Field Studies Evaluating Bait Acceptance and Handling by Dogs in Navajo Nation, USA
Abstract
:1. Introduction
2. Materials and Methods
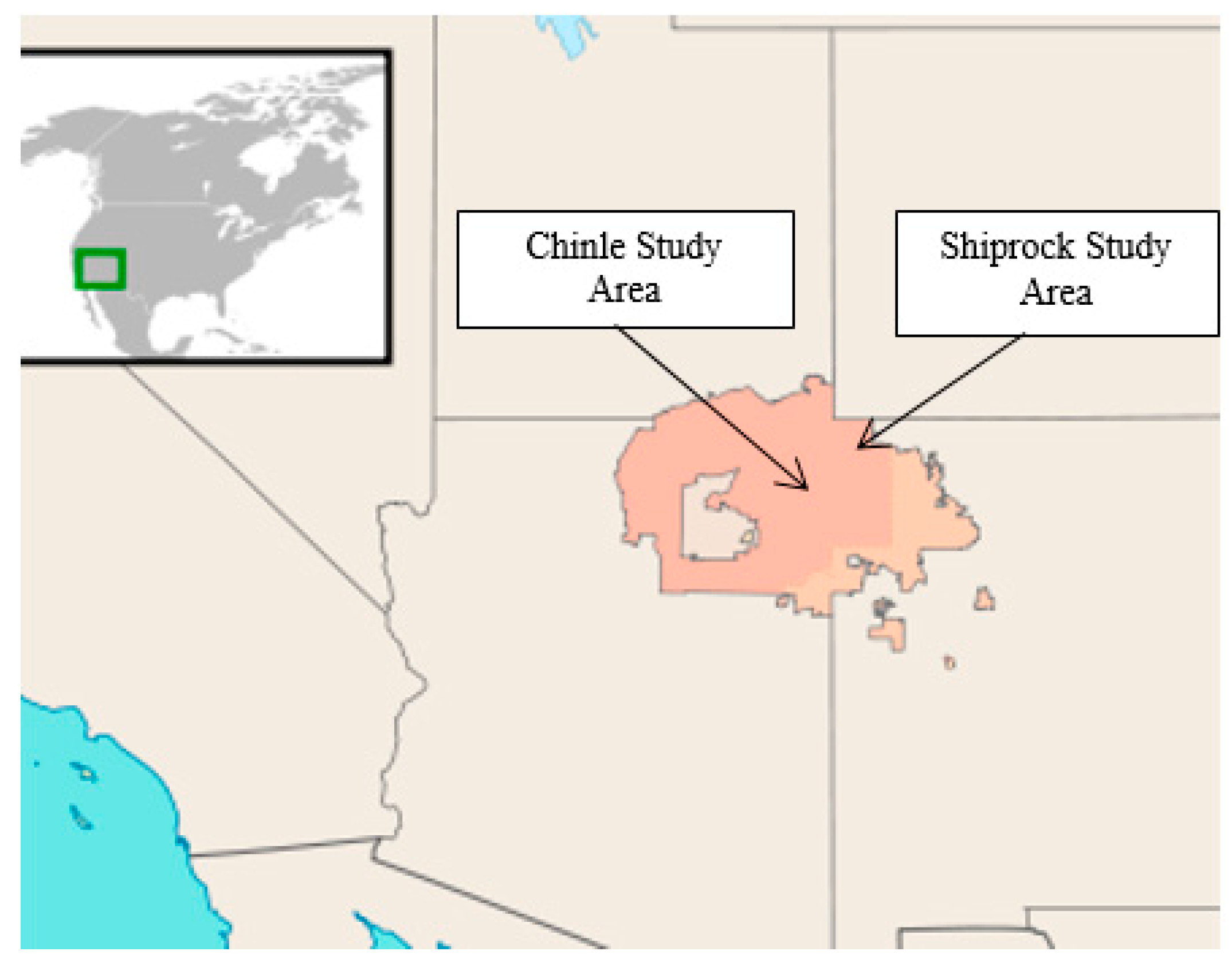
2.1. Study Area
2.2. Field Trial
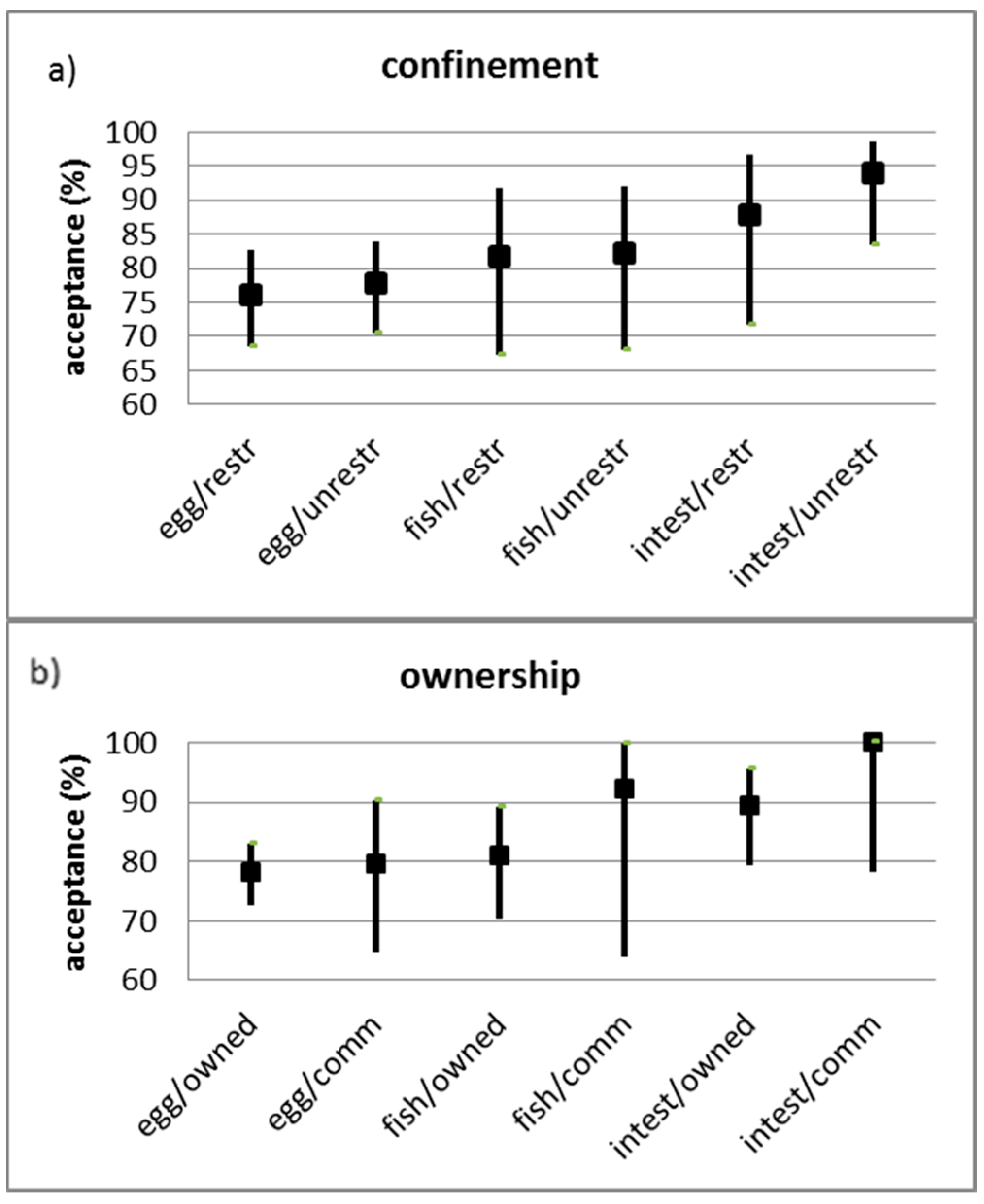
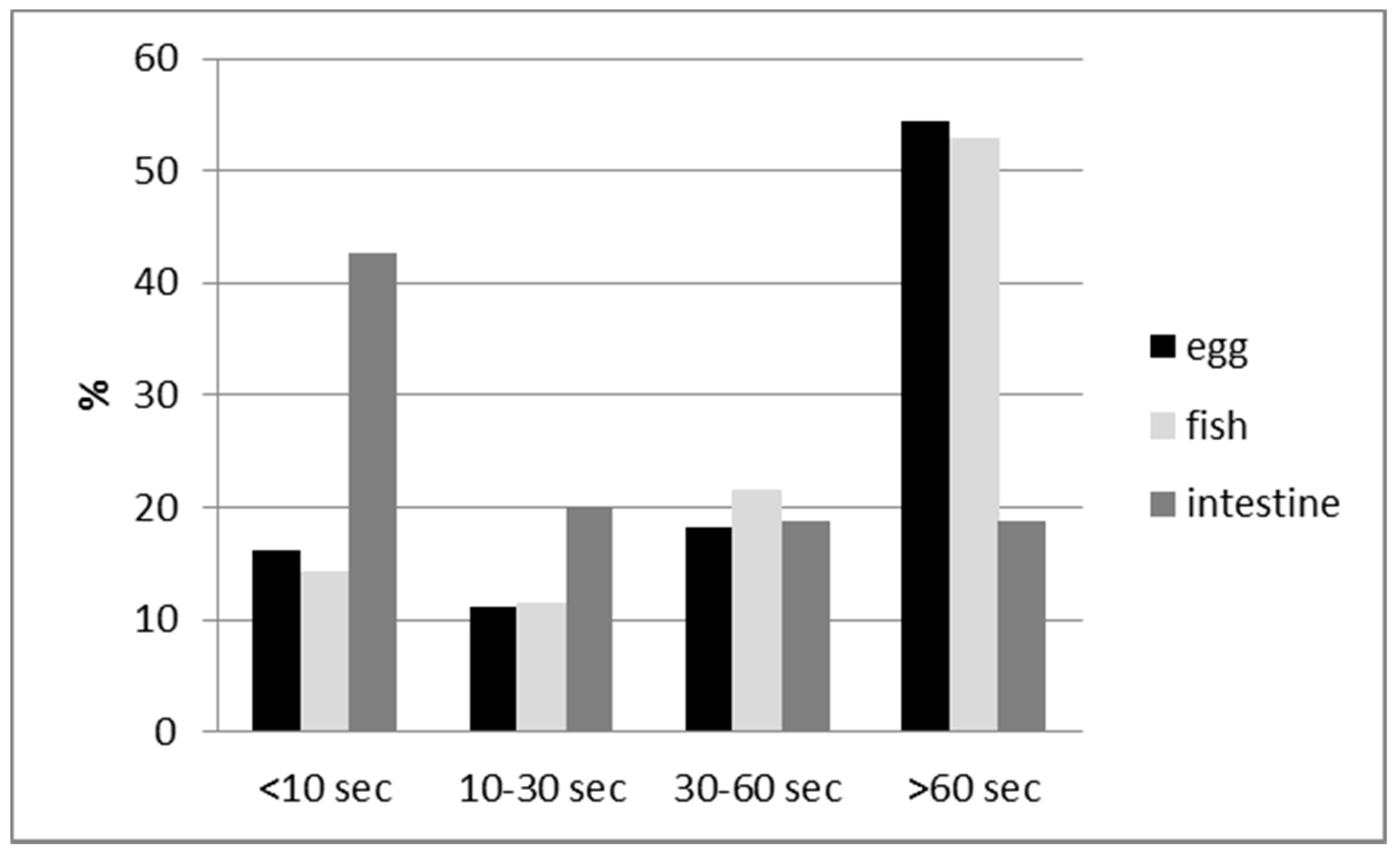
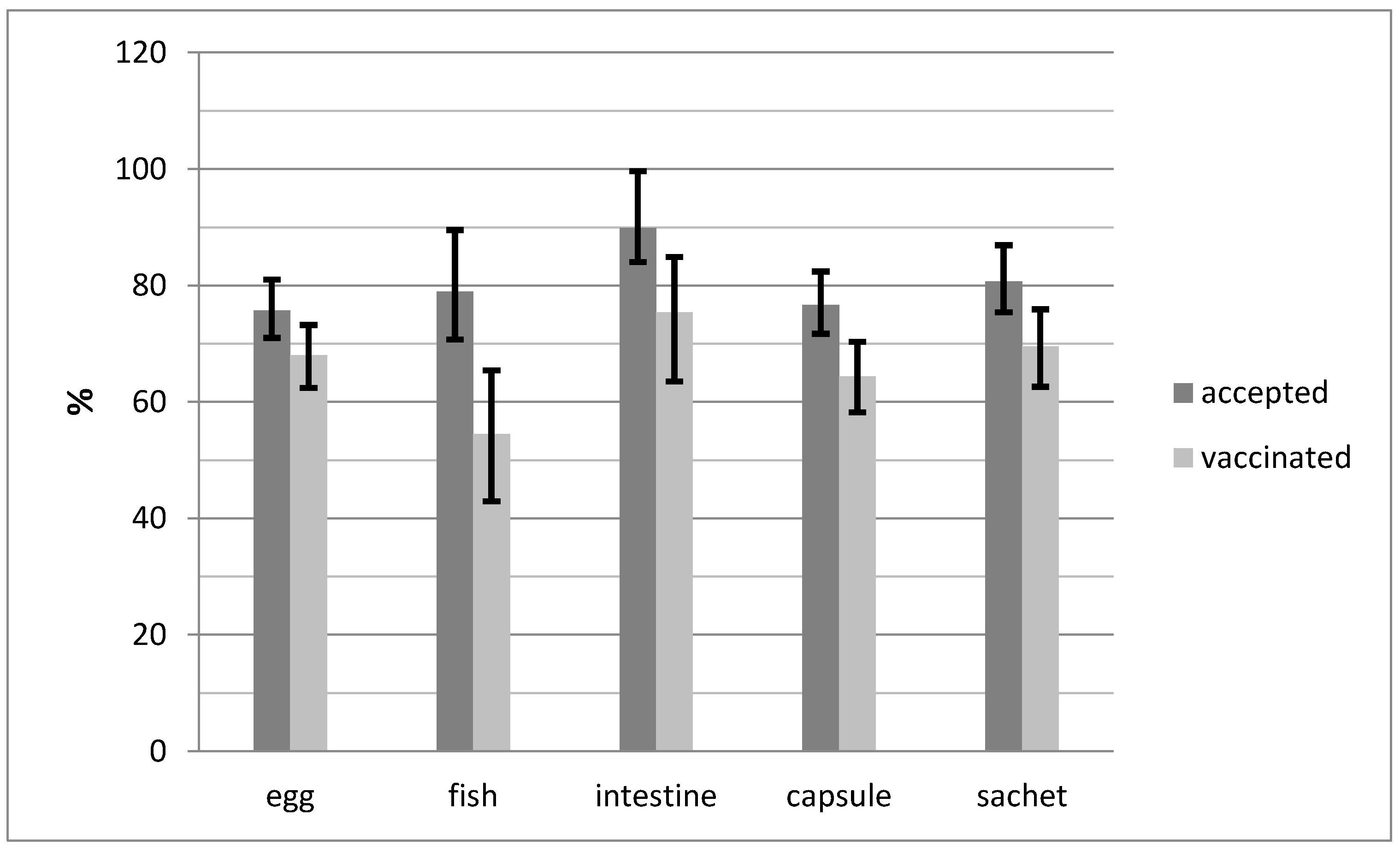
3. Results
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the Global Burden of Endemic Canine Rabies. PLoS Negl. Trop. Dis. 2015, 9, 1–20. [Google Scholar]
- WHO (World Health Organization); OIE (World Organisation for Animal Health). Global Elimination of Dog-Mediated Human Rabies. In Proceedings of the Report of the Rabies Global Conference, Geneva, Switzerland, 10–11 December 2015. [Google Scholar]
- Arief, R.A.; Hampson, K.; Jatikusumah, A.; Widyastuti, M.D.W.; Sunandar; Basri, C.; Putra, A.A.G.; Willyanto, I.; Estoepangestie, A.T.S.; Mardiana, I.W.; et al. Determinants of Vaccination Consequences for Rabies Control in Bali, Indonesia. Front. Vet. Sci. 2017, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Neyra, R.; Brown, J.; Borrini, K.; Arevalo, C.; Levy, M.Z.; Buttenheim, A.; Hunter, G.C.; Becerra, V.; Behrman, J.; Paz-Soldan, V.A. Barriers to Dog Rabies Vaccination during an Urban Rabies Outbreak: Qualitative Findings from Arequipa, Peru. PLoS Neglected Trop. Dis. 2017, 11, e0005460. [Google Scholar] [CrossRef] [PubMed]
