Persistent Biases in Binocular Rivalry Dynamics within the Visual Field
Abstract
:1. Introduction
2. Results
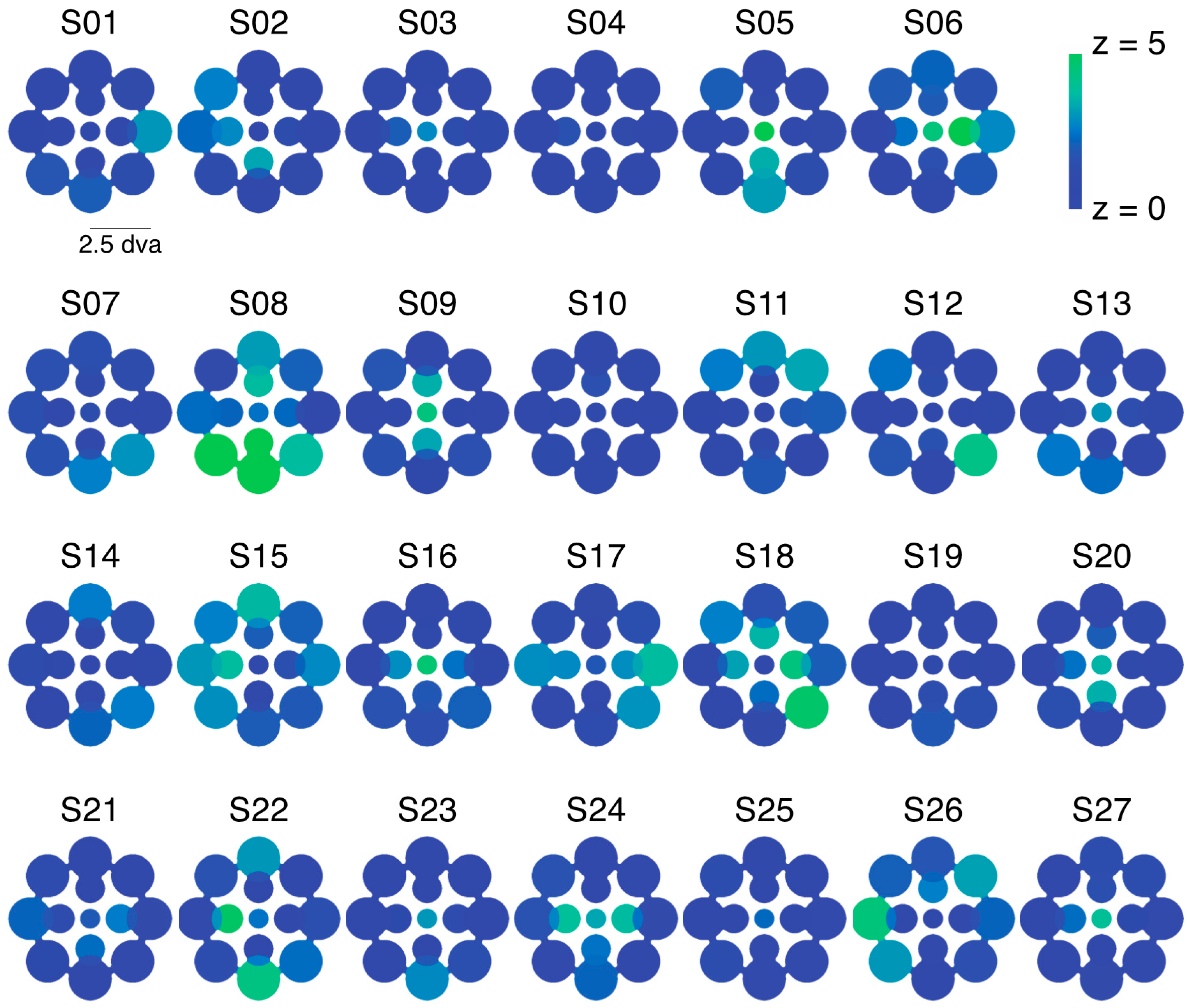
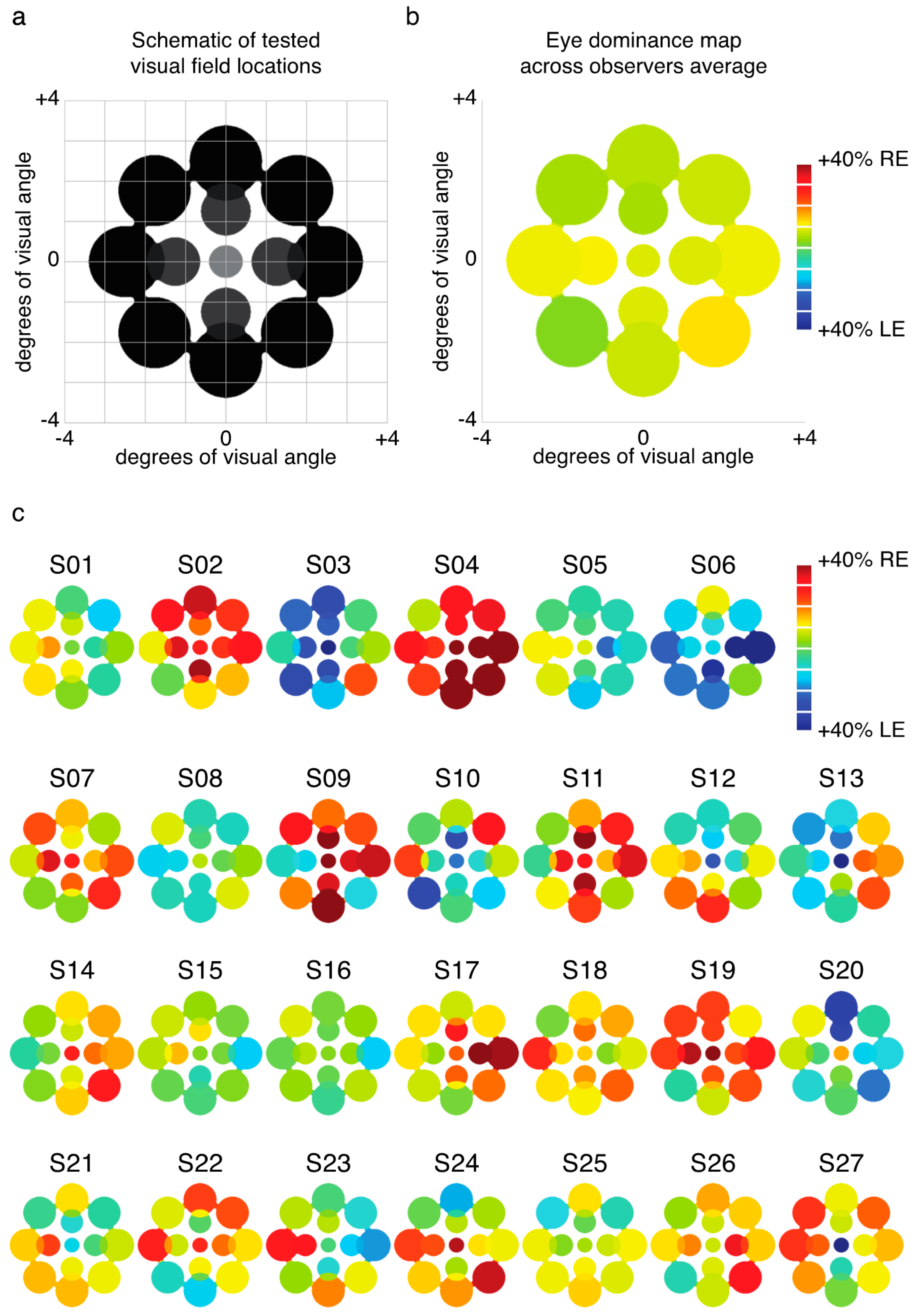
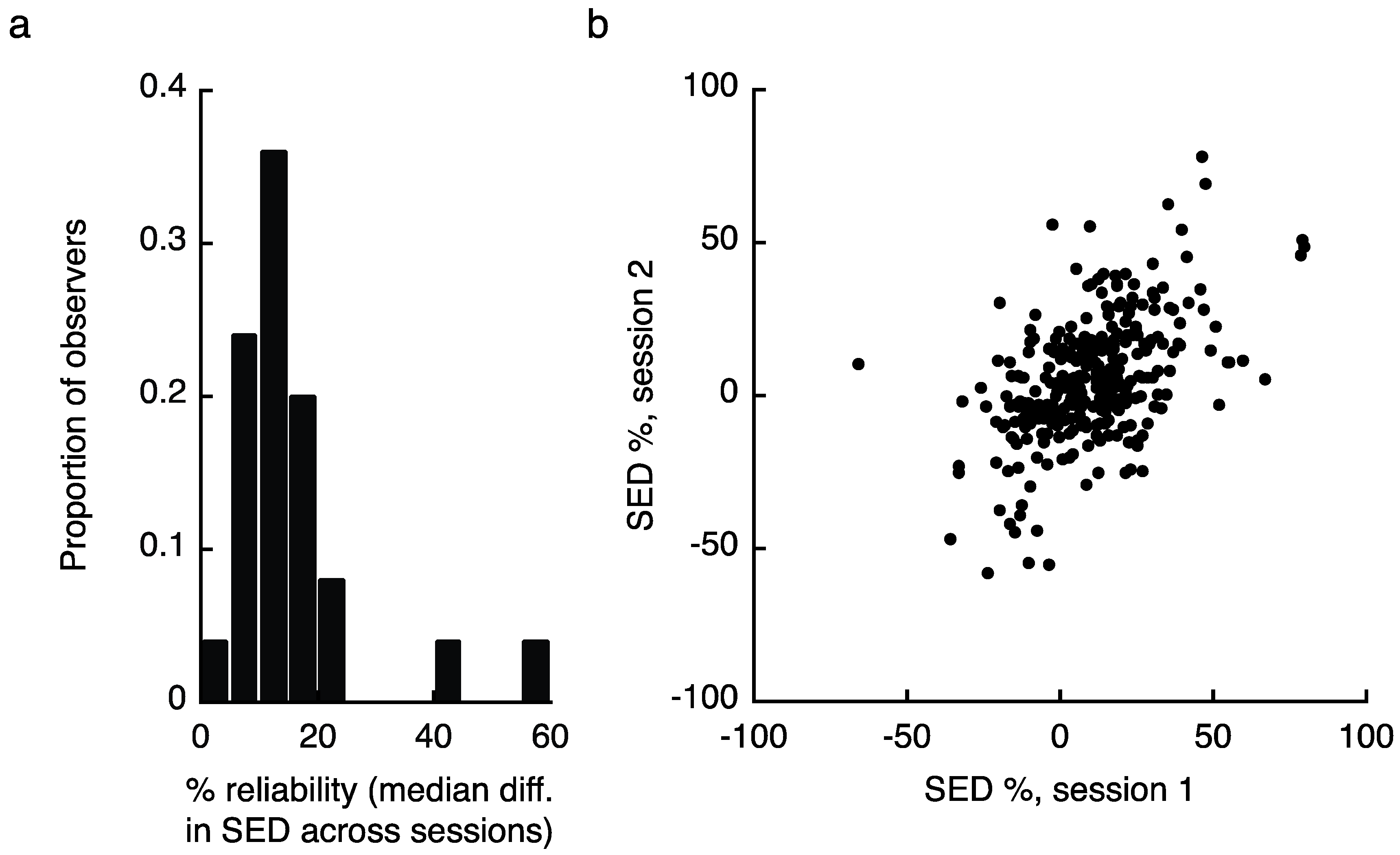
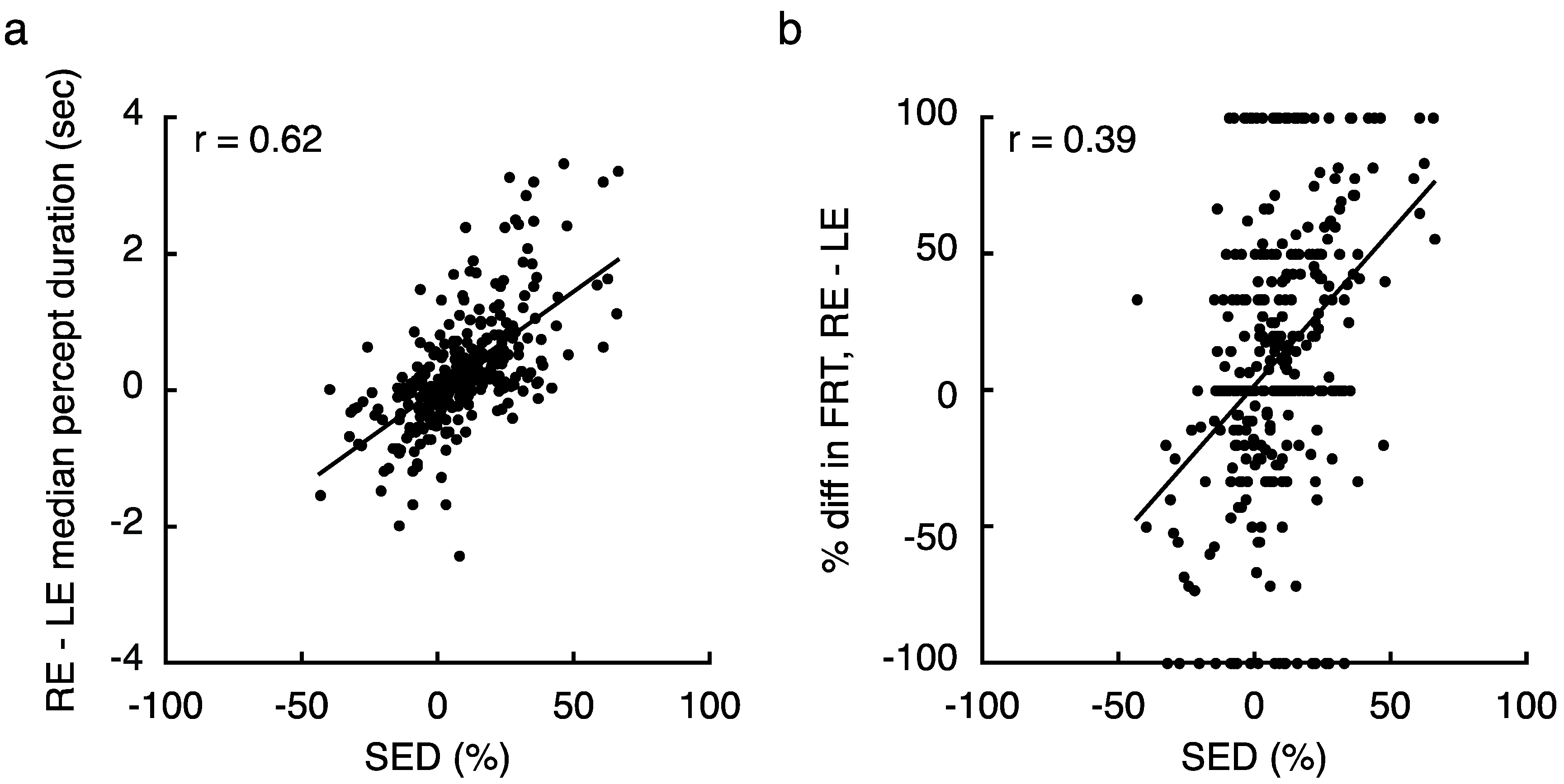
