Temporal Integration of Motion Streaks in Migraine
Abstract
:1. Introduction
2. Results
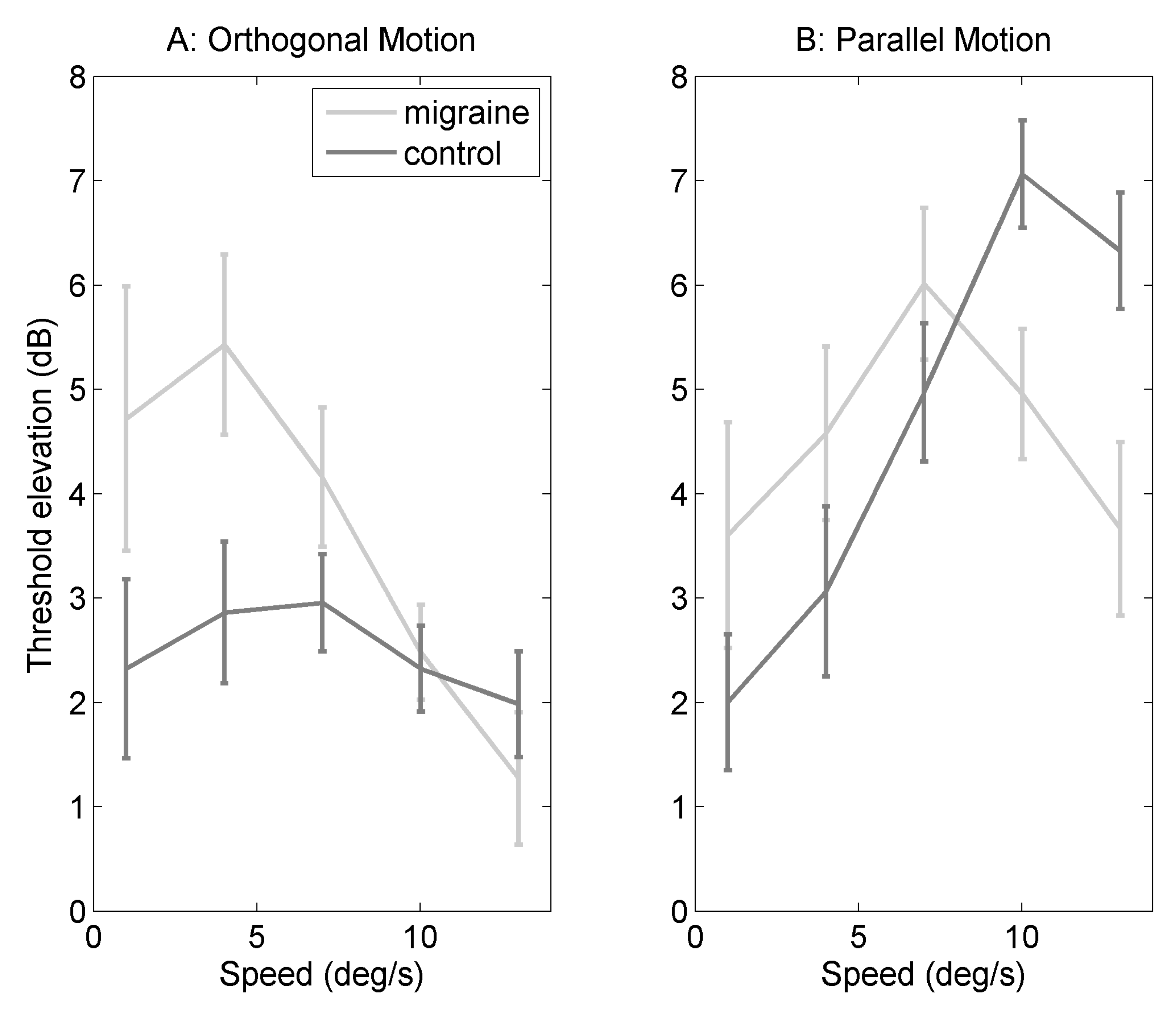
2.1. Orthogonal Motion
2.2. Parallel Motion
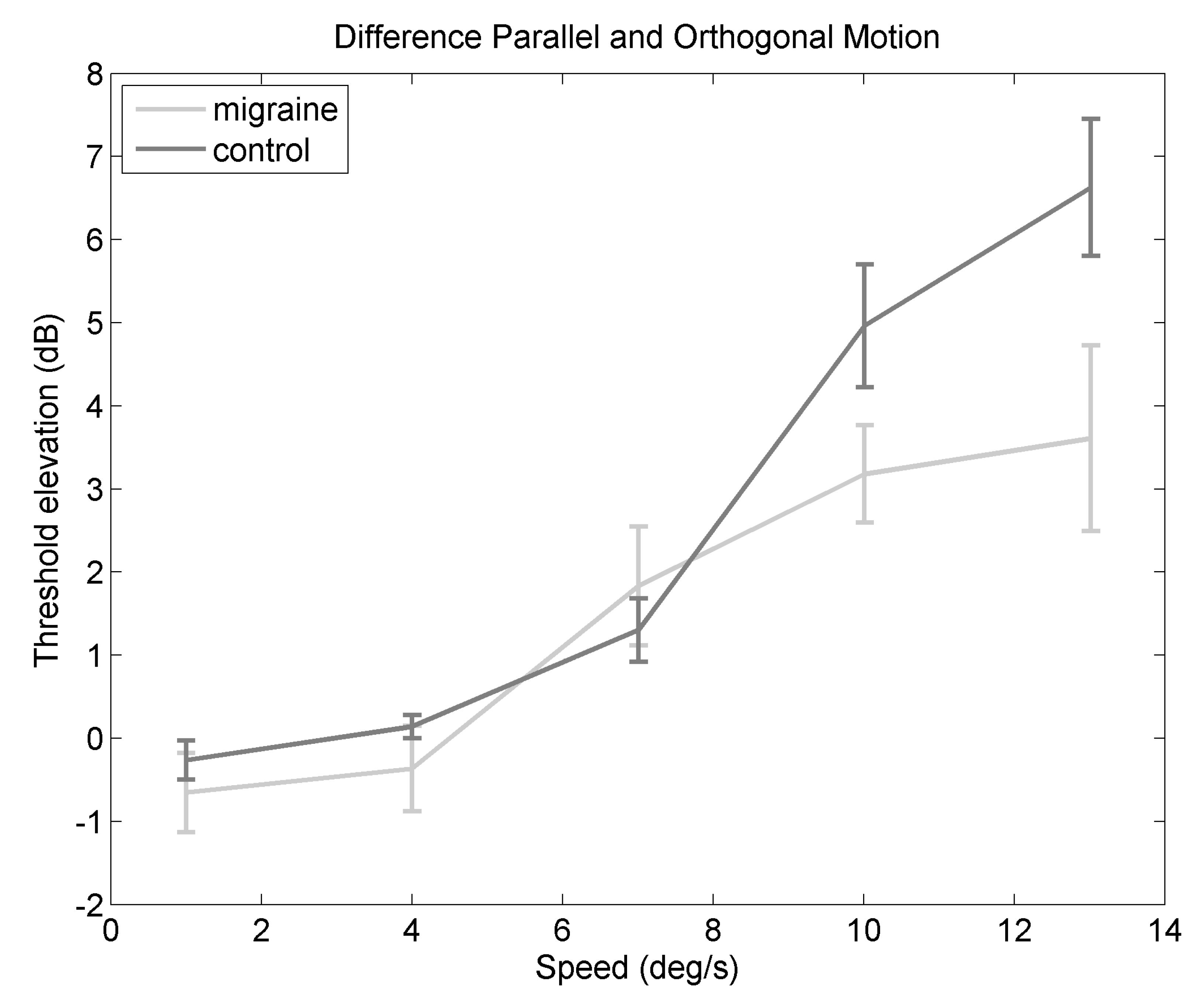
2.3. Difference in Threshold Elevation
2.4. Relationship to Headache Frequency
3. Discussion
3.1. Relationship to Migraine Characteristics
3.2. Temporal Integration Mechanisms
3.3. Limitations of the Current Study
4. Materials and Methods
4.1. Observers
4.2. Apparatus
4.3. Stimuli
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IHS | International Headache Society |
| M | migraine |
| MA | migraine with aura |
| MO | migraine without aura |
| tACS | transcranial alternating current stimulation |
Appendix A. Experiment 1
Appendix A.1. Method
Appendix A.1.1. Observers
| Gender | Age | Monthly Freq | Visual | Speech | Prof Diagnosis | Last Attack |
|---|---|---|---|---|---|---|
| F | 19 | 1–3 | no | no | M | 1 month |
| F | 18 | 10+ | yes | no | MA | 2 months |
| F | 19 | 0 | yes | no | M | 1 week |
| F | 19 | 1–3 | yes | no | MA | 1 week |
| F | 20 | 1–3 | yes | no | no | 2 weeks |
| F | 15 | 1–3 | yes | no | M | 1 week |
| F | 18 | <1 | yes | no | MA | 3 months |
| M | 19 | 3–10 | yes | no | no | 2 weeks |
| F | 19 | <1 | yes | no | no | 2 days |
| F | 20 | 0 | yes | no | MA | 2 weeks |
| F | 18 | 3–10 | no | no | no | 2 months |
| F | 32 | 1–3 | no | no | MO | 1 month |
| M | 24 | 1–3 | no | no | no | 1 week |
| F | 31 | <1 | yes | no | MA | 12 months |
| F | 27 | <1 | yes | yes | MA | 6 weeks |
Appendix A.1.2. Stimuli
Appendix A.2. Results

Appendix B. Experiment 2
Appendix B.1. Method
Appendix B.1.1. Observers
| Gender | Age | Monthly Freq | Visual | Speech | Prof Diagnosis | Last Attack |
|---|---|---|---|---|---|---|
| F | 21 | 3–10 | yes | no | M | 1 week |
| F | 18 | 3–10 | yes | no | M | 2 days |
| F | 19 | 1–3 | no | no | M | 5 days |
| F | 19 | 3–10 | yes | no | no | 3 days |
| F | 19 | <1 | no | no | no | 2 days |
| F | 20 | 1–3 | no | no | no | 2 weeks |
| F | 18 | 1–3 | no | no | no | 1 week |
| F | 20 | 3–10 | yes | no | M | 1 day |
| F | 19 | 1–3 | yes | no | MA | 1 month |
| M | 23 | 1–3 | yes | no | no | 2 weeks |
Appendix B.1.2. Stimuli
Appendix B.2. Results

Appendix C. Diagnosis of Migraine by a Medical Professional Only
| Gender | Age | Monthly Freq | Visual | Speech | Prof Diagnosis | Last Attack |
|---|---|---|---|---|---|---|
| F | 28 | <1 | yes | yes | MA | 2 weeks |
| F | 23 | 1 to 3 | yes | no | M | 8 days |
| F | 48 | <1 | yes | no | MA | 18 months |
| M | 19 | 3 to 10 | yes | no | MA | 2 weeks |
| F | 29 | <1 | yes | yes | MA | 1 month |
| F | 18 | <1 | yes | no | MA | 4 months |
| F | 19 | 0 | yes | no | VM | 8–10 days |
| F | 19 | 3 to 10 | yes | no | M | 5–6 days |
| M | 23 | 0 | yes | yes | M | 7–9 months |
| F | 21 | <1 | yes | no | M | 1 month |
| F | 21 | 1 to 3 | yes | no | MO | 2–3 weeks |
| F | 21 | 10+ | yes | yes | MA | 3–4 days |
| F | 22 | <1 | yes | yes | AB | months |
