1. Introduction
Basic concrete generally consists of five basic ingredients: rock, sand, Portland cement, water, and air. By far, the most expensive and environmentally costly material in this system is the cement. During the production of cement clinker, CaCO
3 (calcium carbonate) is converted into calcium oxide by means of calcination [
1]. The second product, CO
2 (carbon dioxide), is then emitted. A simplified chemical equation is given as Equation (1).
The Intergovernmental Panel on Climate Change (IPCC) states that cement production is the largest emitter of CO
2 in the industrial sector [
2]. Worldwide, it is estimated that for every ton of cement produced approximately 0.82 tons of carbon dioxide are released into the atmosphere [
3]. The significance is that approximately 5% of the world’s anthropogenic carbon emissions can be traced to the production of cement [
4]. However, it is because it is both financially and environmentally costly that reducing the proportion of cement in concrete mixtures has gained popularity for decades. To this end, supplementary cementitious materials (SCMs) have played a large role in reducing the amount of cement needed to meet required concrete quality. When properly specified, SCMs can benefit a concrete mixture in five possible ways:
The inclusion of supplementary cementitious materials (SCMs) has been ubiquitous in North America since the 1970s because of their tendency to reduce cost, enhance concrete performance, and put waste material to reuse [
5]. Until recently, a majority of the ready-mix concrete in the United States utilized three SCMs exclusively: fly ash (Class C or F), slag cement, and silica fume. All three of these SCMs are byproducts of other industries and have enjoyed widespread use as partial replacements of the cement in concrete mixtures.
Metakaolin, however, did not enjoy the same level of popularity initially. Literature on the use of metakaolin as a SCM began to proliferate starting in the early 1990s [
6,
7]. Before then, metakaolin was predominantly known for its use in ceramics, paints, and paper manufacturing industries [
8].
The paint and paper industries utilize the color and plate-like morphology (
Figure 1) of the metakaolin in coating applications [
9], while ceramics benefit from metakaolin’s pozzolanic activity [
10]. Today, the literature is abundant with the benefits of using metakaolin as a SCM in concrete mixtures. Furthermore, the production of metakaolin, compared to Portland cement, requires much lower calcining temperature and emits less CO
2 than Portland cement [
10].
Metakaolin is an aluminous and siliceous natural pozzolan that conforms to ASTM C618, and is the product of thermally or mechanically activating kaolin clay. Typically, kaolin clay that is used in the production of metakaolin contains a high percentage (40–70%) of the clay mineral kaolinite, Al
2Si
2O
5(OH)
4 [
11]. In its dehydroxylated form, metakaolin can be used in concrete to enhance mechanical properties and increase durability [
12,
13,
14,
15,
16], while also improving the aesthetic appeal with its white color. The consensus within the literature is that metakaolin contributes to concrete performance in three ways [
17,
18]. The first is the filler effect. As with silica fume, the fineness of metakaolin densifies the paste matrix. The second is the acceleration of the cement hydration, which is a result of fine metakaolin providing additional surfaces for the nucleation of hydrates [
19]. Lastly, the pozzolanic reactivity of the metakaolin contributes additional structural hydrates (C-S-H, C
2ASH
8, C
4AH
13, and C
3AH
6) to the paste matrix [
20].
The high early-strengths reported in the literature [
14,
15,
21,
22] make metakaolins ideal for prestressing and other high-early strength applications. The most common SCM used for this purpose is silica fume, and metakaolin has shown itself to be comparable to silica fume in ability to achieve high early-strength in concrete [
13,
15]. The aesthetic difference here being that silica fume tends to make the concrete darker with its gray color.
1.1. Background
Currently in the U.S., metakaolin as a Class N pozzolan is permissible for use by 16 state departments of transportation (DOT), although most do not specify approved metakaolin products. The three metakaolin products (MK1, MK2, and MK3) shown in
Table 1 are discussed later.
Although any calcined kaolin can be classified as a metakaolin, the use of metakaolin has typically been restricted to those that can be classified as high-reactivity metakaolins (HRMs). HRMs are frequently defined as a metakaolin that has a total of 90% or greater Al
2O
3 + SiO
2 + Fe
2O
3. Furthermore, unlike other SCMs, metakaolin is not a byproduct of another industry. This status as a primary product ensures that it can be produced with a specific use in mind, and benefit from strict quality control. ASTM C618 requires that the Al
2O
3 + SiO
2 + Fe
2O
3 content of a Class N pozzolan is greater than 70%, and thus it is rarely beneficial to produce a metakaolin that is not a HRM. Therefore, in the U.S. market, most commercially-available metakaolins are HRMs. Some states have already included the metakaolin products used in this study in their approved product lists (
Table 1). As is typical in all concrete mixtures, concrete incorporating metakaolin varies widely in its performance based on a great number of factors including the water-to-cementitious material (w/cm) ratio, cement content and composition, SCM content and composition, aggregate content and size, and mixing and curing conditions.
The result is that the performance of metakaolin in concrete systems is usually contextualized by the metakaolin’s material fineness and oxide composition [
14]. While these two factors are likely the best indicators of performance, the wide range of metakaolin products and mixture designs makes a practical and informed usage of metakaolin based on the literature difficult. Furthermore, little to no research has been conducted comparing commercially-available metakaolin products in the U.S. from different vendors, possibly to guide the practical use of metakaolin in concrete mixtures.
1.2. Objective
This research aims to investigate the variations in performance of concretes incorporating seemingly similar commercially-available metakaolins. To the best of the authors’ knowledge, the comparison of competing metakaolins, that are commercially-available in the U.S. market, has not yet been studied in the literature. Concrete mixtures containing a typical w/cm ratio, cement content, and coarse aggregate content are batched so that the metakaolins can be directly compared to each other at three separate cement-weight replacement levels (10%, 15%, and 20%). Each of these mixtures is tested for mechanical properties, durability, and dimensional stability. The replacement levels presented are chosen such that they contain the optimums for mechanical strengths that are prevalent in the literature (10–20%). In addition, the range was chosen to include higher replacements because durability tends to increase systematically with replacement level [
12,
23,
24,
25,
26,
27]. This concept of an ‘optimum’ replacement level is evoked because of the need to maximize performance and minimize cost. In addition to the hardened properties, some fresh-state properties are measured on concrete mixtures. HRMs are widely known to cause workability issues [
12,
28,
29], hence fresh properties of these mixtures are crucial in informing the usage of metakaolin in U.S. ready-mixed concrete.