2.1. Experiment 1 Results
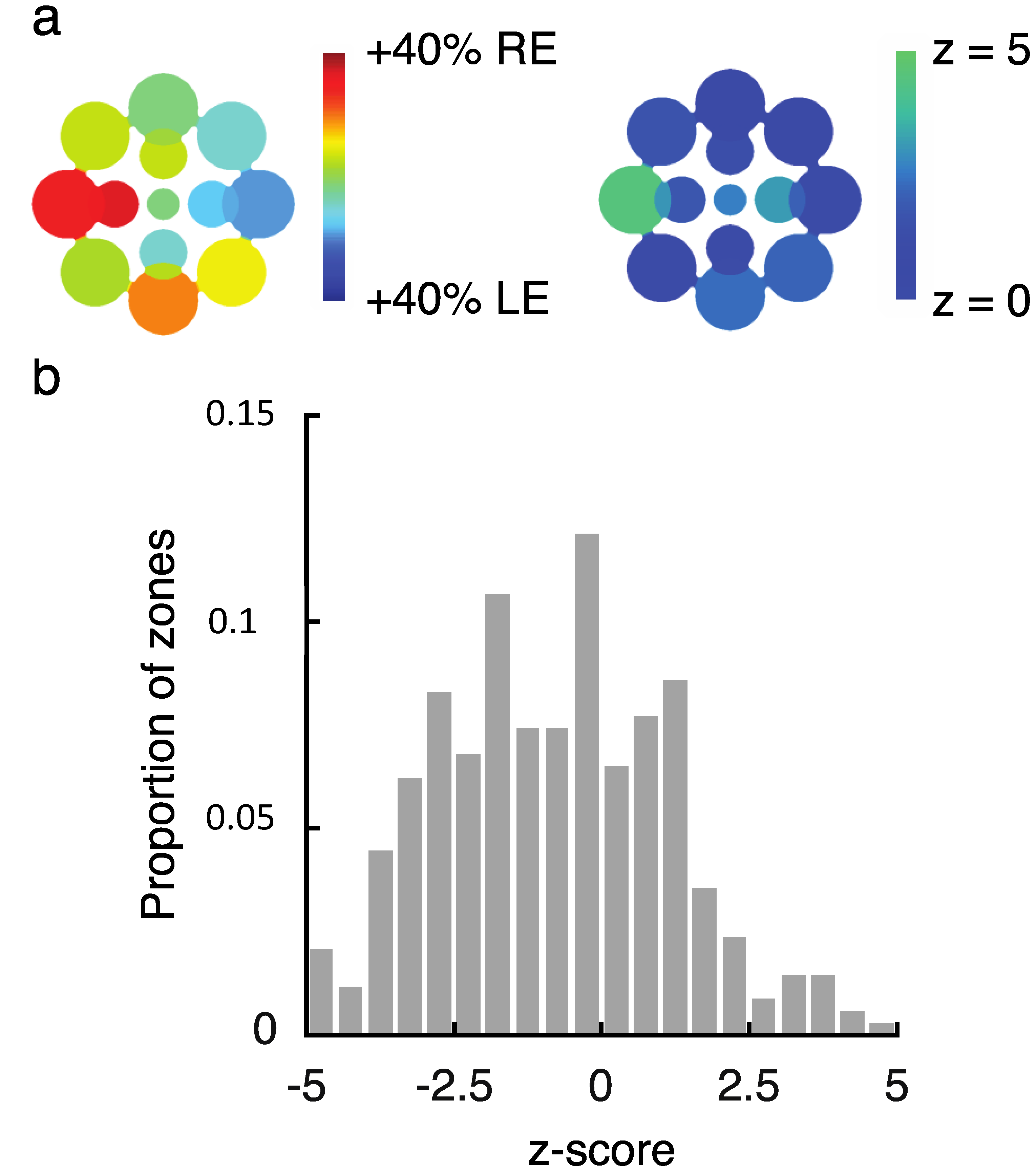
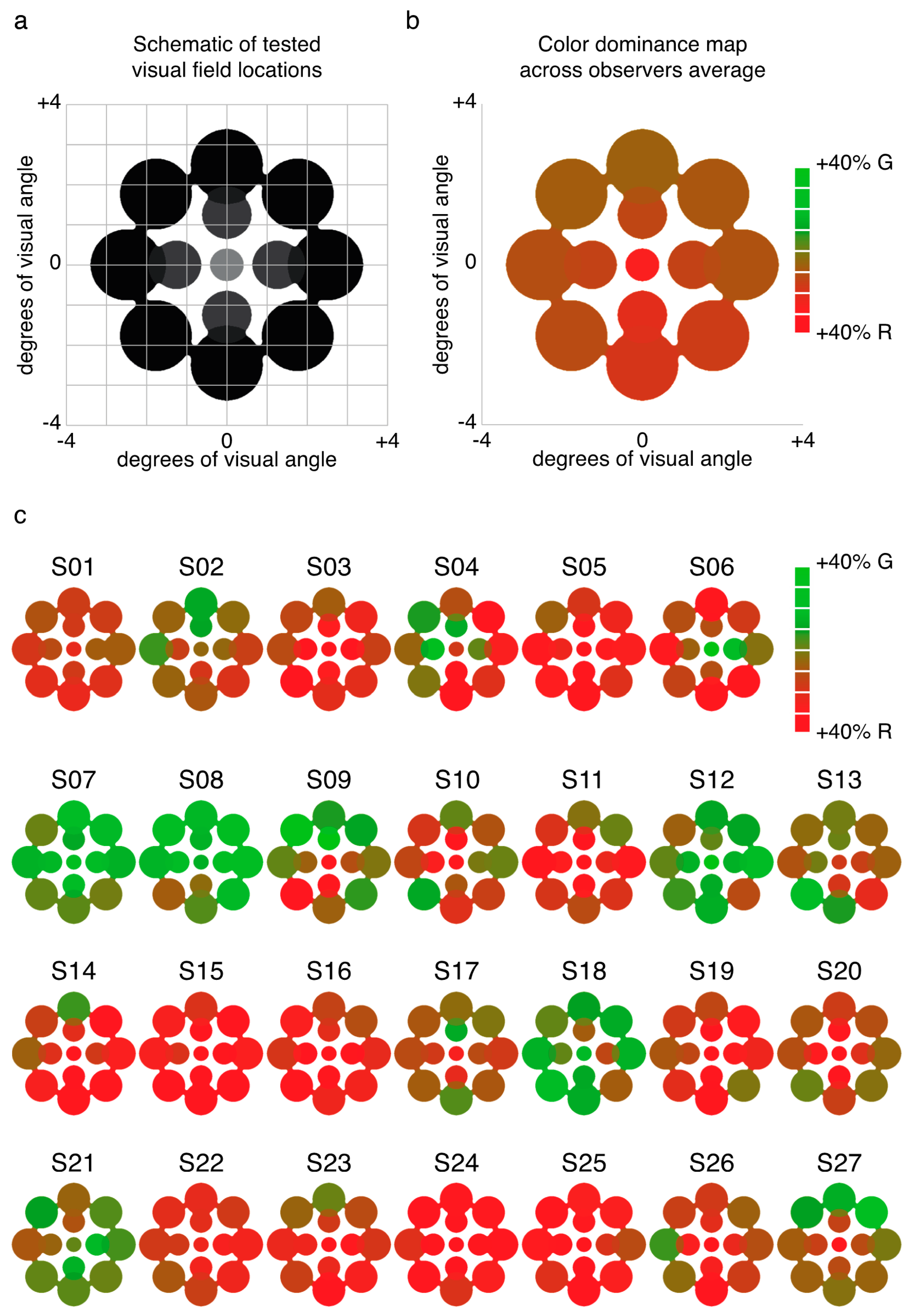
2.2. Experiment 2 Results
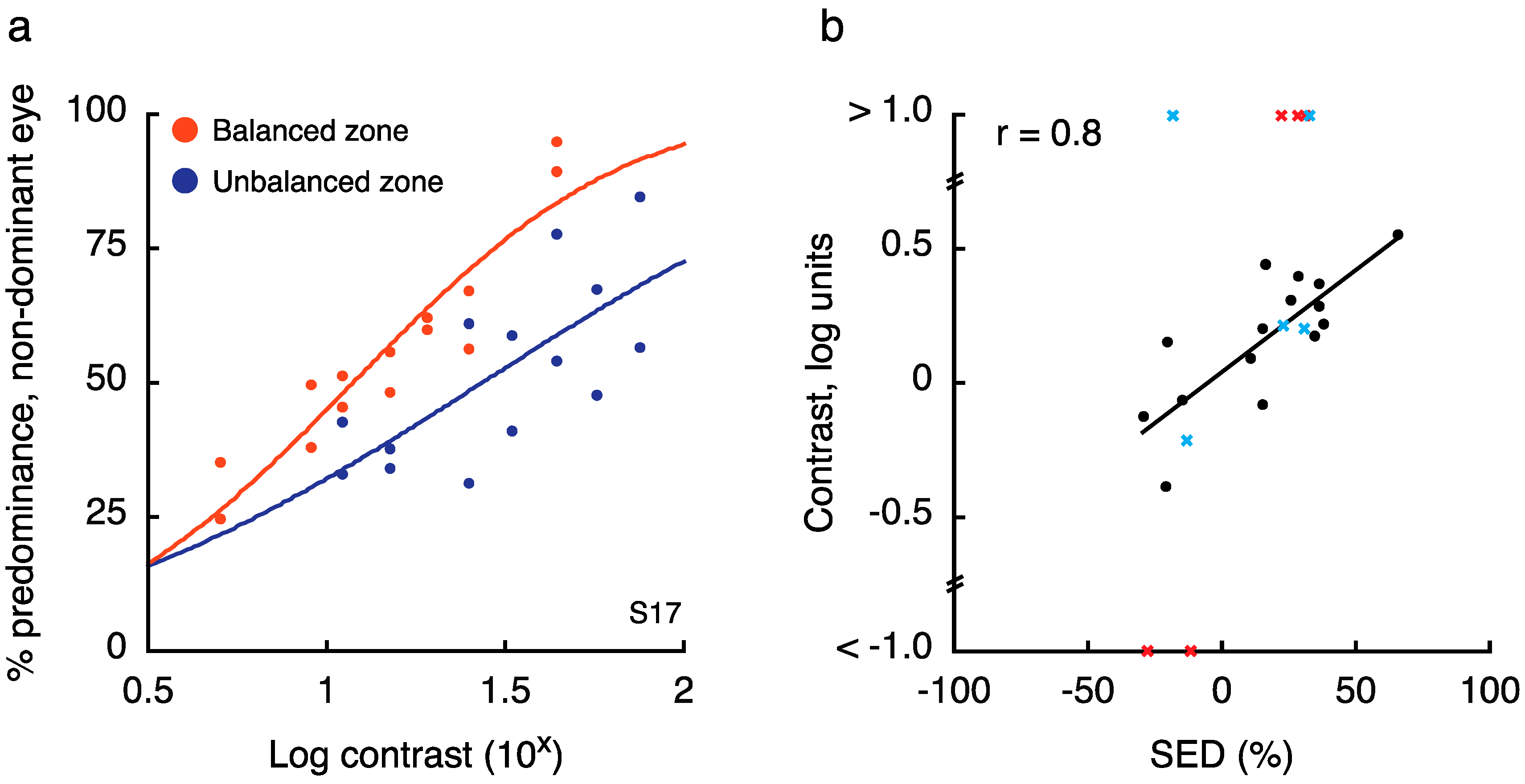
2.3. Experiment 3 Results
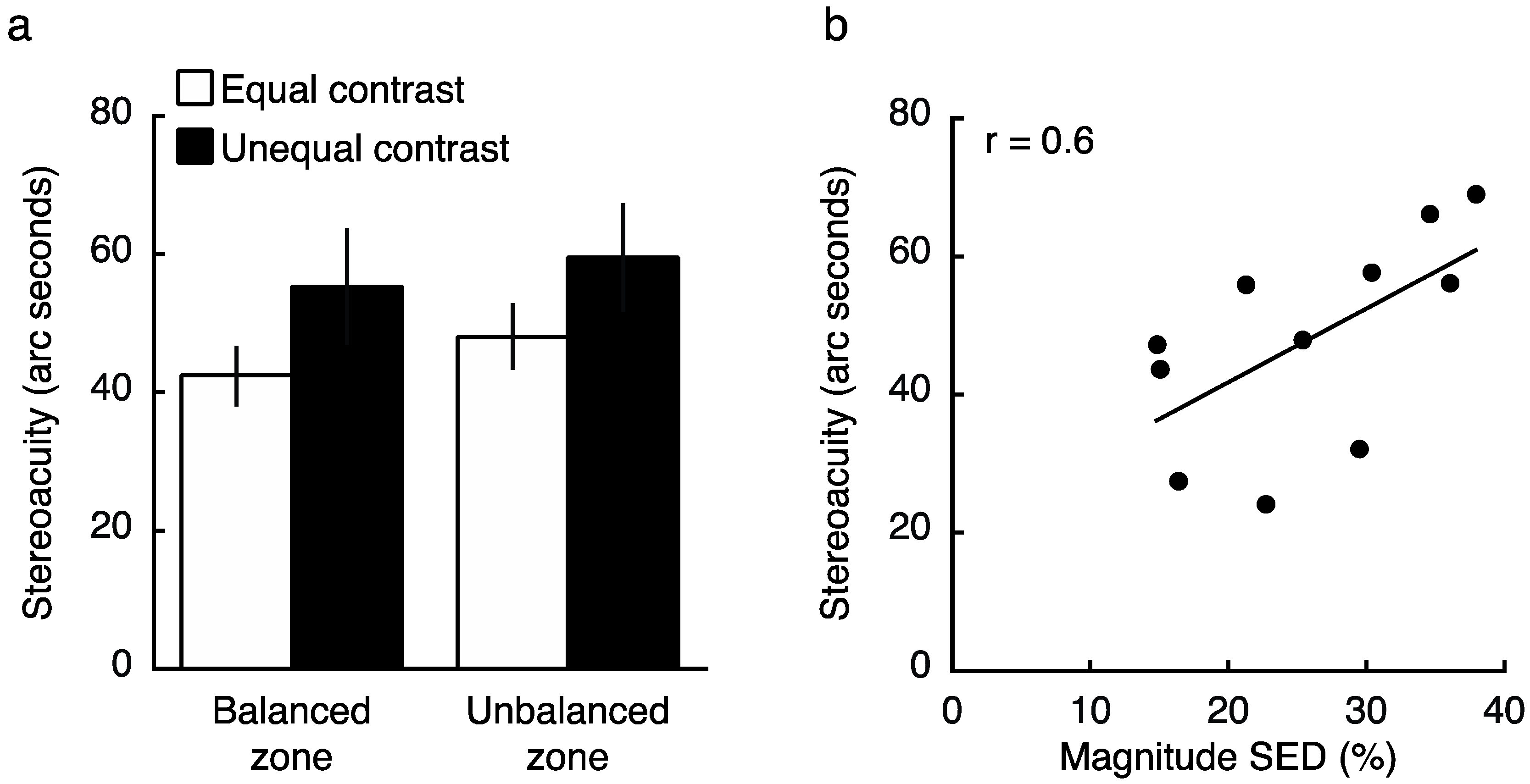
2.4. Experiment 4 Results
3. Discussion
4. Materials and Methods
4.1. Experiment 1 Method
4.1.1. Observers
4.1.2. Apparatus
4.1.3. Stimuli
4.1.4. Procedure
- Practice Block 1:
- Simulation of binocular rivalry at fixation.
- Practice Block 2:
- Real rivalry at fixation.
- Practice Block 3:
- Simulation of rivalry in periphery (1.25 dva eccentricity).
- Practice Block 4:
- Real rivalry in periphery (1.25 dva eccentricity).
- Practice Block 5:
- Real rivalry in periphery (2.5 dva eccentricity).