| F | 19 | 1 to 3 | no | no | M | 6 days |
| F | 20 | 1 to 3 | yes | no | M | 1 week |
| F | 18 | 1 to 3 | yes | no | MA | 2 weeks |
| F | 22 | 1 to 3 | yes | no | M | 6 days |
| F | 21 | 3 to 10 | yes | no | MO | 2 days |
| F | 26 | 1 to 3 | yes | no | M | 2 weeks |
| F | 41 | <1 | no | no | M | 2 weeks |
Appendix C.1. Threshold Elevation for Motion Against Orthogonal Backgrounds

Appendix C.2. Threshold Elevation for Motion Against Parallel Backgrounds
Appendix C.3. Difference in Threshold Elevation between Orthogonal and Parallel Backgrounds

Appendix C.4. Relationship to Headache Frequency
References
- Steiner, T.J.; Scher, A.I.; Stewart, W.F.; Kolodner, K.; Lieberman, J.; Lipton, R.B. The prevalence and disability burden of adult migraine in England and their relationships to age, gender and ethnicity. Cephalalgia 2003, 23, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 2013, 33, 629–808. [Google Scholar]
- Schürks, M.; Buringm, J.E.; Kurth, T. Migraine, migraine features and cardiovascular disease. Headache 2010, 50, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Diamond, S.; Reed, M.; Diamond, M.L.; Stewart, W.F. Migraine diagnosis and treatment: Results from the American Migraine Study II. Headache 2001, 41, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.A.; Soso, M.J. Migraine and stripe-induced visual discomfort. Arch. Neurol. 1989, 46, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, L.; Hibbard, P.B. Visual processing in migraine. Cephalalgia 2016, 36, 1057–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antal, A.; Temme, J.; Nitsche, M.A. Altered motion perception in migraineurs: Evidence for intercortical hyperexcitability. Cephalalgia 2005, 25, 788–794. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, A.M.; Badcock, D.R.; Gurgone, M. Vernier acuity is normal in migraine, whereas global form and motion perception are not. Investig. Opthalmol. Vis. Sci. 2006, 47, 3213–3219. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, J.A.; McKendrick, A.M.; Badcock, D.R. Processing of global form and motion in migraineurs. Vision Res. 2006, 46, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Dickinson, J.E.; Battista, J.; McKendrick, A.M.; Badcock, D.R. Increased internal noise cannot account for motion coherence processing deficits in migraine. Cephalalgia 2011, 31, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Braunitzer, G.; Roksin, A.; Kobor, J.; Benedek, G.; Ngy, A.; Kincses, Z.T. Delayed development of visual motion processing in childhood migraine. Cephalalgia 2012, 32, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J.; Beaumont, H.M.; Hine, T.J. Motion processing deficits in migraine are related to contrast sensitivity. Cephalalgia 2012, 32, 554–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibber, M.S.; Kelly, M.; Jansari, A.; Dakin, S.C.; Shepherd, A.J. An inability to exclude visual noise in migraine. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Dakin, S.C.; Mareschal, I.; Bex, P.J. Local and global limitations on direction integration assessed using equivalen noise analysis. Vis. Res. 2005, 45, 3027–3049. [Google Scholar] [CrossRef] [PubMed]
