Soil-Transmitted Helminths in Children in a Remote Aboriginal Community in the Northern Territory: Hookworm is Rare but Strongyloides stercoralis and Trichuris trichiura Persist
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Population
2.2. Fecal Sample Collection and Processing
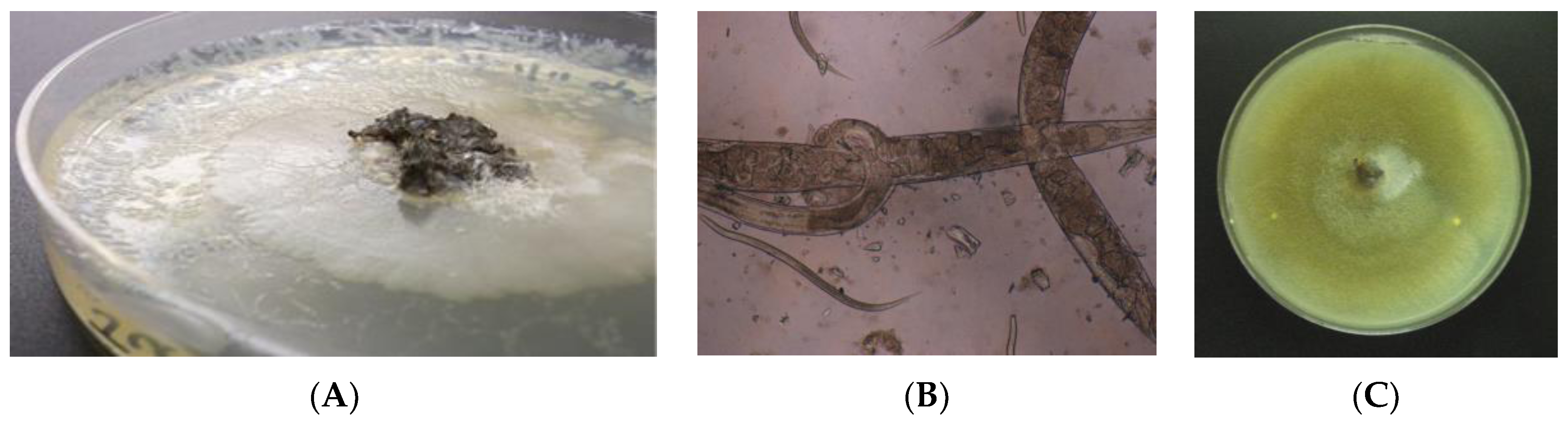
2.3. Agar Plate Culture
2.4. Fecal DNA Extraction and PCR
2.5. Blood Spot Collection and Serological Testing
3. Results
3.1. Comparison of Intestinal Parasites Identified in Children <10 Years in 1994–1996 and 2010–2011
3.2. Comparison of Diagnostic Methods for S. stercoralis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs366/en/ (accessed on 17 July 2017).