4.1.5. Data Analysis
4.1.6. SED with Greyscale Stimuli
4.2. Experiment 2 Method
4.2.1. Observers
4.2.2. Apparatus
4.2.3. Stimuli
4.2.4. Procedure
- Both test locations were always at the same eccentricity
- Observed SED magnitude in the balanced zone must not exceed 10%
- Observed SED magnitude in the unbalanced zone must exceed 10%
4.2.5. Data Analysis
4.3. Experiment 3 Method
4.3.1. Observers
4.3.2. Apparatus
4.3.3. Stimuli
4.3.4. Procedure
4.3.5. Data Analysis
4.4. Experiment 4 Method
4.4.1. Observers
4.4.2. Apparatus
4.4.3. Stimuli
4.4.4. Procedure
4.4.5. Data analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Location (ecc, dir) | S01 | S02 | S03 | S04 | S05 |
| 0, n/a | 1.1 ± 11.5% | 26.8 ± 7.4% | −40.0 ± 9.7% | 47.0 ± 8.4% | 7.9 ± 22.0% |
| 1.25, 0 | 16.3 ± 6.7% | 33.9 ± 5.5% | −26.0 ± 11.0% | 24.6 ± 15.0% | 11.1 ± 7.8% |
| 1.25, 90 | 6.3 ± 8.8% | 19.9 ± 10.1% | −23.1 ± 8.6% | 32.2 ± 9.8% | −3.5 ± 9.6% |
| 1.25, 180 | −5.5 ± 8.2% | 24.5 ± 6.0% | −3.7 ± 10.0% | 62.2 ± 6.4% | −21.3 ± 11.0% |
| 1.25, 270 | 11.1 ± 8.4% | 38.4 ± 7.8% | −29.5 ± 7.3% | 66.0 ± 8.6% | −3.1 ± 13.6% |
| 2.5, 0 | 9.8 ± 6.8% | 9.9 ± 4.8% | −6.0 ± 11.7% | 29.6 ± 9.3% | 10.9 ± 9.5% |
| 2.5, 45 | 9.6 ± 9.3% | 31.1 ± 5.1% | −22.2 ± 9.2% | 5.2 ± 7.3% | −2.5 ± 10.7% |
| 2.5, 90 | −3.6 ± 9.4% | 36.0 ± 6.0% | −28.5 ± 6.5% | 31.9 ± 8.1% | −5.2 ± 9.1% |
| 2.5, 135 | 13.9 ± 7.7% | 24.0 ± 4.4% | −3.6 ± 8.6% | 32.7 ± 9.9% | −7.1 ± 8.2% |
| 2.5, 180 | 2.8 ± 11.1% | 31.1 ± 6.9% | 4.5 ± 7.6% | 43.1 ± 12.7% | −7.6 ± 8.4% |
| 2.5, 225 | −5.3 ± 7.1% | 14.7 ± 6.3% | 21.5 ± 8.0% | 65.7 ± 6.1% | −7.1 ± 7.8% |
| 2.5, 270 | 1.9 ± 7.9% | 11.6 ± 5.9% | −15.2 ± 9.2% | 58.2 ± 9.2% | −15.1 ± 12.4% |
| 2.5, 315 | 12.0 ± 8.2% | −1.2 ± 6.5% | −29.8 ± 7.8% | 22.6 ± 8.4% | 7.8 ± 9.0% |
| Location | S06 | S07 | S08 | S09 | S10 |
| 0, n/a | −11.4 ± 15.7% | 27.2 ± 8.6% | 6.0 ± 14.1% | 60.8 ± 20.4% | −20.1 ± 11.5% |
| 1.25, 0 | −12.1 ± 11.1% | 34.6 ± 8.1% | −11.6 ± 7.7% | −11.6 ± 16.8% | −6.9 ± 12.6% |
| 1.25, 90 | −10.2 ± 11.2% | 8.9 ± 9.2% | −3.2 ± 8.1% | 41.7 ± 16.8% | −27.9 ± 19.2% |
| 1.25, 180 | −52.1 ± 13.9% | 14.1 ± 9.9% | −1.0 ± 10.7% | 34.9 ± 10.5% | −8.9 ± 10.2% |
| 1.25, 270 | −37.2 ± 15.2% | 21.0 ± 9.0% | −7.7 ± 8.7% | 34.8 ± 16.8% | −8.4 ± 12.1% |
| 2.5, 0 | −22.6 ± 11.4% | 6.5 ± 6.7% | −13.1 ± 9.1% | −8.6 ± 9.3% | 23.1 ± 9.8% |
| 2.5, 45 | −13.2 ± 9.7% | 22.4 ± 8.4% | 7.6 ± 8.1% | 29.5 ± 15.0% | −2.0 ± 7.0% |
| 2.5, 90 | 8.8 ± 14.7% | 14.5 ± 8.5% | −6.5 ± 7.9% | 19.7 ± 13.4% | 5.8 ± 10.1% |
| 2.5, 135 | −12.7 ± 9.2% | 4.3 ± 8.1% | −8.4 ± 8.2% | 22.4 ± 11.6% | 30.3 ± 10.6% |
| 2.5, 180 | −40.6 ± 9.7% | 21.3 ± 9.6% | 3.1 ± 9.3% | 35.7 ± 11.1% | 7.0 ± 8.1% |
| 2.5, 225 | 1.3 ± 18.4% | 25.8 ± 6.8% | 8.5 ± 9.3% | −10.6 ± 13.4% | −14.6 ± 9.8% |
| 2.5, 270 | −20.2 ± 10.4% | 1.5 ± 7.0% | −7.9 ± 7.2% | 46.4 ± 10.8% | −3.3 ± 11.1% |
| 2.5, 315 | −21.1 ± 14.9% | 2.1 ± 9.3% | −7.3 ± 7.3 | 18.3 ± 10.1% | −31.1 ± 9.5% |
| Location | S11 | S12 | S13 | S14 | S15 |
| 0, n/a | 29.5 ± 12.1% | −24.4 ± 16.6% | −39.0 ± 12.8% | 27.4 ± 13.1% | 1.4 ± 14.4% |
| 1.25, 0 | 33.0 ± 12.0% | 15.7 ± 9.7% | −14.3 ± 6.7% | 1.0 ± 6.7% | 14.7 ± 7.6% |
| 1.25, 90 | 44.0 ± 14.6% | −14.7 ± 9.0% | −21.0 ± 9.2% | 7.0 ± 8.0% | 11.8 ± 10.7% |
| 1.25, 180 | 14.9 ± 9.3% | −9.2 ± 8.8% | 21.3 ± 15.6% | 18.8 ± 8.0% | 0.8 ± 10.5% |
| 1.25, 270 | 37.9 ± 9.6% | 10.5 ± 11.8% | 4.0 ± 7.7% | 7.9 ± 9.6% | −6.4 ± 8.7% |
| 2.5, 0 | −4.5 ± 24.5% | 11.7 ± 10.0% | −3.9 ± 10.4% | −5.1 ± 7.5% | 5.4 ± 8.5% |
| 2.5, 45 | 2.4 ± 11.1% | −6.5 ± 8.6% | −17.5 ± 8.8% | 3.6 ± 5.8% | 6.9 ± 8.5% |
| 2.5, 90 | 15.2 ± 9.6% | −7.6 ± 9.6% | −10.9 ± 6.6% | 12.0 ± 7.0% | 2.6 ± 7.7% |
| 2.5, 135 | 27.1 ± 10.1% | −1.9 ± 10.4% | 12.8 ± 9.7% | 15.8 ± 7.4% | 0.1 ± 7.5% |
| 2.5, 180 | 34.8 ± 8.0% | 8.8 ± 10.4% | 16.7 ± 9.5% | 15.7 ± 9.2% | −14.0 ± 8.9% |
| 2.5, 225 | 3.9 ± 9.7% | 3.0 ± 10.8% | 21.8 ± 7.3% | 28.1 ± 11.2% | 1.8 ± 8.3% |
| 2.5, 270 | 23.1 ± 7.7% | 26.0 ± 9.1% | −6.2 ± 6.9% | 13.4 ± 7.6% | −2.7 ± 6.9% |
| 2.5, 315 | 11.2 ± 8.0% | 19.4 ± 8.5% | −14.2 ± 7.7% | 2.2 ± 9.5% | −0.8 ± 6.5% |
| Location | S16 | S17 | S18 | S19 | S20 |
| 0, n/a | 2.1 ± 11.3% | 20.5 ± 12.6% | 13.4 ± 5.6% | 60.8 ± 7.8% | 16.0 ± 24.4% |
| 1.25, 0 | 5.5 ± 9.2% | 2.7 ± 8.6% | 12.3 ± 6.4% | 36.7 ± 7.5% | −3.7 ± 10.4 |
| 1.25, 90 | −0.2 ± 6.9% | 28.6 ± 10.2% | 19.2 ± 6.8% | 23.7 ± 7.8% | −32.3 ± 14.5% |
| 1.25, 180 | 2.7 ± 10.1 | 48.1 ± 6.4% | 2.1 ± 5.2% | 21.6 ± 7.9% | −12.7 ± 11.5% |
| 1.25, 270 | −1.2 ± 8.7% | 20.9 ± 6.9% | 16.8 ± 5.0% | 21.4 ± 8.2% | 1.0 ± 10.3% |
| 2.5, 0 | −0.1 ± 6.0% | 11.8 ± 6.5% | 25.3 ± 5.4% | 22.7 ± 7.0% | 6.7 ± 8.5% |