- Schildecker, S.; Millien, M.; Blanton, J.D.; Boone, J.; Emery, A.; Ludder, F.; Fenelon, N.; Crowdis, K.; Destine, A.; Etheart, M.; et al. Dog Ecology and Barriers to Canine Rabies Control in the Republic of Haiti, 2014–2015. Transbound. Emerg. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Matter, H.C. Oral Immunization of Dogs: Analysis of Dog Populations and Bait Delivery Systems. In Rabies Control in Asia; Dodet, B., Meslin, F., Eds.; Elsevier: Paris, France, 1997; pp. 47–59. [Google Scholar]
- Kaare, M.; Lembo, T.; Hampson, K.; Ernest, E.; Estes, A.; Mentzel, C.; Cleaveland, S. Rabies Control in Rural Africa: Evaluating Strategies for Effective Domestic Dog Vaccination. Vaccine 2009, 27, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Widyastuti, M.D.W.; Bardosh, K.L.; Sunandar; Basri, C.; Basuno, E.; Jatikusumah, A.; Arief, R.A.; Putra, A.A.G.; Rukmantara, A.; Estoepangestie, A.T.S.; et al. On Dogs, People, and a Rabies Epidemic: Results from a Sociocultural Study in Bali, Indonesia. Infect. Dis. Poverty 2015, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- WHO. Oral Vaccination of Dogs: Guidance for Research on Oral Rabies Vaccine and Field Application of Oral Vaccination of Dogs against Rabies; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- WHO. WHO Expert Consultation on Rabies; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- WHO. Report of WHO Consultation on Oral Immunization of Dogs against Rabies; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Rupprecht, C.E.; Barrett, J.; Briggs, D.; Cliquet, F.; Fooks, A.R.; Lumlertdacha, B.; Meslin, F.X.; Müler, T.; Nel, L.H.; Schneider, C.; et al. Can Rabies Be Eradicated? Dev. Biol. 2008, 131, 95–121. [Google Scholar]