- Geisler, W.S. Motion streaks provide a spatial code for motion direction. Nature 1999, 400, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.C.; Ross, J. Direct evidence that speedlines influence motion mechanisms. J. Neurosci. 2002, 22, 8661–8664. [Google Scholar] [CrossRef] [PubMed]
- Apthorp, D.; Wenderoth, P.; Alais, D. Motion streaks in fast motion rivalry cause orientation-selective suppression. J. Vis. 2009, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Apthorp, D.; Cass, J.; Alais, D. Orientation tuning of contrast masking cuased by motion streaks. J. Vis. 2010, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Apthorp, D.; Cass, J.; Alais, D. The spatial tuning of motion streak mechanisms revealed by masking and adaptation. J. Vis. 2011, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alais, D.; Apthorp, D.; Karmann, A.; Cass, J. Temporal integration of movement: the time-course of motion streaks revealed by masking. PLoS ONE 2011, 6, e28675. [Google Scholar] [CrossRef] [PubMed]
- Zhaoping, L. Understanding Vision: Theory, Models and Data.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Series, P.; Latham, P.E.; Pouget, A. Tuning curve sharpening for orientation selectivity: Coding efficiency and the impact of correlations. Nat. Neurosci. 2004, 7, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Crane, M.F. Motion streaks improve detection. Vis. Res. 2007, 47, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Rogalinski, S.; Rambold, H.A. Probing early motion processing with eye movements: Differences of vestibular migraine, migraine with and without aura in headache-free interval. Headache 2017, 58, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Drummond, P.D. Motion sickness and migraine: optokinetic stimulation increases scalp tenderness, pain sensitivity in the fingers and photophobia. Cephalalgia 2002, 22, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Igarashi, M. Comparison of vertical and horizontal optokinetic nystagmus in the squirrel monkey. ORL 1977, 39, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Sakurai, S.; Kanzaki, J. Horizontal and vertical optokinetic nystagmus in man. ORL 1979, 40, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Winter, B. Linear models and linear mixed effects models in R with linguistic applications. arXiv, 2013; arXiv:1308.5499. [Google Scholar]
