Scrub Typhus: No Longer Restricted to the Tsutsugamushi Triangle
Abstract
:1. Introduction
2. Scrub Typhus outside of the Tsutsugamushi Triangle
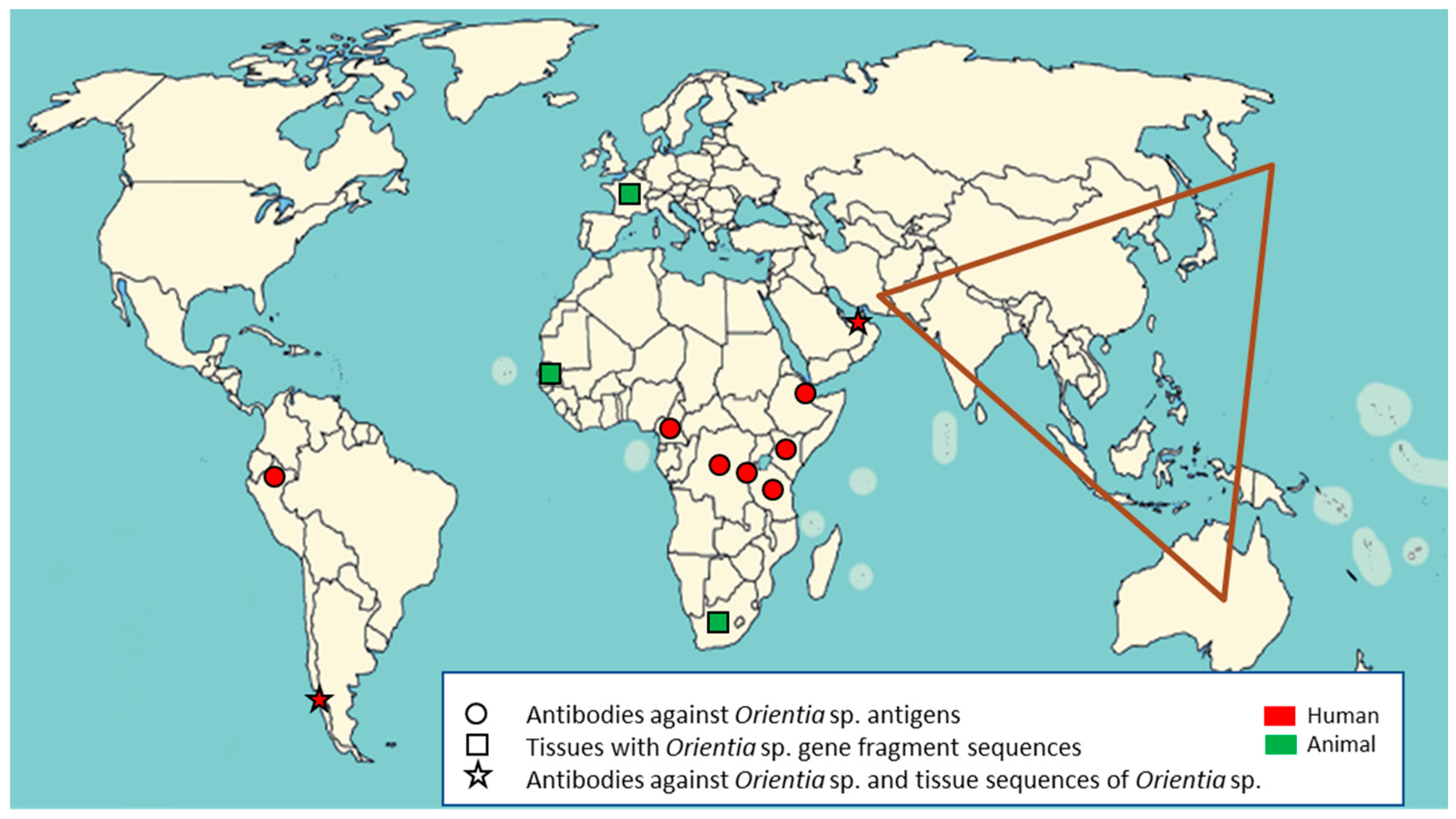
2.1. Serological Evidence of Orientia Species Infection in Africa
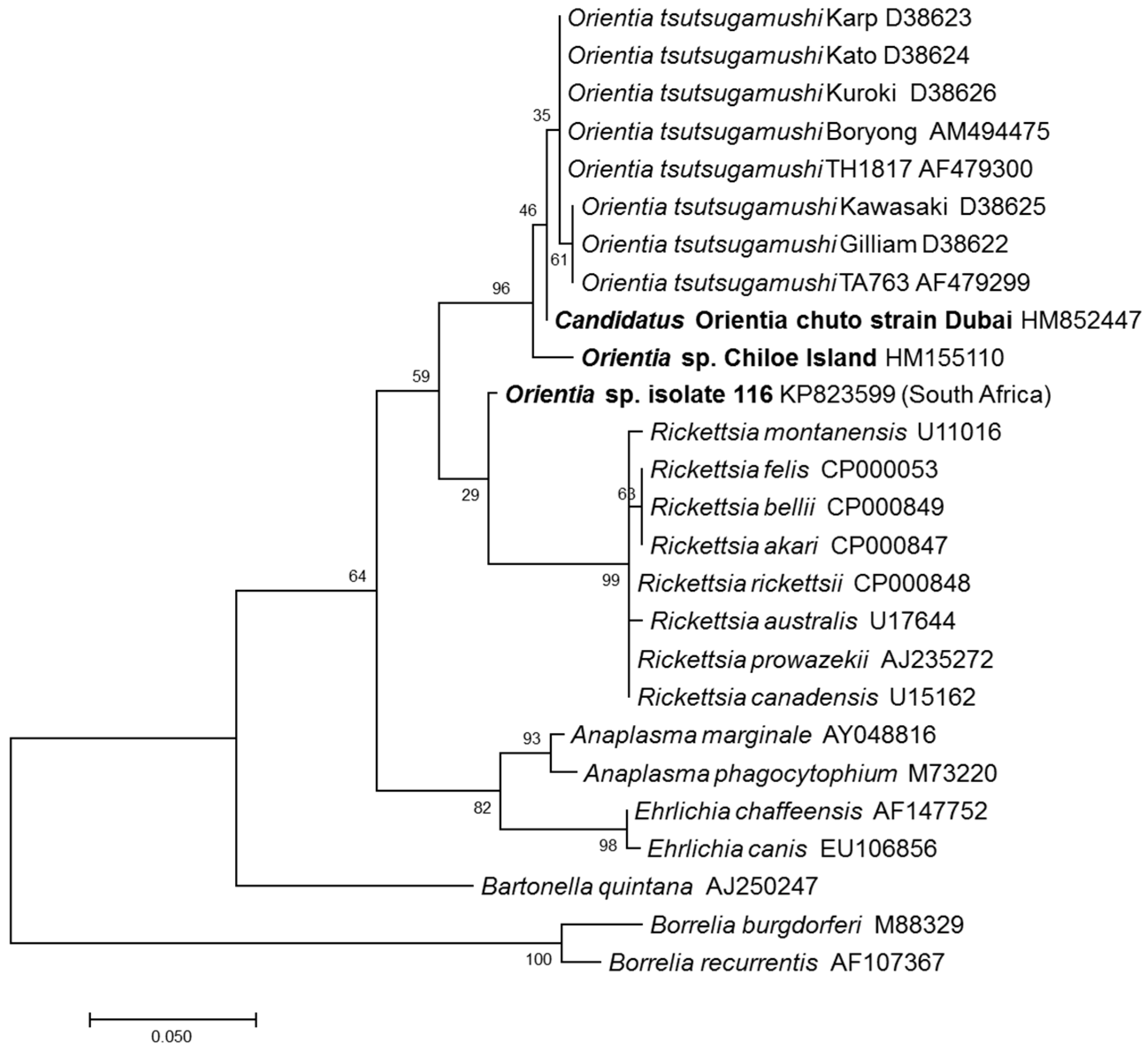
2.2. Molecular Evidence of Orientia Species in Africa and Europe
2.3. Scrub Typhus Cases in the Middle East and South America
3. Conclusions
Acknowledgments
Conflicts of Interest
Disclaimers
References
- Watt, G.; Parola, P. Scrub typhus and tropical rickettsioses. Curr. Opin. Infect. Dis. 2003, 16, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Paris, D.H.; Shelite, T.R.; Day, N.P.; Walker, D.H. Review article: Unresolved problems related to scrub typhus: A seriously neglected life-threatening disease. Am. J. Trop. Med. Hyg. 2013, 89, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Richards, A.L.; Temenak, J.J.; Strickman, D.; Dasch, G.A. The past and present threat of rickettsial diseases to military medicine and international public health. Clin. Infect. Dis. 2002, 34 (Suppl. s4), 145–169. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Fuerst, P.A.; Ching, W.M.; Richards, A.L. Scrub typhus: The geographical distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin. Infect. Dis. 2009, 48, S203–S230. [Google Scholar] [CrossRef] [PubMed]
- Bonell, A.; Lubell, Y.; Newton, P.N.; Crump, J.A.; Paris, D.H. Estimating the burden of scrub typhus: A systematic review. PLoS Negl. Trop. Dis. 2017, 11, e0005838. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A review of the global epidemiology of scrub typhus. PLoS Negl. Trop. Dis. 2017, 11, e0006062. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Foley, D.; Richards, A.L. A spatiotemporal database to track human scrub typhus using the VectorMap application. PLoS. Negl. Trop. Dis. 2015, 9, e0004161. [Google Scholar] [CrossRef] [PubMed]