- Schuster, P.; Gülsen, N.; Neubert, A.; Vos, A. Field Trials Evaluating Bait Uptake by an Urban Dog Population in Turkey. J. Etlik Vet. Microbiol. 1998, 9, 73–81. [Google Scholar]
- Yakobson, B.A.; King, R.; Sheichat, N.; Eventov, B.; David, D. Assessment of the Efficacy of Oral Vaccination of Livestock Guardian Dogs in the Framework of Oral Rabies Vaccination of Wild Canids in Israel. Dev. Biol. 2008, 131, 151–156. [Google Scholar]
- Darkaoui, S.; Boué, F.; Demerson, J.M.; Fassi Fihri, O.; Yahia, K.I.S.; Cliquet, F. First Trials of Oral Vaccination with Rabies SAG2 Dog Baits in Morocco. Clin. Exp. Vaccine Res. 2014, 3, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Bergman, D.; Bender, S.; Wenning, K.; Slate, D.; Rupprecht, C.; Heuser, C.; Delibertoi, T. Bait Acceptability for Delivery of Oral Rabies Vaccine to Free-Ranging Dogs on the Navajo and Hopi Nations. Dev. Biol. 2008, 131, 145–150. [Google Scholar]
- Frontini, M.G.; Fishbein, D.B.; Garza Ramos, J.; Flores Collins, E.; Balderas Torres, J.M.; Quiroz Huerta, G.; Gamez Rodriguez, J.J.; Belotto, A.J.; Dobbins, J.G.; Linhart, S.B.; et al. A Field Evaluation in Mexico of Four Baits for Oral Rabies Vaccination of Dogs. Am. J. Trop. Med. Hyg. 1992, 47, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Kharmachi, H.; Haddad, N.; Matter, H. Tests of Four Baits for Oral Vaccination of Dogs against Rabies in Tunisia. Vet. Rec. 1992, 130, 494. [Google Scholar] [CrossRef] [PubMed]