- Calandre, E.P.; Bembibre, J.; Arnedo, M.L.; Becerra, D. Cognitive disturbances and regional cerebral blood flow abnormalities in migraine patients: their relationship with the clinical manifestations of the illness. Cephalalgia 2002, 22, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.; Kaltenhüser, M.; Neundörfer, B.; Siedler, S. Hyperexcitability of the primary somatosensory cortex in migraine—A megnetoencephalographic study. Brain 2004, 127, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Suhr, J.A.; Seng, E.K. Neurophysiological functioning in migraine: clinical and research implications. Cephalalgia 2012, 32, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.G.; Hamid, M.A.A.; Mansour, D.E. Correlation of visual field defects and optical coherence tomography finding in migraine patients. Saudi J. Opthalmol. 2015, 29, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Yener, A.U.; Korucu, O. Visual field losses in patients with migraine with and without aura and tension-type headache. Neuroopthalmology 2017, 41, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.N.; Vingrys, A.J.; McKendrick, A.M. The effect of duration post-migraine on visual electrophysiological and visual field performance in people with migraine. Cephalalgia 2014, 34, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M. Investigations of Visual Function in Migraine Using Visual Evoked Potentials and Visual Psychophysical Tests. Ph.D. Thesis, University of London, London, UK, 1991. [Google Scholar]
- Siniatchkin, M.; Averkina, N.; Andrasik, F.; Stephani, U.; Gerber, W.D. Neurophysiological reactivity before a migraine attack. Neurosci. Lett. 2006, 400, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Sand, T.; Zhitniy, N.; White, L.R.; Stovner, L.J. Visual evoked potential latency, amplitude and habituation in migraine: A longitudinal study. Clin. Neurophysiol. 2008, 119, 1020–7102. [Google Scholar] [CrossRef] [PubMed]
- Sand, T.; White, L.R.; Hagen, K.; Stovner, L.J. Visual evoked potential and spatial frequency in migraine: A longitudinal study. Acta Neurol. Scand Suppl. 2009, 189, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Karovanic, O.; Thabet, M.; Wilson, H.R.; Wilkinson, F. Detection and discrimination of flicker contrast in migraine. Cephalalgia 2011, 31, 723–736. [Google Scholar] [Green Version]
- Thabet, M.; Wilkinson, F.; Wilson, H.R.; Karanovic, O. The locus of flicker adaptation in the migraine visual system: A dichoptic study. Cephalalgia 2013, 33, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Coleston, D.M.; Kennard, C. Responses to temporal visual stimulation in migraine: the critical flicker fusion test. Cephalalgia 1995, 15, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Kowacs, P.A.; Piovesan, E.J.; Werneck, L.C.; Famelie, H.; Zani, A.N.; da Silva, H.P. Critical flicker frequency in migraine: A controlled study in patients without prophylactic therapy. Cephalalgia 2005, 25, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J.; Wyatt, G.; Tibber, M.S. Visual metacontrast masking in migraine. Cephalalgia 2011, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Schoenen, J.; Wang, W.; Albert, A.; Delwaide, P.J. Potentiation instead of habituation characterizes visual evoked potentials in migraine patients between attacks. Eup. J. Neurosci. 