| 2.5, 45 | 8.0 ± 6.1% | 12.3 ± 9.0% | 1.1 ± 5.9% | 23.5 ± 5.7% | 8.8 ± 7.9% |
| 2.5, 90 | 0.4 ± 7.0% | 7.3 ± 10.6% | 11.3 ± 5.6% | 23.7 ± 5.9% | −32.9 ± 10.5% |
| 2.5, 135 | 3.5 ± 6.9% | 11.7 ± 8.2% | 15.3 ± 5.6% | 10.9 ± 9.4% | −5.0 ± 7.3% |
| 2.5, 180 | −14.9 ± 6.4% | 37.9 ± 6.3% | 8.2 ± 4.7% | 27.8 ± 6.9% | −10.8 ± 6.7% |
| 2.5, 225 | 5.3 ± 7.6% | 19.3 ± 6.8% | 20.9 ± 6.3% | 21.9 ± 6.5% | −20.6 ± 7.9% |
| 2.5, 270 | −4.7 ± 7.5% | 0.2 ± 7.8% | 10.2 ± 5.4% | 7.5 ± 7.8% | −1.8 ± 13.1% |
| 2.5, 315 | 3.1 ± 7.3% | 6.9 ± 5.7% | 11.4 ± 4.7% | −6.1 ± 8.2% | −9.9 ± 8.0% |
| Location | S21 | S22 | S23 | S24 | S25 |
| 0, n/a | −11.3 ± 7.0% | 25.8 ± 9.3% | −2.5 ± 12.8% | 36.4 ± 12.7% | 3.9 ± 14.6% |
| 1.25, 0 | 22.5 ± 5.2% | 7.5 ± 8.0% | 31.3 ± 12.2% | 22.2 ± 11.7% | 1.6 ± 8.3% |
| 1.25, 90 | −9.1 ± 6.4% | −1.8 ± 9.9% | 6.1 ± 7.8% | 5.8 ± 10.1% | −0.4 ± 8.3% |
| 1.25, 180 | −3.5 ± 5.2% | 19.3 ± 8.1% | −14.3 ± 7.7% | 11.6 ± 10.7% | 6.5 ± 7.0% |
| 1.25, 270 | 9.3 ± 5.6% | 10.3 ± 7.5% | −9.6 ± 8.4% | 3.9 ± 10.3% | 10.1 ± 10.6% |
| 2.5, 0 | 13.7 ± 4.3% | 27.2 ± 8.6% | 28.5 ± 8.4% | 22.7 ± 9.0% | 9.2 ± 8.5% |
| 2.5, 45 | −5.0 ± 4.4% | 8.0 ± 7.3% | 5.3 ± 7.6% | −0.7 ± 9.2% | −10.2 ± 9.2% |
| 2.5, 90 | 12.5 ± 4.3% | 22.7 ± 7.6% | −3.0 ± 9.4% | −16.7 ± 7.2% | 11.3 ± 7.2% |
| 2.5, 135 | −6.0 ± 4.9% | 22.5 ± 7.1% | −9.2 ± 8.5% | 3.8 ± 7.9% | −7.4 ± 7.9% |
| 2.5, 180 | 6.4 ± 5.5% | 11.5 ± 7.4% | −18.4 ± 9.0% | 9.5 ± 7.8% | 10.1 ± 6.3% |
| 2.5, 225 | 9.1 ± 4.4% | 10.3 ± 6.7% | 9.9 ± 8.9% | 36.0 ± 9.1% | 8.2 ± 6.2% |
| 2.5, 270 | 14.3 ± 4.2% | −12.5 ± 9.3% | 17.8 ± 9.6% | 16.3 ± 9.8% | 12.5 ± 9.2% |
| 2.5, 315 | 15.0 ± 4.8% | −8.6 ± 7.3% | 3.5 ± 8.6% | 13.6 ± 8.2% | 9.0 ± 8.0% |
| Location | S26 | ||||
| 0, n/a | 7.4 ± 13.2% | ||||
| 1.25, 0 | 17.8 ± 11.7% | ||||
| 1.25, 90 | 6.9 ± 10.5% | ||||
| 1.25, 180 | 32.9 ± 8.6% | ||||
| 1.25, 270 | 7.8 ± 9.3% | ||||
| 2.5, 0 | 12.5 ± 8.5% | ||||
| 2.5, 45 | 2.5 ± 8.9% | ||||
| 2.5, 90 | 16.0 ± 9.8% | ||||
| 2.5, 135 | 13.7± 9.6% | ||||
| 2.5, 180 | 12.7 ± 8.2% | ||||
| 2.5, 225 | 31.3 ± 9.3 | ||||
| 2.5, 270 | 6.8 ± 12.1% | ||||
| 2.5, 315 | −6.5 ± 9.2 |

| Location (ecc, dir) | S01 | S02 | S03 | S04 | S05 |
| 0, n/a | 22.0 ± 11.2% | 5.6 ± 7.8% | 51.8 ± 8.5% | 17.6 ± 13.0% | 99.5 ± 0.1% |
| 1.25, 0 | 12.5 ± 6.8% | 15.2 ± 6.4% | 33.4 ± 10.2% | −29.9 ± 10.9% | 24.4 ± 6.6% |
| 1.25, 90 | 18.0 ± 8.2% | −9.5 ± 9.9% | 28.5 ± 8.5% | −11.8 ± 13.1% | 31.5 ± 7.0% |
| 1.25, 180 | 8.6 ± 8.2% | 5.8 ± 6.7% | 37.4 ± 7.7% | −1.8 ± 12.8% | 29.5 ± 9.8% |
| 1.25, 270 | 17.4 ± 7.7% | 18.7 ± 9.3% | 28.5 ± 7.3% | 38.9 ± 15.4% | 53.5 ± 7.0% |
| 2.5, 0 | 18.6 ± 6.4% | −6.0 ± 4.8% | 13.0 ± 11.4% | 6.1 ± 10.7% | 30.9 ± 8.2% |
| 2.5, 45 | 10.5 ± 9.5% | 6.4 ± 6.4% | 13.3 ± 9.0% | −7.7 ± 7.3% | 7.6 ± 10.7% |
| 2.5, 90 | 16.3 ± 9.2% | −10.6 ± 7.8% | 9.0 ± 6.8% | 11.3 ± 9.0% | 18.9 ± 8.2% |
| 2.5, 135 | 16.4 ± 8.2% | 5.0 ± 4.8% | 24.4 ± 7.9% | 34.9 ± 12.1% | 25.0 ± 7.2% |
| 2.5, 180 | 8.8 ± 11.2% | 15.1 ± 8.9% | 15.9 ± 7.2% | 27.0 ± 11.3% | 27.6 ± 6.7% |
| 2.5, 225 | 20.5 ± 6.4% | 19.8 ± 6.3% | 21.0 ± 7.9% | 21.0 ± 11.0% | 20.3 ± 7.1% |
| 2.5, 270 | 23.7 ± 7.0% | 9.1 ± 5.8% | 21.8 ± 8.4% | 38.4 ± 11.5% | 53.4 ± 6.8% |
| 2.5, 315 | 21.3 ± 8.0% | 6.8 ± 6.2% | 34.8 ± 7.1% | 2.2 ± 9.7% | 29.0 ± 7.7% |
| Location (ecc, dir) | S06 | S07 | S08 | S09 | S10 |
| 0, n/a | −52.6 ± 11.1% | −27.0 ± 8.2% | −12.6 ± 14.0% | 31.6 ± 28.8% | 31.9 ± 11.5% |
| 1.25, 0 | 7.1 ± 11.6% | −15.1 ± 9.6% | −17.1 ± 7.2% | 7.8 ± 18.4% | 28.0 ± 12.2% |
| 1.25, 90 | 12.0 ± 10.5% | −10.8 ± 9.3% | −13.2 ± 7.7% | −45.4 ± 14.9% | 41.5 ± 17.8% |
| 1.25, 180 | −19.0 ± 18.0% | −19.7 ± 8.8% | −24.3 ± 9.8% | 11.3 ± 13.9% | 1.5 ± 10.6% |
| 1.25, 270 | 11.8 ± 21.6% | −19.8 ± 9.2% | 4.1 ± 8.7% | 41.5 ± 14.1% | 9.4 ± 11.9% |
| 2.5, 0 | 30.5 ± 10.1% | −13.8 ± 6.5% | −14.1 ± 9.7% | −1.8 ± 9.2% | 13.7 ± 11.4% |
| 2.5, 45 | 11.9 ± 10.4% | −0.9 ± 9.6% | −23.0 ± 7.8% | −36.0 ± 11.4% | 18.5 ± 6.7% |
| 2.5, 90 | 63.7 ± 8.8% | −18.8 ± 8.1% | −15.8 ± 7.8% | −7.4 ± 13.3% | −1.7 ± 9.6% |
| 2.5, 135 | 17.4 ± 8.8% | −16.6 ± 7.6% | −24.2 ± 7.0% | −9.1 ± 12.3% | 10.7 ± 12.3% |
| 2.5, 180 | 3.8 ± 13.2% | −13.9 ± 10.2% | −22.6 ± 8.1% | 2.9 ± 15.3% | −0.4 ± 8.2% |
| 2.5, 225 | 40.9 ± 11.8% | 0.8 ± 7.9% | −17.0 ± 9.1% | −7.0 ± 12.6% | 15.9 ± 10.6% |
| 2.5, 270 | 44.8 ± 8.8% | −2.9 ± 7.0% | −3.4 ± 7.3% | 7.4 ± 15.5% | 20.8 ± 10.6% |
| 2.5, 315 | 14.2 ± 16.0% | −1.3 ± 9.2% | 8.0 ± 7.2% | 35.2 ± 8.6% | −10.8 ± 12.4% |
| Location (ecc, dir) | S11 | S12 | S13 | S14 | S15 |
| 0, n/a | 43.1 ± 9.0% | −39.8 ± 11.8% | 17.8 ± 16.7% | 42.7 ± 11.3% | 65.9 ± 6.7% |