This research attempts to address the following research questions:
For each metakaolin product, what is the effect of replacement level on fresh and hardened concrete performance?
Are there significant differences in effect on concrete performance between metakaolin products? If so, how large is the variation, and what are the primary contributors to the change in concrete properties?
Can general guidelines be given for the inclusion of commercially-available metakaolin in concrete?
2. Materials and Methods
2.1. Materials
A type I/II cement was used for all mixtures, for which a portion was replaced by a commercially-available metakaolin product. Three metakaolin products produced by different companies were chosen. The first and third products are sourced from Georgia (processing and calcination performed by different vendors). The second product is sourced from South Carolina. Selected physical and chemical properties for all of the cementing materials are provided in
Table 2. They are obtained from the manufacturers. The coarse aggregate is a standard size #57 (NMAS = 25 mm) graded granite stone sourced from Athens, Georgia. The fine aggregate is an alluvial river sand sourced from Watkinsville, Georgia and has shown no signs of deterioration from alkali reactivity when used for concrete projects. The gradations and composition of both meet ASTM C33 requirements. In addition, a superplasticizer has been included in each mixture to ensure adequate workability. The superplasticizer is a polycarboxylate ether (PCE) based plasticizer with 33% solids, and conforms to ASTM C494 Type A and F requirements. All material data for the chemical and mineral admixtures were provided by the material vendors.
2.2. Mixture Design
The concrete mixtures used in this study are designed such that the total cementitious content would equal 422 kg/m
3. Each metakaolin product replaced cement at rates equaling 10%, 15%, and 20% by weight. The coarse aggregate content of the mixtures was maintained at a constant 985 kg/m
3, and the sand contents slightly varied by mixture to attain an equal volume between mixtures in accordance with ACI 211 [
29]. A w/cm ratio of 0.43 was used for all mixtures. Finally, the mixtures were designed with an air content of 4%. As previously mentioned, a PCE-based superplasticizer was used to retain workability of the mixtures, and the dosage needed in each mixture was determined upon mixing. Saturated surface dry mixture proportions can be found in
Table 3. Batch quantities were such that the specimens were manufactured using the same materials to successfully conduct the following tests: compression, split-cylinder tension, modulus of rupture (MOR), dynamic modulus of elasticity (MOE), rapid chloride-ion penetrability test (RCPT), and the coefficient of thermal expansion (CTE).
Tests for sulfate resistance and alkali–silica reactivity (ASR) were performed on mortar specimens according to ASTM testing methods (C1012 and C1567, respectively). These testing standards require specific mixture proportions.
Sulfate specimens required 1:2.75 ratio of cementitious material to sand content, and a w/cm equal to 0.485. As with the concrete mixtures, the metakaolins replaced percentages of the cement by weight. In addition, a superplasticizer was used to retain a consistent workability between mixtures. This addition is a deviation from ASTM C1012. Currently, the standard specifies that the w/cm be increased in view of workability concerns, however, the superplasticizer was included because of the documented negative effects of increasing w/cm when using metakaolin [
30].
As per ASTM C1567, the batching of ASR specimens included 990 g of sand (saturated surface-dry condition), 440 g of total cementitious material, and a w/cm ratio equal to 0.43. As with the sulfate mixtures, a superplasticizer was used to retain a consistent workability across all mixtures.
2.3. Batching and Molding
Concretes were machine mixed using a portable revolving drum mixer with a 0.35 m3 capacity. Saturated surface dry (SSD) mixture proportions were altered according to the volumetric method to accommodate the moisture condition of the aggregates upon mixing, and then weighed using a scale with a 136 kg capacity and 0.05 kg accuracy. Materials were added and mixed per ASTM C192. The superplasticizer was added to the mixing water and thoroughly agitated before the water was added to the mixer. Contents of the mixer were then discharged into a wheelbarrow so that fresh concrete tests could be performed, and molds could be filled. Material from the same batched filled the specimen molds for all hardened concrete tests excluding sulfate and ASR.
Mortar mixtures were mixed using a portable table-top mixer with a nominal capacity of 4.8 L, and according to ASTM C305 mixing procedure. Mixture proportions were slightly altered to accommodate for non-SSD sand moisture contents at the time of mixing. The superplasticizer was added separately after the mixing water as the paddle rotated. The plastic mixture was then discharged into molds.