- Hopkins, R.M.; Gracey, M.S.; Hobbs, R.P.; Spargo, R.M.; Yates, M.; Thompson, R.C. The prevalence of hookworm infection, iron deficiency and anaemia in an aboriginal community in north-west Australia. Med. J. Aust. 1997, 166, 241–244. [Google Scholar] [PubMed]
- Shield, J.; Aland, K.; Kearns, T.; Gongdjalk, G.; Holt, D.; Currie, B.; Prociv, P. Intestinal parasites of children and adults in a remote Aboriginal community of the Northern Territory, Australia, 1994–1996. Western Pac. Surveill. Response J. 2015, 6, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Fryar, D.; Hagan, S. Pilot screening program for intestinal parasites and anaemia in adults in a Top End Aboriginal community. NT Comm Dis Bull. 1997, 4, 20–21. [Google Scholar]
- Johnston, F.H.; Morris, P.S.; Speare, R.; McCarthy, J.; Currie, B.; Ewald, D.; Page, W.; Dempsey, K. Strongyloidiasis: A review of the evidence for Australian practitioners. Aust. J. Rural Health 2005, 13, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kearns, T.M.; Currie, B.J.; Cheng, A.C.; McCarthy, J.; Carapetis, J.R.; Holt, D.C.; Page, W.; Shield, J.; Gundjirryirr, R.; Mulholland, E.; et al. Strongyloides seroprevalence before and after an ivermectin mass drug administration in a remote Australian Aboriginal community. PLoS Negl. Trop. Dis. 2017, 11, e0005607. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Berk, S.L. Diagnosis of Strongyloides stercoralis infection. Clin. Infect. Dis. 2001, 33, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Speare, R.; Durrheim, D.N. Strongyloides serology – useful for diagnosis and management of strongyloidiasis in rural Indigenous populations, but important gaps in knowledge remain. Rural Remote Health 2004, 4, 264. [Google Scholar] [PubMed]
- Grove, D.I. Human strongyloidiasis. Adv. Parasitol. 1996, 38, 251–309. [Google Scholar] [PubMed]
- Dreyer, G.; Fernandes-Silva, E.; Alves, S.; Rocha, A.; Albuquerque, R.; Addiss, D. Patterns of detection of Strongyloides stercoralis in stool specimens: Implications for diagnosis and clinical trials. J. Clin. Microbiol. 1996, 34, 2569–2571. [Google Scholar] [PubMed]
- Tanaka, H. Experimental and epidemiological studies on strongyloidiasis of Amami Oshima island. Jpn. J. Exp. Med. 1958, 28, 159–182. [Google Scholar] [PubMed]
- Kearns, T.M.; Speare, R.; Cheng, A.C.; McCarthy, J.; Carapetis, J.R.; Holt, D.C.; Currie, B.J.; Page, W.; Shield, J.; Gundjirryirr, R.; et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian Aboriginal community. PLoS Negl. Trop. Dis. 2015, 9, e0004151. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Available online: http://www.censusdata.abs.gov.au/census_services/getproduct/census/2016/quickstat/SSC70106 (accessed on 31 August 2017).
- VassarStats: Website for Statistical Computation. Available online: http://vassarstats.net/ (accessed on 30 September 2017).
- Garcia, L. Diagnostic Medical Parasitology, 5th ed.; American Society for Microbiology Press: Washington DC, USA, 2007; pp. 837–840. [Google Scholar]
- Sultana, Y.; Jeoffreys, N.; Watts, M.R.; Gilbert, G.L.; Lee, R. Real-time polymerase chain reaction for detection of Strongyloides stercoralis in stool. Am. J. Trop. Med. Hyg. 2013, 88, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J.J.; Canales, M.; Polman, K.; Ziem, J.; Brienen, E.A.; Polderman, A.M.; van Lieshout, L. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Ramachandran, S.; Thompson, R.W.; Andersen, J.F.; Neva, F.A. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Mol. Biochem. Parasitol. 2002, 125, 73–81. [Google Scholar] [CrossRef]