| 1.25, 0 | 28.5 ± 12.3% | −13.6 ± 9.9% | 0.2 ± 7.0% | 20.7 ± 6.0% | 19.8 ± 7.4% |
| 1.25, 90 | 38.8 ± 15.5% | −1.0 ± 9.5% | −0.5 ± 10.8% | 21.9 ± 7.4% | 33.1 ± 8.8% |
| 1.25, 180 | 23.4 ± 8.7% | −13.7 ± 8.6% | 15.4 ± 16.6% | 17.1 ± 8.6% | 45.5 ± 7.5% |
| 1.25, 270 | 45.3 ± 7.7% | −11.0 ± 11.3% | 18.1 ± 7.6% | 36.0 ± 7.3% | 37.8 ± 6.0% |
| 2.5, 0 | 49.6 ± 15.7% | −1.4 ± 10.3% | 10.7 ± 10.3% | 7.6 ± 7.5% | 26.5 ± 6.9% |
| 2.5, 45 | 19.8 ± 10.0% | 7.0 ± 8.6% | 4.6 ± 10.1% | 15.7 ± 5.5% | 26.1 ± 7.4% |
| 2.5, 90 | 3.9 ± 10.2% | −9.2 ± 9.5% | 0.7 ± 7.1% | −5.6 ± 7.0% | 19.9 ± 7.0% |
| 2.5, 135 | −0.4 ± 11.6% | −10.0 ± 10.3% | 6.1 ± 10.3% | 35.9 ± 6.7% | 38.5 ± 5.7% |
| 2.5, 180 | 30.0 ± 9.7% | −18.3 ± 9.5% | 7.2 ± 10.7% | 27.4 ± 7.8% | 27.7 ± 8.5% |
| 2.5, 225 | 19.3 ± 8.4% | 12.2 ± 10.7% | 22.4 ± 7.7% | 45.2 ± 8.7% | 36.2 ± 6.6% |
| 2.5, 270 | 12.6 ± 7.2% | −12.6 ± 9.3% | −7.8 ± 7.0% | 39.9 ± 5.3% | 34.2 ± 5.2% |
| 2.5, 315 | 18.7 ± 7.4% | −5.3 ± 9.0% | −18.0 ± 7.5% | 26.9 ± 8.0% | 34.5 ± 5.3% |
| Location (ecc, dir) | S16 | S17 | S18 | S19 | S20 |
| 0, n/a | 51.0 ± 6.4% | −34.9 ± 10.5% | −16.6 ± 5.3% | 48.9 ± 14.8% | 77.1 ± 6.8% |
| 1.25, 0 | 37.2 ± 6.3% | 22.5 ± 8.3% | −1.3 ± 6.5% | 14.0 ± 9.3% | 29.7 ± 8.1% |
| 1.25, 90 | 21.6 ± 6.4% | −12.9 ± 10.6% | 9.0 ± 7.3% | 36.2 ± 6.2% | 32.0 ± 14.5% |
| 1.25, 180 | 48.4 ± 5.8% | 4.9 ± 8.9% | 13.3 ± 5.1% | 29.7 ± 7.6% | 20.5 ± 10.9% |
| 1.25, 270 | 40.1 ± 5.9% | −4.1 ± 7.5% | −10.2 ± 5.2% | 32.2 ± 7.2% | 34.7 ± 7.2% |
| 2.5, 0 | 18.2 ± 5.5% | 14.6 ± 6.3% | −13.5 ± 5.8% | 9.3 ± 6.8% | 11.3 ± 8.1% |
| 2.5, 45 | 27.1 ± 5.1% | −11.9 ± 9.0% | −1.6 ± 5.9% | 17.8 ± 6.1% | 9.5 ± 8.0% |
| 2.5, 90 | 14.9 ± 6.7% | −11.3 ± 10.5% | −8.5 ± 5.7% | 13.5 ± 6.0% | 16.9 ± 13.8% |
| 2.5, 135 | 11.1 ± 6.6% | −4.2 ± 8.2% | −11.1 ± 5.9% | 29.5 ± 8.8% | 11.8 ± 7.0% |
| 2.5, 180 | 22.2 ± 6.2% | 25.0 ± 6.1% | −13.1 ± 4.6% | 24.3 ± 7.1% | 5.8 ± 6.7% |
| 2.5, 225 | 25.3 ± 6.4% | 13.9 ± 6.4% | 8.4 ± 6.6% | 2.8 ± 7.1% | 4.0 ± 8.7% |
| 2.5, 270 | 24.2 ± 6.1% | 13.5 ± 7.1% | −11.1 ± 5.4% | 45.0 ± 5.8% | 15.7 ± 12.3% |
| 2.5, 315 | 44.2 ± 4.9% | −0.3 ± 5.7% | −17.7 ± 4.4% | 21.3 ± 7.7% | −0.4 ± 8.4% |
| Location (ecc, dir) | S21 | S22 | S23 | S24 | S25 |
| 0, n/a | −1.7 ± 7.2% | 24.4 ± 10.0% | 50.3 ± 9.2% | 37.9 ± 10.9% | 58.9 ± 8.1% |
| 1.25, 0 | 7.0 ± 5.8% | 17.2 ± 7.9% | 24.6 ± 13.7% | 52.0 ± 7.9% | 31.1 ± 6.6% |
| 1.25, 90 | 10.1 ± 6.1% | 21.5 ± 9.4% | 17.2 ± 7.7% | 32.1 ± 10.1% | 30.1 ± 6.7% |
| 1.25, 180 | −16.3 ± 4.9% | 26.1 ± 7.8% | 30.5 ± 7.6% | 47.8 ± 7.1% | 19.9 ± 6.3% |
| 1.25, 270 | −13.8 ± 5.4% | 25.9 ± 7.6% | 23.7 ± 8.2% | 33.7 ± 7.9% | 33.4 ± 8.2% |
| 2.5, 0 | 9.8 ± 4.5% | 17.5 ± 9.3% | 24.0 ± 9.7% | 20.3 ± 9.3% | 29.2 ± 7.0% |
| 2.5, 45 | −8.0 ± 4.3% | 21.4 ± 7.4% | 6.2 ± 7.3% | 37.8 ± 7.1% | 41.6 ± 6.9% |
| 2.5, 90 | 6.9 ± 4.4% | 22.3 ± 7.7% | −0.6 ± 9.1% | 31.1 ± 7.2% | 27.8 ± 6.6% |
| 2.5, 135 | −3.2 ± 4.9% | 17.9 ± 7.0% | 12.4 ± 8.5% | 30.3 ± 6.7% | 27.1 ± 6.4% |
| 2.5, 180 | −4.5 ± 5.5% | 25.2 ± 6.4% | 18.1 ± 9.4% | 32.4 ± 6.0% | 12.9 ± 6.2 |
| 2.5, 225 | −0.2 ± 4.5% | 24.7 ± 6.8% | 26.8 ± 8.4% | 27.7 ± 10.0% | 27.5 ± 4.9% |
| 2.5, 270 | −2.8 ± 4.7% | 15.2 ± 10.2% | 36.6 ± 7.7% | 19.8 ± 10.3% | 35.1 ± 7.0% |
| 2.5, 315 | −1.5 ± 5.2% | 9.1 ± 7.4% | 15.9 ± 8.2% | 32.6 ± 7.1% | 20.0 ± 7.8% |
| Location (ecc, dir) | S26 | S27 | |||
| 0, n/a | 44.2 ± 8.7% | 51.1 ± 15.3 | |||
| 1.25, 0 | 27.6 ± 10.2% | 15.9 ± 8.0% | |||
| 1.25, 90 | 21.0 ± 8.8% | 14.9 ± 8.2% | |||
| 1.25, 180 | 23.3 ± 9.7% | 12.2 ± 8.5% | |||
| 1.25, 270 | 26.9 ± 7.4% | 12.3 ± 8.0% | |||
| 2.5, 0 | −5.1 ± 9.1% | 8.5 ± 7.6% | |||
| 2.5, 45 | 15.0 ± 8.4% | −8.7 ± 7.6% | |||
| 2.5, 90 | 17.8 ± 9.6% | −12.4 ± 7.5% | |||
| 2.5, 135 | 7.3 ± 10.7% | −26.1 ± 6.7% | |||
| 2.5, 180 | 11.6 ± 8.2% | 2.0 ± 9.6% | |||
| 2.5, 225 | 16.8 ± 10.7% | 6.2 ± 7.0% | |||
| 2.5, 270 | 34.3 ± 8.9% | 3.8 ± 6.4% | |||
| 2.5, 315 | 4.4 ± 9.3% | 7.4 ± 7.1% |
References
- Barlow, H.B.; Blakemore, C.; Pettigrew, J.D. The neural mechanism of binocular depth discrimination. J. Physiol. 1967, 193, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Howard, I.P.; Rogers, B.J. Binocular Vision and Stereopsis; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Johansson, J.; Pansell, T.; Ygge, J.; Seimyr, G.O. The effect of contrast on monocular versus binocular reading performance. J. Vis. 2014, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Servos, P.; Goodale, M.A.; Jakobson, L.S. The role of binocular vision in prehension: A kinematic analysis. Vis. Res. 1992, 32, 1513–1521. [Google Scholar] [CrossRef]