2.4. Experimental Plan
2.4.1. Fresh Properties
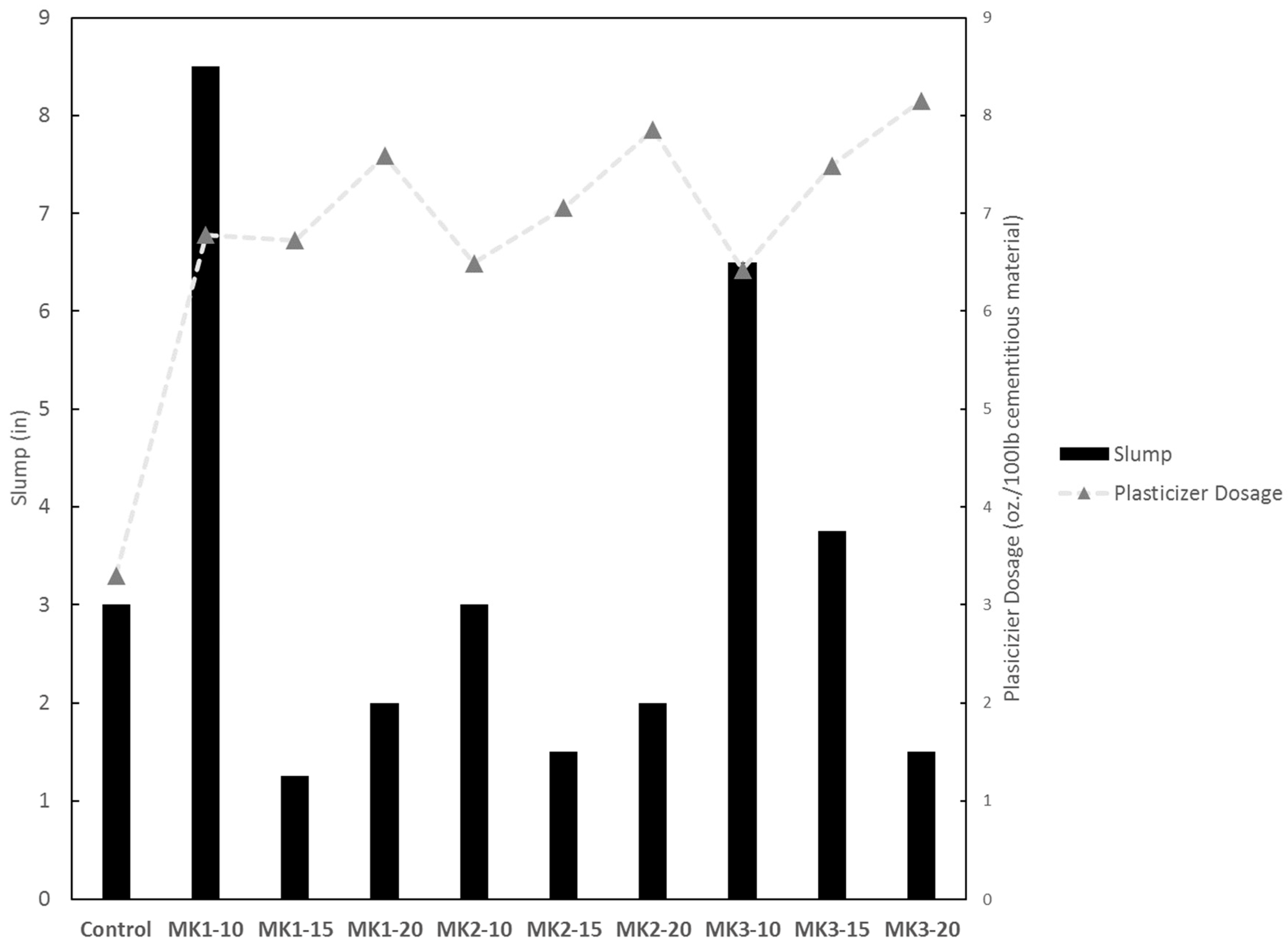
Concrete temperature, unit weight, air content, and slump were measured directly after batching. A thermometer was used to record the temperature per ASTM C1064. Unit weight and air content were measured as outlined in ASTM C138 and C231, respectively. Slump was measured in accordance with ASTM C143. Temperature and air content were measured to ensure concrete quality, and the unit weight was measured to ensure that metakaolin did not have a significant effect on concrete density. Slumps and superplasticizer dosages were measured to understand the extent of workability reduction that is associated with using metakaolin.
2.4.2. Mechanical Properties
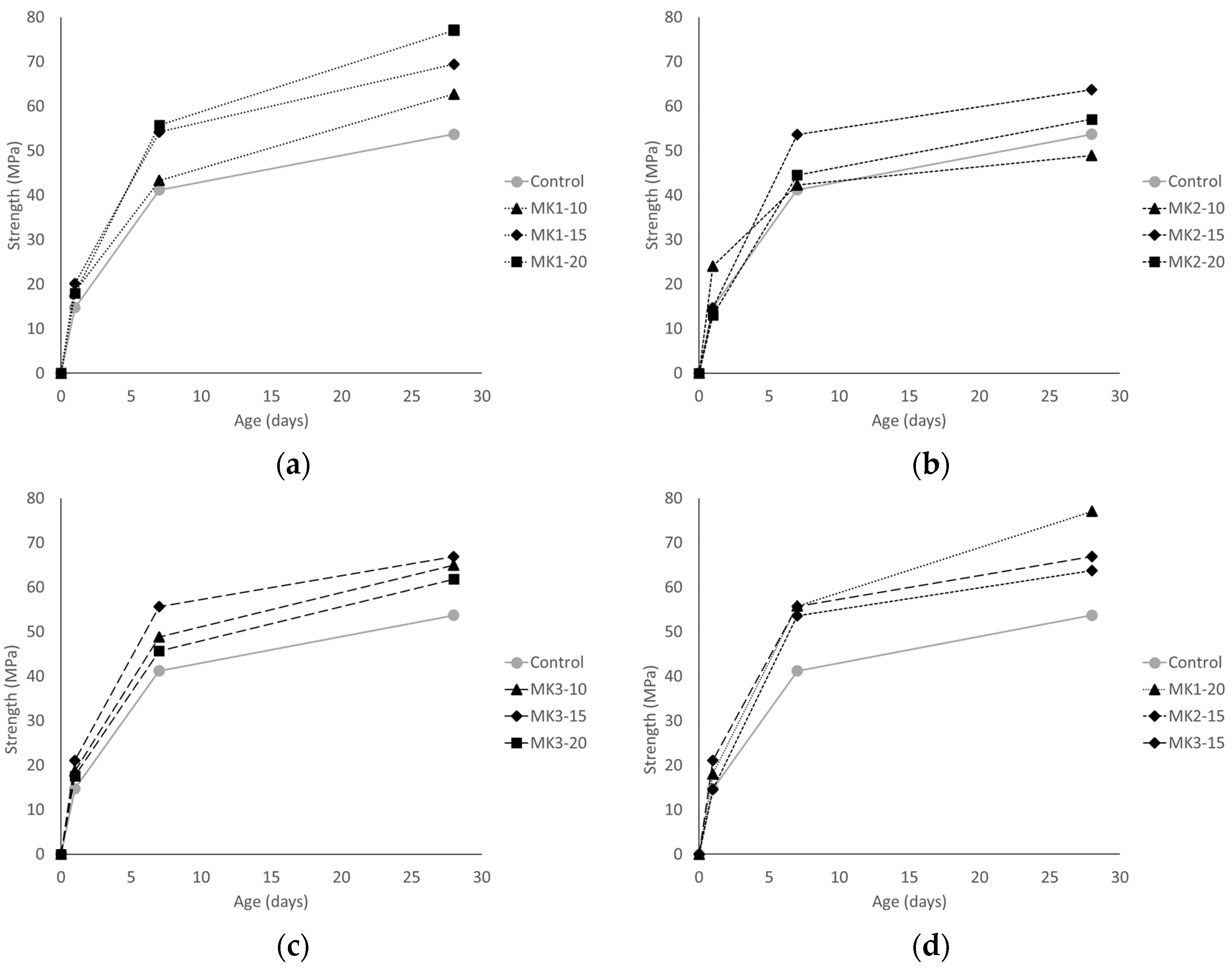
All specimens used in mechanical property testing were laboratory-molded in accordance with ASTM C192, and cured in a lime-saturated bath until the day of testing per ASTM C511. Nine 100 mm × 200 mm cylinders were made during each batching to test for compression: three each for 1-day, 7-day, and 28-day strengths. At the age of 1, 7, and 28 days, three cylinders from each mixture were broken in a universal testing machine with a capacity of 3.5 MN, and were constrained by neoprene caps. Cylinders were loaded at a rate of 2000 N/s until fracture per ASTM C39. The average strength of three specimens was taken as the compressive strength.