1995, 2, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, D.J.; Veggeberg, R.; Kucyi, A.; van Dijk, K.R.A.; Wilcox, S.L.; Scrivani, S.J.; Burstein, R.; Becerra, L.; Borsook, D. Cortico-cortical connections of primary sensory areas and associated symptoms in migraine. eNeuro 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, G.; Russo, A.; Conte, F.; Caiazzo, G.; Giordano, A.; Conforti, R.; Esposito, F.; Tessitore, A. Increased interictal visual network connectivity in patients with migraine with visual aura. Cephalalgia 2016, 36, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Keil, J.; Müller, N.; Ihssen, N.; Weisz, N. On the variability of the McGurk effect: Audiovisual integration depends on prestimulus brain states. Cereb. Cortex 2012, 22, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.G.; Halder, T.; Jaiswal, A.K.; Mukherjee, A.; Roy, D.; Banerjee, A. Large scale functional brain networks underlying temporal integration of audio-visual speech perception: An EEG study. Front. Psychol. 2016, 7, 1558. [Google Scholar] [CrossRef] [PubMed]
- De Tommaso, M.; Stamaglia, S.; Marinazzo, D.; Trotta, G.; Pellicoro, M. Functional and effective connectivity in EEG alpha and beta bands during intermittent flash stimulation in migraine with and without aura. Cephalalgia 2013, 33, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Gips, B.; Bergmann, T.O.; Bonneford, M. Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci. 2014, 37, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Dugue, L.; MArque, P.; VanRullen, R. The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. J. Neurosci. 2011, 31, 11889–11893. [Google Scholar] [CrossRef] [PubMed]
- Ergenoglu, T.; Demiralp, T.; Bayrataroglu, Z.; Ergen, M.; Beydagi, H.; Uresin, Y. Alpha rhythm of the EEG modulates visual detection performance in humans. Cognit. Brain Res. 2004, 20, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Hanslmayr, S.; Klimesch, W.; Sauseng, P.; Gruber, W.; Doppelmayr, M.; Freunberger, R.; Pecherstorfer, T. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci. Lett. 2005, 375, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Hanslmayr, S.; Aslan, A.; Staudigl, T.; Klimesch, W.; Herrmann, C.S.; Bauml, K.H. Prestimulus oscillations predict visual perception performance between and within subjects. NeuroImage 2007, 37, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Cecere, R.; Rees, G.; Romei, V. Individual differences in alpha frequency drive crossmodal illusory perception. Curr. Biol. 2015, 25, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Zaehle, T.; Rach, S.; Herrmann, C.S. Transcranial alternating current stimulation ehances alpha activity in human EEG. PLoS ONE 2010, 5, e13766. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chu, B.; Yang, J.; Yu, Y.; Wu, J.; Yu, S. Elevated audiotemporal interaction in patients with migraine without aura. J. Head. Pain 2014, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- De Tommaso, M.; Ambrosini, A.; Brighina, F.; Coppola, G.; Perrotta, A.; Pierelli, F.; Sandrini, G.; Valeriani, M.; Marinazzo, D.; Stramaglia, S.; et al. Altered processing of sensory stimuli in patients with migraine. Nat. Rev. Neurosci. 