- Matter, H.C.; Kharmachi, H.; Haddad, N.; Youssef, S.B.; Sghaier, C.; Khelifa, R.B.; Jemli, J.; Mrabet, L.; Meslin, F.X.; Wandeler, A.I. Test of Three Bait Types for Oral Immunization of Dogs against Rabies in Tunisia. Am. J. Trop. Med. Hyg. 1995, 52, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Linhart, S.B.; Kappeler, A.; Windberg, L.A. A Review of Baits and Bait Delivery Systems for Free-Ranging Carnivores and Ungulates. In Contraception in Wildlife: Animal and Plant Health Inspection Service; United States Department of Agriculture: Oakland, CA, USA, 1993; pp. 69–132. [Google Scholar]
- Estrada, R.; Vos, A.; De Leon, R. Acceptability of Local-Made Baits for Oral Vaccination of Dogs against Rabies in the Philippines. BMC Infect. Dis. 2001, 1, 5. [Google Scholar] [CrossRef]
- Corn, J.L.; Méndez, J.R.; Catalán, E.E. Evaluation of Baits for Delivery of Oral Rabies Vaccine to Dogs in Guatemala. Am. J. Trop. Med. Hyg. 2003, 69, 155–158. [Google Scholar] [PubMed]
- Bishop, G.C. Increasing Dog Vaccination Coverage in South Africa: Is Oral Vaccination the Answer? In Rabies Control in Asia; Dodet, B., Meslin, F., Eds.; John Libbey Eurotext: Paris, France, 2001; pp. 105–109. [Google Scholar]