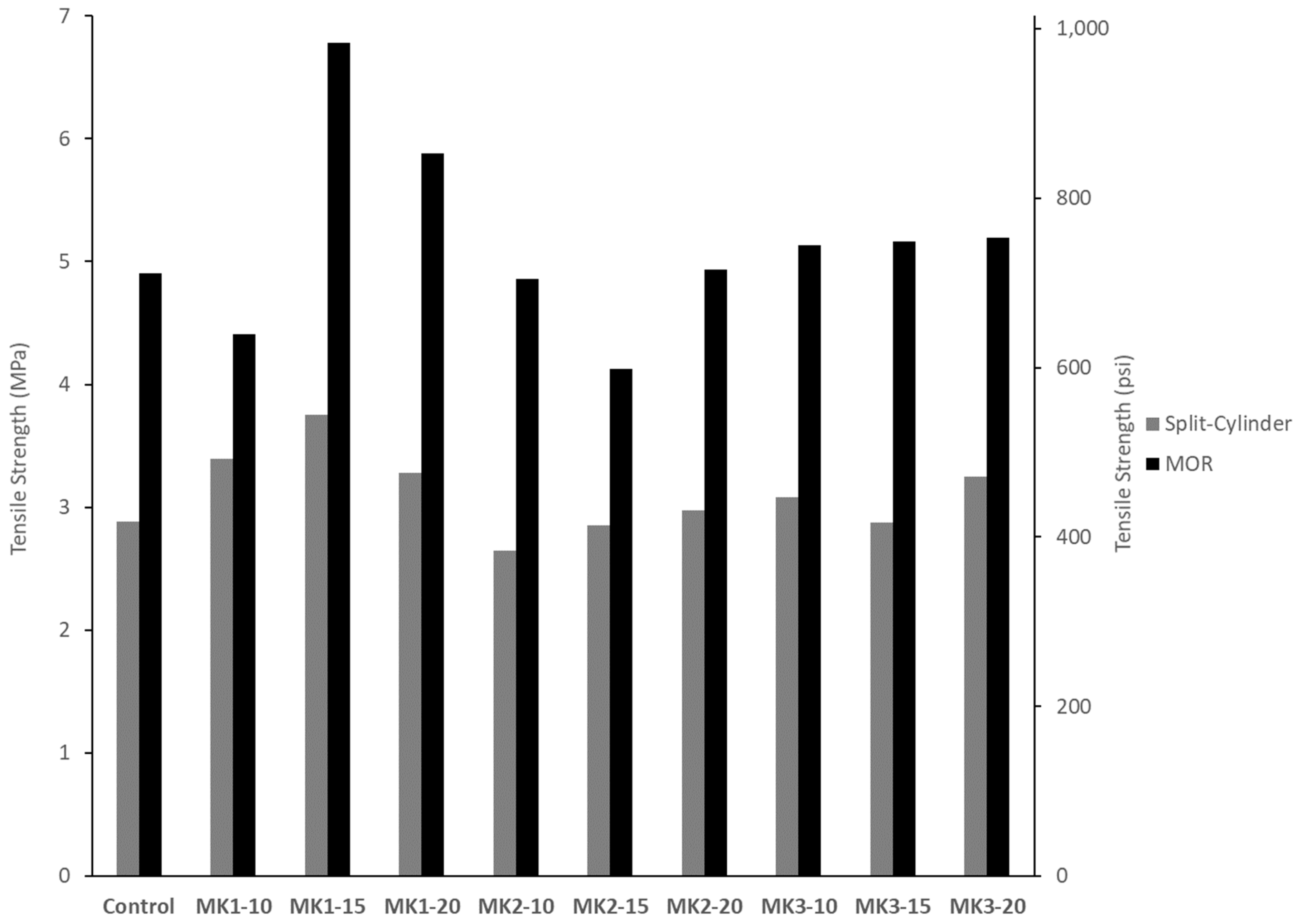
Two tests of tensile strength were performed. Split-cylinder tensile strength was tested using 100 mm × 200 mm cylinders, and in a procedure consistent with ASTM C496. Three specimens were broken at an age of 28 days, and the tensile strength was taken as the average. The loading rate was 130 N/s. The second test of tensile strength was the MOR. Three prisms of dimension 150 mm × 150 mm × 550 mm were broken by third-point loading (ASTM C78) at an age of 28 days, and the average breaking strength was taken as the MOR.