- Mounsey, K.; Kearns, T.; Rampton, M.; Llewellyn, S.; King, M.; Holt, D.; Currie, B.J.; Andrews, R.; Nutman, T.; McCarthy, J. Use of dried blood spots to define antibody response to the Strongyloides stercoralis recombinant antigen NIE. Acta Trop. 2014, 138, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Barker, B.; Begg, S.; Stanley, L.; Lopez, A.D. Burden of disease and injury in Aboriginal and Torres Strait Islander Peoples: the Indigenous health gap. Int. J. Epidemiol. 2009, 38, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Bailie, R.S.; McDonald, E.L.; Stevens, M.; Guthridge, S.; Brewster, D.R. Evaluation of an Australian indigenous housing programme: Community level impact on crowding, infrastructure function and hygiene. J. Epidemiol. Community Health 2011, 65, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Bailie, R.S.; Stevens, M.; McDonald, E.L. The impact of housing improvement and socio-environmental factors on common childhood illnesses: A cohort study in Indigenous Australian communities. J. Epidemiol. Community Health 2012, 66, 821–831. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.; Bailie, R. Hygiene improvement: Essential to improving child health in remote Aboriginal communities. J. Paediatr. Child. Health 2010, 46, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Central Australian Rural Practitioners Association. CARPA Standard Treatment Manual, 2nd ed.; Central Australian Rural Practitioners Association: Alice Springs, NT, Australia, 1994. [Google Scholar]
- Davies, J.; Majumdar, S.S.; Forbes, R.T.; Smith, P.; Currie, B.J.; Baird, R.W. Hookworm in the Northern Territory: down but not out. Med. J. Aust. 2013, 198, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Coverdale, J.K.; Crowe, A.; Smith, P.; Baird, R.W. Trends in Strongyloides stercoralis faecal larvae detections in the Northern Territory, Australia: 2002–2012. Trop. Med. Infect. Dis. 2017, 2, 18. [Google Scholar] [CrossRef]
- Crowe, A.L.; Smith, P.; Ward, L.; Currie, B.J.; Baird, R. Decreasing prevalence of Trichuris trichiura (whipworm) in the Northern Territory from 2002 to 2012. Med. J. Aust. 2014, 200, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Horton, J. Albendazole: A review of anthelmintic efficacy and safety in humans. Parasitology 2000, 121, S113–S132. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Utzinger, J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. J.A.M.A. 2008, 299, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, P.; Utzinger, J.; Du, Z.W.; Jiang, J.Y.; Chen, J.X.; Hattendorf, J.; Zhou, H.; Zhou, X.N. Efficacy of single-dose and triple-dose albendazole and mebendazole against soil-transmitted helminths and Taenia spp.: A randomized controlled trial. PLoS One 2011, 6, e25003. [Google Scholar] [CrossRef] [PubMed]
- Willcocks, B.; McAuliffe, G.N.; Baird, R.W. Dwarf tapeworm (Hymenolepis nana): Characteristics in the Northern Territory 2002–2013. J. Paediatr. Child. Health 2015, 51, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Central Australian Rural Practitioners Association. CARPA Standard Treatment Manual, 5th ed.; Central Australian Rural Practitioners Association: Alice Springs, NT, Australia, 2014. [Google Scholar]
- Sato, Y.; Kobayashi, J.; Toma, H.; Shiroma, Y. Efficacy of stool examination for detection of Strongyloides infection. Am. J. Trop. Med. Hyg. 1995, 53, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Beknazarova, M.; Millsteed, S.; Robertson, G.; Whiley, H.; Ross, K. Validation of DESS as a DNA preservation method for the detection of Strongyloides spp. in canine feces. Int. J. Environ. Res. Public Health 2017, 14. [Google Scholar] [CrossRef]