- Giroud, P. Jadin, J. Presence des anticorps vis-a-vis de Rickettsia orientalis chez les indigenes et des Asiatiques vivant au Ruanda-urundi (Congo Belge). Bull. Soc. Pathol. Exot. 1951, 44, 50–51. [Google Scholar]
- Osuga, K.; Kimura, M.; Goto, H.; Shimada, K.; Suto, T. A case of tsutsugamushi disease probably contracted in Africa. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, R.P.; Ghorbani, A.J.; Jain, M.K.; Walker, D.H. A case of scrub typhus probably acquired in Africa. Clin. Infect. Dis. 1997, 25, 1473–1474. [Google Scholar] [CrossRef]
- Groen, J.; Nur, Y.A.; Osterhaus, M.E. Scrub and murine typhus among Dutch travellers. Infection 1999, 27, 291–292. [Google Scholar] [PubMed]
- Thiga, J.W.; Mutai, B.; Eyako, W.; Ng’ang’a, Z.; Jiang, J.; Richards, A.L.; Waitumbi, J.N. High sero-prevalence and IgG titers for spotted fever and scrub typhus in patients with febrile illness in Kenya. Emerg. Infect. Dis. 2015, 21, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Maina, A.N.; Farris, C.M.; Odhiambo, A.; Jiang, J.; Laktabai, J.; Armstrong, J.; Holland, T.; Richards, A.L.; O’Meara, W.P. Q fever, scrub typhus, and rickettsial diseases in children, 2011–2012 Kenya. Emerg. Infect. Dis. 2016, 22, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Horton, K.C.; Jiang, J.; Maina, A.; Dueger, E.; Zayed, A.; Ahmed, A.A.; Pimentel, G.; Richards, A.L. Evidence of Rickettsia and Orientia infections among abattoir workers in Djibouti. Am. J. Trop. Med. Hyg. 2016, 95, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Cosson, J.F.; Galan, M.; Bard, E.; Razzauti, M.; Bernard, M.; Morand, S.; Brouat, C.; Dalecky, A.; Bâ, K.; Charbonnel, N.; et al. Detection of Orientia sp. DNA in rodents from Asia, West Africa and Europe. Parasites Vectors 2015, 8, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolo, A.O.; Sibeko-Matjila, K.P.; Maina, A.N.; Richards, A.L.; Knobel, D.L.; Matjila, P.T. Molecular detection of zoonotic rickettsiae and Anaplasma spp. in domestic dogs and their ectoparasites in Bushbuckridge, South Africa. Vector Borne Zoonotic Dis. 2016, 16, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Izzard, L.; Fuller, A.; Blacksell, S.D.; Paris, D.H.; Richards, A.L.; Aukkanit, N.; Nguyen, C.; Jiang, J.; Fenwick, S.; Day, N.P.J.; Graves, S.; Stenos, J. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 2010, 48, 4404–4409. [Google Scholar] [CrossRef] [PubMed]
- Balcells, M.E.; Rabagliati, R.; García, P.; Poggi, H.; Oddó, D.; Concha, M.; Abarca, K.; Jiang, J.; Kelly, D.J.; Richards, A.L.; et al. Endemic scrub typhus-like illness, Chile. Emerg. Infect. Dis. 2011, 17, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Dittrich, S.; Lopez, J.; Phuklia, W.; Martinez-Valdebenito, C.; Velasquez, K.; Blacksell, S.D.; Paris, D.H.; Abarca, K. Endemic scrub typhus in South America. N. Engl. J. Med. 2016, 375, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Kocher, C.; Jiang, J.; Morrison, A.C.; Castillo, R.; Leguia, M.; Loyola, S.; Ampuero, S.; Bausch, D.G.; Richards, A.L. Scrub typhus in the Peruvian Amazon Basin. Emerg. Infect. Dis. 2017, 23, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
| Patient | Country of Origin | Area/Country Acquired Diseases | Signs and Symptoms | Laboratory Results | Treatment/Time to Defervescence | Reference |
|---|---|---|---|---|---|---|
| 46-year-old male | United States | Rural village/Cameroon, West Africa | Eschar on left lower leg, fever (39.6 °C), rash initially on legs, headaches, myalgias, and malaise | Decreased platelet count and WBC (leukopenia) | Doxycycline/24 h | [8] |
| 44-year-old female | Netherlands | Rural areas/Tanzania, East Africa | Eschar on right foot, fever (39.8 °C) and headache | Elevated erythrocyte sedimentation rate | Ciprofloxacin 1 week after noticing the eschar, no effect; doxycycline/not reported | [9] |
| 52-year-old female | Australia | Dubai (stable)/United Arab Emirates (UAE), West Asia | Eschar on abdomen, fever and rash, lymphadenopathy, myalgia, headache, pain behind eyes, backache | Elevated WBC and C-reactive proteins (CRP); abnormal liver function: elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP), and elevated gamma-glutamyl transpeptidase (GGT) | Doxycycline/10 days | [15] |