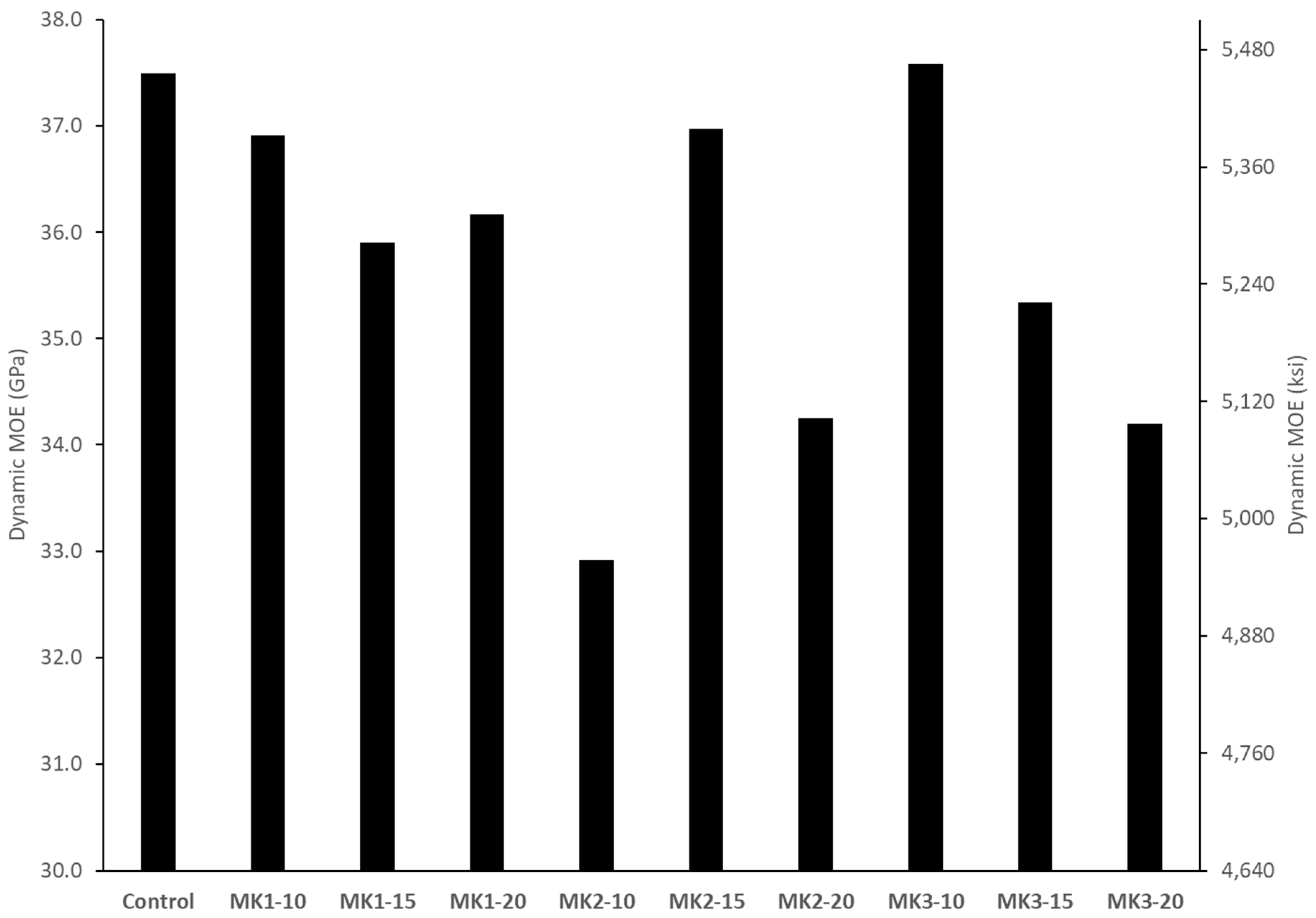
Tests to obtain the dynamic modulus of elasticity (E
d) were performed on 100 mm × 200 mm cylinders at an age of 28 days. The method used a longitudinal resonant frequency to calculate the dynamic modulus, which was obtained by the forced resonance method outlined in ASTM C215. The method for calculating E
d is provided in Equation. 2, where n′ = longitudinal frequency [Hz], M = mass of specimen [kg], and D = 5.093 × (length/diameter
2) [m
−1]. Dimensions for length and diameter were taken as the average of three measurements.
2.4.3. Durability
Three tests for durability were performed: one on concrete specimens, and two on mortar specimens. Concrete specimens were laboratory-molded in accordance with ASTM C192, and cured in a lime-saturated bath until the day of testing per ASTM C511.
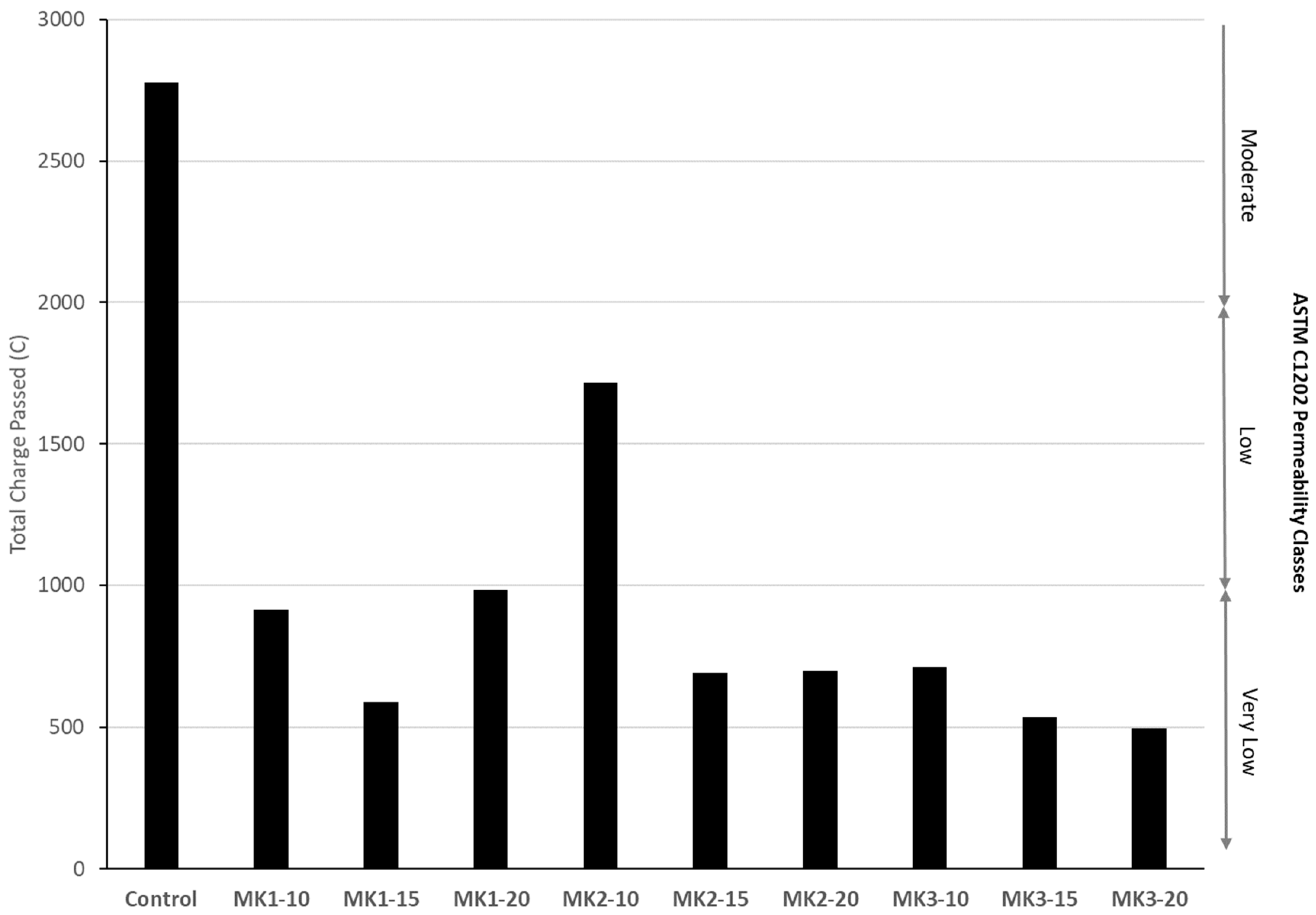
Permeability of concrete specimens were tested indirectly via rapid chloride-ion penetrability testing (RCPT) at an age of 28 days. Two 100 mm × 50 mm discs were cut from the top (most permeable section) of 100 mm × 200 mm cylinders by a diamond-tipped saw. RCPT was performed in accordance with ASTM C1202 for 6 h. The total charge, Coulombs (C), passed through each of the specimens was measured, and the average of the two was used as the measure of permeability.