2014, 10, 144–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battista, J.; Badcock, D.R.; McKendrick, A.M. Migraine increases centre-surround suppression for drifting visual stimuli. PLoS ONE 2011, 6, e18211. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Parisi, V.; Lorenzo, C.D.; Serrao, M.; Magis, D.; Schoenen, J. Lateral inhibition in visual cortex of migraine patients between attacks. Head. Pain 2013, 14, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asher, J.M.; O’Hare, L.; Romei, V.; Hibbard, P.B. Typical lateral interactions, but increased contrast sensitivity, in migraine-with-aura. Vision 2018, 2, 7. [Google Scholar] [CrossRef]
- Adelson, E.H.; Bergen, J.R. Spatiotemporal energy models for the perception of motion. J. Opt. Soc. Am. A 1985, 2, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Granziera, C.; DaSilva, A.F.M.; Snyder, J.; Tuch, D.S.; Hadjikhani, N. Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Med. 2006, 3, e402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmeads, J.; Findlay, H.; Tugwell, P.; Pryse-Phillips, W.; Nelson, R.F.; Murray, T.J. A Canadian population survey on the clinical, epidemiologic and societal impact of migraine and tension-type headache. Can. J. Neurol. Sci. 1993, 19, 333–339. [Google Scholar]
- MacGregor, E.A.; Brandes, J.; Eikermann, A. Migraine prevalence and treatment patterns: The Global Migraine and Zolmitriptan Evaluation Survey. Headache 2003, 43, 19–26. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, A.M.; Badcock, D.R. Motion processing deficits in migraine. Cephalalgia 2004, 24, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Battista, J.; Badcock, D.R.; McKendrick, A.M. Centre-surround visual motion processing in migraine. Invest. Ophthalmol. Vis. Sci. 2010, 51, 6070–6076. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Renso, A.D.; Tnelli, E.; Lepre, C.; Lorenzo, C.D.; Lorenzo, G.D.; Scapeccia, M.; Parisi, V.; Serrao, M.; Colonnese, C.; et al. Thalamo-cortical network activity between migraine attacks: Insights from MRI-based microstructural and functional resting-state network correlation analysis. J. Head. Pain 2016, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Renzo, A.D.; Tinelli, E.; Lorenzo, C.D.; Lorenzo, G.D.; Parisi, V.; Serrao, M.; Schoenen, J.; Pierelli, F. Thalamo-cortical network activity during spontaneous migraine attacks. Neurology 2016, 87, 2154–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faragó, P.; Tuka, B.; Tóth, E.; Szabó, N.; Király, A.; Csete, G.; Szok, D.; Tajti, J.; Párdutz, A.; Vécsei, L.; Kincses, Z. Interictal brain activity differs in migraine with and without aura: resting state fMRI study. J. Head. Pain 2017, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Brainard, D.H. The psychophysics toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Pelli, D.G. The videotoolbox for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, M.; Brainard, D.; Pelli, D. What’s new in Psychtoolbox-3? Perception 2007, 36 (Suppl. 36), 1–16. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Bolker, H.S.; Westfall, J.; Aust, F.; R Core Team. Afex: Analysis of Factorial Experiments. R package version 0.18-0. 2017. [Google Scholar]