| 54-year-old male | Chile | Chiloé Island/Chile, South America | Eschar on left leg, high-grade fever (39.0 °C), headache, myalgia, and scanty dry cough, rash; bilateral conjunctival suffusion | Abnormal liver function: elevated ALT and AST | Doxycycline/3 days | [16] |
| 38-year-old female | Chile | Chiloé Island/Chile, South America | Eschar on abdomen, maculopapular rash, fever (40.0 °C), headaches, and intense myalgia especially in the calves, malaise and confusion, apathetic, bilateral conjunctivitis | Elevated erythrocyte sedimentation rate, abnormal liver function: elevated CRP, ALT, AST and GGT | Doxycycline/24 h | [17] |
| 40-year-old male | Chile | Chiloé Island/Chile, South America | Eschar on right leg, rash, high fever, chills, night sweats, headaches, myalgia, retro-orbital pain and photophobia | Abnormal liver function: elevated CRP, ALT and AST | Cloxacillin and anti-inflammatory, no effect; doxycycline/24 h | [17] |
| 55-year-old male | Chile | Chiloé Island/Chile, South America | Eschar on upper left thigh, rash, high fever, chills, night sweats, intense headaches, myalgia, and arthralgia | Elevated CRP | Fever and systemic symptoms resolved spontaneously after 1 week; doxycycline/not reported | [17] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Richards, A.L. Scrub Typhus: No Longer Restricted to the Tsutsugamushi Triangle. Trop. Med. Infect. Dis. 2018, 3, 11. https://doi.org/10.3390/tropicalmed3010011
Jiang J, Richards AL. Scrub Typhus: No Longer Restricted to the Tsutsugamushi Triangle. Tropical Medicine and Infectious Disease. 2018; 3(1):11. https://doi.org/10.3390/tropicalmed3010011
Chicago/Turabian StyleJiang, Ju, and Allen L. Richards. 2018. "Scrub Typhus: No Longer Restricted to the Tsutsugamushi Triangle" Tropical Medicine and Infectious Disease 3, no. 1: 11. https://doi.org/10.3390/tropicalmed3010011