- Berentsen, A.R.; Bender, S.; Bender, P.; Bergman, D.; Hausig, K.; VerCauteren, K.C. Preference among 7 Bait Flavors Delivered to Domestic Dogs in Arizona: Implications for Oral Rabies Vaccination on the Navajo Nation. J. Vet. Behav. Clin. Appl. Res. 2014, 9, 169–171. [Google Scholar] [CrossRef]
- Berentsen, A.R.; Bender, S.; Bender, P.; Bergman, D.; Gilbert, A.T.; Rowland, H.M.; VerCauteren, K.C. Bait Flavor Preference and Immunogenicity of ONRAB Baits in Domestic Dogs on the Navajo Nation, Arizona. J. Vet. Behav. 2016, 15, 20–24. [Google Scholar] [CrossRef]
- Gräßer, N. Beitrag Zur Entwicklung Eines Köders Für Wölfe (Canis Lupus) Zur Oralen Vakzination Gegen Tollwut; University of Veterinary Medicine: Hannover, Germany, 2016. [Google Scholar]
- Aylan, O.; Vos, A. Efficacy of Oral Rabies Vaccine Baits in Indigenous Turkish Dogs. Rev. Infect. Dis. 2000, 2, 74–77. [Google Scholar]
- Linhart, S.B. Bait Formulation and Distribution for Oral Rabies Vaccination of Domestic Dogs: An Overview. Onderstepoort J. Vet. Res. 1993, 60, 479–490. [Google Scholar] [PubMed]
- Harischindra, P.A.L. Dog Vaccination Coverage and Oral Rabies Vaccination in Sri Lanka, an Update. In Rabies Control in Asia; Dodet, B., Meslin, F.X., Eds.; John Libbey Eurotext: Paris, France, 2001; pp. 97–100. [Google Scholar]
- Perry, B.D.; Johnston, D.H.; Jenkins, S.R.; Foggin, C.M.; Garner, N.; Brooks, R.; Bleakley, J. Studies on the Delivery of Oral Rabies Vaccines to Wildlife and Dog Populations. Acta Vet. Scand. 1988, 84, 303–305. [Google Scholar]