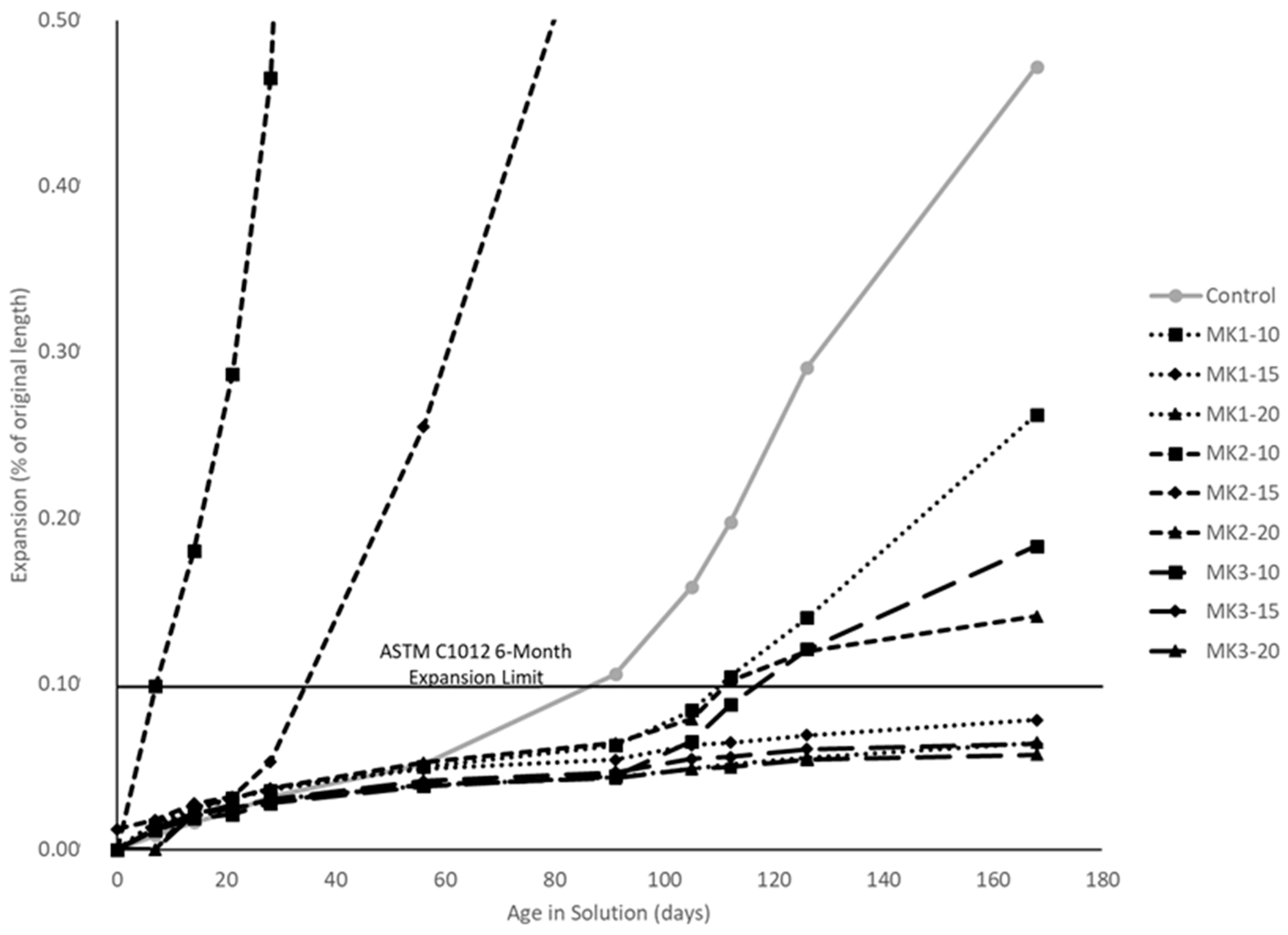
Resistance to sulfate attack was tested per ASTM C1012. Six mortar prisms of dimension 25 mm × 25 mm × 280 mm, and six mortar cubes of dimension 50 mm × 50 mm × 50 mm were batched and stored at 35 °C for 24 h. The specimens were then released, their lengths measured, and immersed in a lime saturated bath. The cubes were periodically broken per ASTM C109 until two cubes averaged above 20 MPa. Once the cubes tested above the required strength they were immersed in plastic containers with 0.75 l of aqueous Na2SO4 solution per mortar bar. The concentration of Na2SO4 was 40 g/L. Measurements were taken at ages of 7, 14, 21, 28, 56, 91, 105, 112, 126, and 168 days after first being immersed in solution. At each of the standard intervals (excluding 126 days), the solution was replaced. The expansion at any time was taken as the average of the six specimens. Expansion was measured as percentage length change. Any mixture with a percent expansion less than the standard limit of 0.01% at the end of the 6-month testing period was determined to effectively reduce the risk of sulfate attack.
