMed]
- Nganda, R.Y.; Drakeley, C.; Reyburn, H.; Marchant, T. Knowledge of malaria influences the use of insecticide treated nets but not intermittent presumptive treatment by pregnant women in Tanzania. Malar. J. 2004, 3, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.C.; Harvey, S.A.; Diarra, H.; Hurley, E.A.; Rao, N.; Diop, S.; Doumbia, S. “There is no free here, you have to pay”: Actual and perceived costs as barriers to intermittent preventive treatment of malaria in pregnancy in Mali. Malar. J. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Malaria Continues to Threaten Pregnant Women and Children. Available online: http://www.prb.org/Publications/Articles/2001/MalariaContinuestoThreatenPregnantWomenandChildren.aspx (accessed on 27 December 2017).
- Sangaré, L.R.; Weiss, N.S.; Brentlinger, P.E.; Richardson, B.A.; Staedke, S.G.; Kiwuwa, M.S.; Stergachis, A. Patterns of anti-malarial drug treatment among pregnant women in Uganda. Malar. J. 2011, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- World Malaria Day: Which Countries Are the Hardest Hit? Get the Full Data. Available online: http://www.theguardian.com/global-development/datablog/2011/apr/25/world-malaria-day-data (accessed on 27 December 2017).

| Received at Least 3 Doses of IPTp-SP in Last Pregnancy | p-Value | |||||
|---|---|---|---|---|---|---|
| N = 18,603 | % | % | 95% CI Lower | 95% CI Upper | ||
| Age Group (Mean = 28.76) | 0.061 | |||||
| 15–19 | 1372 | 7.4 | 7.0 | 6.2 | 7.8 | |
| 20–24 | 4184 | 22.5 | 22.1 | 20.8 | 23.5 | |
| 25–29 | 4829 | 26.0 | 25.7 | 24.4 | 27.2 | |
| 30–34 | 3919 | 21.1 | 21.3 | 20.0 | 22.7 | |
| 35–39 | 2697 | 14.5 | 14.5 | 13.5 | 15.7 | |
| 40–44 | 1208 | 6.5 | 7.0 | 6.3 | 7.8 | |
| 45–49 | 394 | 2.1 | 2.3 | 1.9 | 2.9 | |
| Setting | <0.001 | |||||
| Urban | 5271 | 28.3 | 30.6 | 27.6 | 33.7 | |
| Rural | 13,332 | 71.7 | 69.4 | 66.3 | 72.4 | |
| Religion | <0.001 | |||||
| Islam | 8964 | 48.2 | 43.8 | 40.9 | 46.8 | |
| Christian | 6082 | 32.7 | 32.0 | 29.6 | 34.5 | |
| Other | 3557 | 19.1 | 24.2 | 21.3 | 27.3 | |
| Education | <0.001 | |||||
| No education | 8960 | 48.2 | 44.3 | 41.9 | 46.8 | |
| Primary | 3895 | 20.9 | 22.4 | 20.9 | 24.1 | |
| Secondary | 3676 | 19.8 | 25.4 | 23.5 | 27.4 | |
| Higher | 2072 | 11.1 | 7.8 | 6.5 | 9.4 | |
| Wealth index | <0.001 | |||||
| Poorest | 4071 | 21.9 | 19.0 | 17.4 | 20.8 | |
| Poorer | 3910 | 21.0 | 21.1 | 19.4 | 23.0 | |
| Middle | 3850 | 20.7 | 20.7 | 19.1 | 22.3 | |
| Richer | 3683 | 19.8 | 21.2 | 19.3 | 23.1 | |
| Richest | 3089 | 16.6 | 18.1 | 15.9 | 20.4 | |
| TV * | 0.05 | |||||
| No | 15,013 | 80.7 | 22.4 | 20.3 | 24.7 | |
| Yes | 3590 | 19.3 | 77.6 | 75.3 | 79.7 | |
| Radio * | <0.001 | |||||
| No | 10,008 | 53.8 | 45.6 | 43.3 | 48.0 | |
| Yes | 8595 | 46.2 | 54.4 | 52.0 | 56.7 | |
| Health worker * | <0.001 | |||||
| No | 11,515 | 61.90 | 34.7 | 32.1 | 37.4 | |
| Yes | 7088 | 38.11 | 65.3 | 62.6 | 67.9 | |
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI Lower | 95% CI Upper | OR | 95% CI Lower | 95% CI Upper | OR | 95% CI Lower | 95% CI Upper | |
| Education (Nil) | |||||||||
| Primary | 1.791 | 1.590 | 2.018 | 1.307 | 1.104 | 1.547 | |||
| Secondary | 2.043 | 1.796 | 2.324 | 1.431 | 1.202 | 1.705 | |||
| Higher | 2.374 | 2.086 | 2.701 | 1.658 | 1.401 | 1.962 | |||
| Wealth index (Richest) | |||||||||
| Poorest | 1.185 | 1.069 | 1.314 | 1.320 | 1.140 | 1.529 | |||
| Poorer | 1.137 | 1.025 | 1.262 | 1.235 | 1.069 | 1.425 | |||
| Middle | 1.085 | 0.977 | 1.205 | 1.210 | 1.054 | 1.388 | |||
| Richer | 1.127 | 1.014 | 1.253 | 1.212 | 1.069 | 1.374 | |||
| Nagelkerke R Square | 0.179 | 0.134 | 0.484 | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaya, S.; Uthman, O.A.; Amouzou, A.; Bishwajit, G. Use of Intermittent Preventive Treatment among Pregnant Women in Sub-Saharan Africa: Evidence from Malaria Indicator Surveys. Trop. Med. Infect. Dis. 2018, 3, 18. https://doi.org/10.3390/tropicalmed3010018
Yaya S, Uthman OA, Amouzou A, Bishwajit G. Use of Intermittent Preventive Treatment among Pregnant Women in Sub-Saharan Africa: Evidence from Malaria Indicator Surveys. Tropical Medicine and Infectious Disease. 2018; 3(1):18. https://doi.org/10.3390/tropicalmed3010018
Chicago/Turabian StyleYaya, Sanni, Olalekan A. Uthman, Agbessi Amouzou, and Ghose Bishwajit. 2018. "Use of Intermittent Preventive Treatment among Pregnant Women in Sub-Saharan Africa: Evidence from Malaria Indicator Surveys" Tropical Medicine and Infectious Disease 3, no. 1: 18. https://doi.org/10.3390/tropicalmed3010018