- Bates, D.; Maechler, M.; bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Norman, G. Likert scales, levels of measurement and the “laws” of statistics. Adv. Health Sci. Edu. Theory Pract. 2010, 15, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Nimon, K.F. Statistical assumptions of substantive analyses across the general linear model: A mini-review. Front. Psychol. 2012, 3, 322. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rayner, G.D. Robustness to non-normality of common tests for the many-sample location problem. J. Appl. Math. Dec. Sci. 2003, 7, 187–206. [Google Scholar] [CrossRef]
- Noguchi, K.; Gel, Y.R.; Brunner, E.; Konietschke, F. nparLD: An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar] [CrossRef]
- Naito, T.; Sato, H.; Osaka, N. Direction anisotropy of human motion perception depends on stimulus speed. Vis. Res. 2010, 50, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, Y.; Ejima, Y. Anisotropy for direction discrimination in a two-frame apparent motion display. Vis. Res. 1997, 37, 765–767. [Google Scholar] [CrossRef]
| Gender | Age | Monthly Freq | Visual | Speech | Prof Diagnosis | Last Attack |
|---|---|---|---|---|---|---|
| F | 28 | <1 | yes | yes | MA | 2 weeks |
| M | 41 | <1 | yes | no | no | 2 months |
| F | 23 | 1 to 3 | yes | no | M | 8 days |
| F | 48 | <1 | yes | no | MA | 18 months |
| M | 19 | 3 to 10 | yes | no | MA | 2 weeks |
| F | 29 | <1 | yes | yes | MA | 1 month |
| F | 18 | <1 | yes | no | MA | 4 months |
| F | 22 | 1 to 3 | no | yes | no | 2 weeks |
| F | 19 | 3 to 10 | yes | no | M | 5–6 days |
| F | 21 | 1 to 3 | yes | no | MO | 2–3 weeks |
| F | 21 | 10+ | yes | yes | MA | 3–4 days |
| F | 22 | <1 | yes | yes | AB | months |
| F | 19 | 1 to 3 | no | no | M | 6 days |
| F | 20 | 1 to 3 | yes | no | M | 1 week |
| F | 21 | 3 to 10 | yes | no | no | 1 week |
| F | 18 | 1 to 3 | yes | no | MA | 2 weeks |
| F | 22 | 1 to 3 | yes | no | M | 6 days |
| F | 23 | 1 to 3 | yes | no | no | 3 weeks |
| F | 21 | 3 to 10 | yes | no | MO | 2 days |
| F | 26 | 1 to 3 | yes | no | M | 2 weeks |
| M | 24 | <1 | yes | no | no | 2 months |
| F | 41 | <1 | no | no | M | 2 weeks |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Hare, L. Temporal Integration of Motion Streaks in Migraine. Vision 2018, 2, 27. https://doi.org/10.3390/vision2030027
O’Hare L. Temporal Integration of Motion Streaks in Migraine. Vision. 2018; 2(3):27. https://doi.org/10.3390/vision2030027
Chicago/Turabian StyleO’Hare, Louise. 2018. "Temporal Integration of Motion Streaks in Migraine" Vision 2, no. 3: 27. https://doi.org/10.3390/vision2030027