- Watts, M.R.; James, G.; Sultana, Y.; Ginn, A.N.; Outhred, A.C.; Kong, F.; Verweij, J.J.; Iredell, J.R.; Chen, S.C.; Lee, R. A loop-mediated isothermal amplification (LAMP) assay for Strongyloides stercoralis in stool that uses a visual detection method with SYTO-82 fluorescent dye. Am. J. Trop. Med. Hyg. 2014, 90, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Soto, P.; Sanchez-Hernandez, A.; Gandasegui, J.; Bajo Santos, C.; Lopez-Aban, J.; Saugar, J.M.; Rodriguez, E.; Vicente, B.; Muro, A. Strong-LAMP: A LAMP Assay for Strongyloides spp. detection in stool and urine samples. Towards the diagnosis of human strongyloidiasis starting from a rodent model. PLoS Negl. Trop. Dis. 2016, 10, e0004836. [Google Scholar] [CrossRef]
- Mejia, R.; Vicuna, Y.; Broncano, N.; Sandoval, C.; Vaca, M.; Chico, M.; Cooper, P.J.; Nutman, T.B. A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. Am. J. Trop. Med. Hyg. 2013, 88, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Basuni, M.; Muhi, J.; Othman, N.; Verweij, J.J.; Ahmad, M.; Miswan, N.; Rahumatullah, A.; Aziz, F.A.; Zainudin, N.S.; Noordin, R. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am. J. Trop. Med. Hyg. 2011, 84, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, S.; Inpankaew, T.; Nery, S.V.; Gray, D.J.; Verweij, J.J.; Clements, A.C.; Gomes, S.J.; Traub, R.; McCarthy, J.S. Application of a multiplex quantitative PCR to assess prevalence and intensity of intestinal parasite infections in a controlled clinical trial. PLoS Negl. Trop. Dis. 2016, 10, e0004380. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, N.; Papaiakovou, M.; Grant, J.R.; Bierwert, L.A.; Llewellyn, S.; McCarthy, J.S.; Williams, S.A. Improved PCR-based detection of soil transmitted helminth infections using a next-generation sequencing approach to assay design. PLoS Negl. Trop. Dis. 2016, 10, e0004578. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Perandin, F.; Formenti, F.; Bisoffi, Z. A retrospective study comparing agar plate culture, indirect immunofluorescence and real-time PCR for the diagnosis of Strongyloides stercoralis infection. Parasitology 2017, 144, 812–816. [Google Scholar] [CrossRef] [PubMed]
| n | 1994–1996 84 | 2010–2011 85 | Fisher Exact Probability Test p (Two-tail) |
|---|---|---|---|
| Average age (years) | 5.6 | 3.7 | |
| Strongyloides stercoralis | 11 (13.1%) | 4 (4.7%) | 0.063 |
| Hookworm | 11 (13.1%) | 1 (1.2%) | 0.002 * |
| Rodentolepis nana | 20 (23.8%) | 19 (22.4%) | 0.857 |
| Trichuris trichiura | 57 (67.9%) | 41 (48.2%) | 0.012 * |
| Method | +ve/n (%) |
|---|---|
| Direct smear | 4/85 (4.7%) |
| Culture and formalin sedimentation | 5/77 (6.5%) |
| S. stercoralis 18S rDNA PCR | 6/83 (7.2%) |
| Serology on dried blood spots | 25/154 (16.2%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holt, D.C.; Shield, J.; Harris, T.M.; Mounsey, K.E.; Aland, K.; McCarthy, J.S.; Currie, B.J.; Kearns, T.M. Soil-Transmitted Helminths in Children in a Remote Aboriginal Community in the Northern Territory: Hookworm is Rare but Strongyloides stercoralis and Trichuris trichiura Persist. Trop. Med. Infect. Dis. 2017, 2, 51. https://doi.org/10.3390/tropicalmed2040051
Holt DC, Shield J, Harris TM, Mounsey KE, Aland K, McCarthy JS, Currie BJ, Kearns TM. Soil-Transmitted Helminths in Children in a Remote Aboriginal Community in the Northern Territory: Hookworm is Rare but Strongyloides stercoralis and Trichuris trichiura Persist. Tropical Medicine and Infectious Disease. 2017; 2(4):51. https://doi.org/10.3390/tropicalmed2040051
Chicago/Turabian StyleHolt, Deborah C., Jennifer Shield, Tegan M. Harris, Kate E. Mounsey, Kieran Aland, James S. McCarthy, Bart J. Currie, and Therese M. Kearns. 2017. "Soil-Transmitted Helminths in Children in a Remote Aboriginal Community in the Northern Territory: Hookworm is Rare but Strongyloides stercoralis and Trichuris trichiura Persist" Tropical Medicine and Infectious Disease 2, no. 4: 51. https://doi.org/10.3390/tropicalmed2040051