- Cliquet, F.; Gurbuxani, J.P.; Pradhan, H.K.; Pattnaik, B.; Patil, S.S.; Regnault, A.; Begouen, H.; Guiot, A.L.; Sood, R.; Mahl, P.; et al. The Safety and Efficacy of the Oral Rabies Vaccine SAG2 in Indian Stray Dogs. Vaccine 2007, 25, 3409–3418. [Google Scholar] [CrossRef] [PubMed]
- Nokireki, T.; Nevalainen, M.; Sihvonen, L.; Gadd, T. Adverse Reactions from Consumption of Oral Rabies Vaccine Baits in Dogs in Finland. Acta Vet. Scand. 2016, 58, 1–4. [Google Scholar] [CrossRef]


| Material | Size (cm) | Weight (gr) | |
|---|---|---|---|
| Bait | |||
| Intestine | boiled local cow intestine sections | 8–12 cm long | 20–30 |
| Fishmeal | vegetable fats + fishmeal | 8.5 × 4.0 × 1.2 | 43 |
| Egg | gelatin + egg powder | 8.5 × 4.0 × 1.2 | 43 |
| Blister | |||
| Capsule | PVC + aluminum foil | 6.5 × 3.0 × 0.7 | 3.5 mL |
| Sachet | Biodegradable foil | 5.0 × 3.0 × 0.4 | 3.5 mL |
| Bait Accepted | Bait Consumed Completely | Blister Swallowed | Blister Perforated | ‘Vaccinated’ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bait | Blister | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | N | % | 95% CI | N | % | 95% CI |
| egg | capsule | 140 | 74.5 | 69.3–82.1 | 106 | 86.9 | 79.6–92.3 | 57 | 42.2 | 33.8–51.0 | 112 | 94.9 | 89.3–98.1 | 114 | 91.2 | 84.8–95.5 |
| sachet | 110 | 81.5 | 73.9–87.6 | 90 | 90.0 | 82.4–95.1 | 82 | 80.4 | 71.4–87.6 | 90 | 94.7 | 88.1–98.3 | 90 | 88.2 | 80.4–93.8 | |