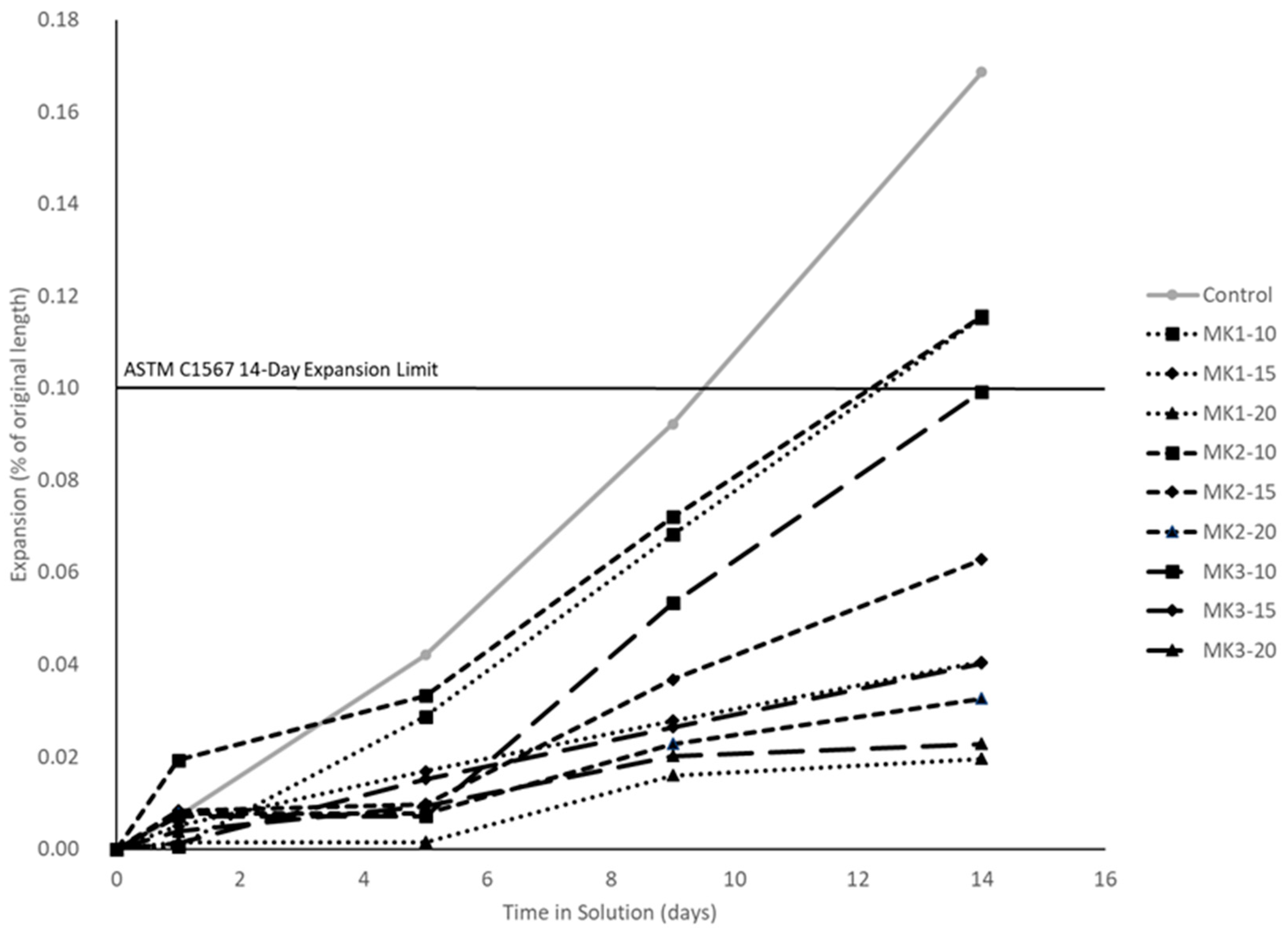
Resistance to ASR was determined by ASTM C1567. Three 25 mm × 25 mm × 280 mm mortar bars per mixture were batched, and burlap cured for 24 h where the ambient temperature was 23 °C. The bars were released from the molds at 24 h of age and immersed in 80 °C tap water for a period of 24 h. An initial comparator reading was then taken, and the specimens were immersed in an aqueous NaOH solution (40 g/L) at 80 °C for the remainder of the testing period. After this time, the specimens are measured with a length comparator and immersed in the NaOH solution for 14 days. Standard measurements were made at 1, 5, 9, and 14 days after immersion into solution. The expansion at any time was taken as the average of the three specimens. Any mixture with an expansion less than the expansion limit of 0.01% at the end of the 14-day testing period was the determined to effectively reduce the risk of ASR expansion.
2.4.4. Dimensional Stability
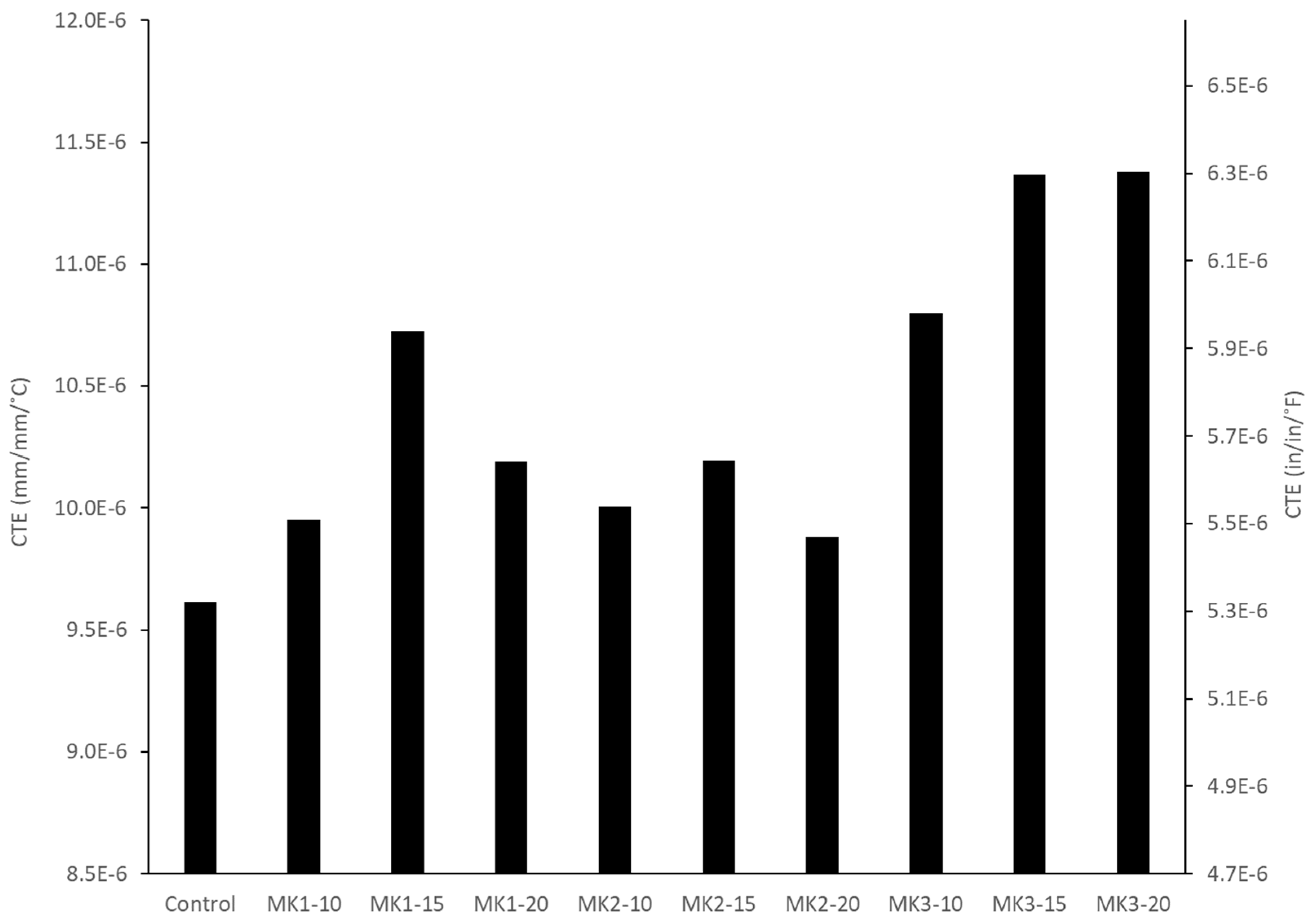
Testing for the coefficient of thermal expansion (CTE) of concretes was performed in accordance with AASHTO T336. Three 100 mm × 200 mm concrete cylinders were subjected to three heating and cooling cycles (10 °C to 50 °C) in a water bath, and the dimensional change was measured using a LVDT (sensitivity = 0.001 mm). Linear expansion was assumed, and the CTE (mm/mm/°C) was taken as the average thermal strain experienced over the 40 °C temperature gradient. The average of three specimens was taken as the CTE value for a given mixture.
Drying shrinkage was performed in accordance with ASTM C157. Three 100 mm × 100 mm × 280 mm rectangular prisms were cast at the time of batching, and cured in a lime saturated bath after being released from the molds at 24 h of age. At an age of 28 days, the prisms were removed from the bath and allowed to air dry on a shelf while being supported at either end by wooden blocks. The room containing the specimens maintained an ambient temperature of 22 °C and 50% relative humidity. Drying shrinkage measurements were taken at concrete ages of 32, 35, 42, 56, 84, 140, and 252 days using a length comparator. Length change as a percentage of the nominal gauge length (250 mm) were calculated and reported as the average percent change of the three prisms.
5. Conclusions
Metakaolin (MK) is a naturally-occurring pozzolan and is included as an ASTM C618 Class N Pozzolan. MK provides improved concrete performance in the form of decreased permeability, improved strength and durability, and reduced shrinkage [
32]. Low permeability and reduced shrinkage are two performance characteristics of metakaolin supplemented concrete that can prolong the service life of transportation infrastructure that is subjected to severe exposure conditions, although the increased coefficient of thermal expansion levels are observed and need further investigation. This paper concludes with the following key findings and answers to the research questions:
MK1 achieves an optimum compressive strength at a 20% replacement, tensile strength at a 15% replacement and, overall, measures of durability increase systematically with increasing replacement levels. MK2 achieves an optimum compressive strength at a 15% replacement, while tensile strengths are maximized at a 20% replacement. Measures of durability of MK2 concretes vary too greatly for its use to be recommended for increased durability. MK3 performance was the most consistent amongst the metakaolins. Optimum replacements for compressive strength and tensile strengths were 15% and 20%, respectively. All measures of durability increased systematically with increasing replacement levels.
MK2 was an obvious outlier in both mechanical and durability performance. Aside from this, MK1 performance greatly depends on the replacement level, while MK3 does not. Mechanical strengths varied at most 29% (MK1-20 and MK3-20 compression, by % of control) between MK1 and MK3. Measures of durability indicate that a replacement of 15% or higher by either MK1 or MK3 will be sufficient to ensure very low permeability and mitigate deleterious expansion resulting from ASR or sulfate attack.
All concrete mixtures incorporating metakaolin as a replacement of cement require a high level dosage of superplasticizer (≥4 mL per 100 kg of cementitious material), with higher dosage requirements at higher replacement levels. Increased mechanical strengths are seen at all replacement levels, and are generally optimized at a 15–20% replacement level. Resistance to chlorides and other chemicals is achieved by all well-performing metakaolins at a replacement level of 15% or higher.
It is also concluded that commercially available metakaolin products that conform to the threshold composition limit (Al2O3 + SiO2 + Fe2O3 ≥ 90%) could significantly vary in performance although the requirement ensures that the vast majority of the kaolin base material was kaolinite. Based on the findings of this study, a breakdown of oxides by weight and material fineness (11,000 m2/kg ≤ BET ≤ 14,200 m2/kg) is a typically good predictor of quality although the reactivity of any metakaolin product is dependent on the percentage of kaolinite that is dehydroxylated during calcination and product quality assurance.