| fish | capsule | 40 | 87.0 | 73.7–95.1 | 34 | 87.2 | 72.6–95.7 | 6 | 15.8 | 6.0–31.3 | 29 | 78.4 | 61.8–90.2 | 20 | 55.6 | 38.1–72.1 |
| sachet | 33 | 75.0 | 59.7–86.8 | 28 | 90.3 | 74.2–98.0 | 20 | 66.7 | 47.2–82.7 | 22 | 88.0 | 68.8–97.5 | 24 | 85.7 | 67.3–96.0 | |
| intestine | capsule | 39 | 88.6 | 75.4–96.2 | 34 | 97.1 | 75.2–97.1 | 27 | 73.0 | 55.9–86.2 | 28 | 96.6 | 82.2–99.9 | 29 | 87.9 | 71.8–96.6 |
| sachet | 40 | 95.2 | 83.8–99.4 | 37 | 97.4 | 86.2–99.9 | 37 | 94.9 | 82.7–99.4 | 19 | 76.0 | 54.9–90.6 | 23 | 79.3 | 60.3–92.0 | |
| Total | 402 | 80.6 | 76.8–83.9 | 329 | 90.1 | 86.6–93.0 | 229 | 60.3 | 55.0–65.1 | 300 | 91.2 | 87.6–94.0 | 300 | 85.0 | 80.8–88.5 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, S.; Bergman, D.; Vos, A.; Martin, A.; Chipman, R. Field Studies Evaluating Bait Acceptance and Handling by Dogs in Navajo Nation, USA. Trop. Med. Infect. Dis. 2017, 2, 17. https://doi.org/10.3390/tropicalmed2020017
Bender S, Bergman D, Vos A, Martin A, Chipman R. Field Studies Evaluating Bait Acceptance and Handling by Dogs in Navajo Nation, USA. Tropical Medicine and Infectious Disease. 2017; 2(2):17. https://doi.org/10.3390/tropicalmed2020017
Chicago/Turabian StyleBender, Scott, David Bergman, Adrian Vos, Ashlee Martin, and Richard Chipman. 2017. "Field Studies Evaluating Bait Acceptance and Handling by Dogs in Navajo Nation, USA" Tropical Medicine and Infectious Disease 2, no. 2: 17. https://doi.org/10.3390/tropicalmed2020017