- Cagenello, R.; Arditi, A.; Halpern, D.L. Binocular enhancement of visual acuity. J. Opt. Soc. Am. A 1993, 10, 1841–1848. [Google Scholar] [CrossRef]
- Blake, R.; Fox, R. The psychophysical inquiry into binocular summation. Percept. Psychophys. 1973, 14, 161–185. [Google Scholar] [CrossRef]
- Blake, R.; Sloane, M.; Fox, R. Further developments in binocular summation. Percep. Psychophys. 1981, 30, 266–276. [Google Scholar] [CrossRef]
- Halpern, D.L.; Blake, R.R. How contrast affects stereoacuity. Perception 1988, 17, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Legge, G.E. Binocular contrast summation—II. Quadratic summation. Vis. Res. 1984, 24, 385–394. [Google Scholar] [CrossRef]
- Odell, N.V.; Hatt, S.R.; Leske, D.A.; Adams, W.E.; Holmes, J.M. The effect of induced monocular blur on measures of stereoacuity. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2009, 13, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.T.; Romano, P.E. Stereoacuity degradation by experimental and real monocular and binocular amblyopia. Investig. Ophthalmol. Vis. Sci. 1985, 26, 917–923. [Google Scholar]
- Schmidt, P.P. Sensitivity of random dot stereoacuity and snellen acuity to optical blur. Optom. Vis. Sci. 1994, 71, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Campos, E. Amblyopia. Surv. Ophthalmol. 1995, 40, 23–39. [Google Scholar] [CrossRef]
- Ooi, T.L.; He, Z.J. Sensory eye dominance. Optometry 2001, 72, 168. [Google Scholar] [PubMed]
- Dieter, K.C.; Sy, J.L.; Blake, R. Individual differences in sensory eye dominance reflected in the dynamics of binocular rivalry. Vis. Res. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Zheleznyak, L.; Alarcon, A.; Dieter, K.C.; Tadin, D.; Yoon, G. The role of sensory ocular dominance on through-focus visual performance in monovision presbyopia corrections. J. Vis. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Al-Dossari, M.; Blake, R.; Brascamp, J.W.; Freeman, A.W. Chronic and acute biases in perceptual stabilization. J. Vis. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Blake, R.; McDonald, J.E., II. A new interocular suppression technique for measuring sensory eye dominance. Investig. Ophthalmol. Vis. Sci. 2010, 51, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.P.; He, Z.J.; Ooi, T.L. Effectively reducing sensory eye dominance with a push-pull perceptual learning protocol. Curr. Biol. 2010, 20, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Engel, S.A. Interocular rivalry revealed in the human cortical blind-spot representation. Nature 2001, 411, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Blake, R. A neural theory of binocular rivalry. Psychol. Rev. 1989, 96, 145. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.; Tadin, D.; Sobel, K.V.; Raissian, T.A.; Chong, S.C. Strength of early visual adaptation depends on visual awareness. Proc. Natl. Acad. Sci. USA 2006, 103, 4783–4788. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, K.; Schneider, K.A.; Kastner, S. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nat. Neurosci. 2005, 8, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.L.; He, Z.J. A distributed intercortical processing of binocular rivalry: Psychophysical evidence. Perception 2003, 32, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.P.; He, Z.J.; Ooi, T.L. A binocular perimetry study of the causes and implications of sensory eye dominance. Vis. Res. 2011, 51, 2386–2397. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Forte, J.; Cavanagh, P.; Carter, O. Onset rivalry: The initial dominance phase is independent of ongoing perceptual alternations. Front. Hum. Neurosci. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Carter, O.; Cavanagh, P. Onset rivalry: Brief presentation isolates an early independent phase of perceptual competition. PLoS ONE 2007, 2, e343. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.; Yang, Y.; Westendorf, D. Discriminating binocular fusion from false fusion. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2821–2825. [Google Scholar] [PubMed]
- Wolfe, J.M. Influence of spatial frequency, luminance, and duration on binocular rivalry and abnormal fusion of briefly presented dichoptic stimuli. Perception 1983, 12, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Dieter, K.C.; Tadin, D. Understanding attentional modulation of binocular rivalry: A framework based on biased competition. Front. Hum. Neurosci. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.A.B.; Bosker, R.J. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling, 2nd ed.; Sage: London, UK, 2012. [Google Scholar]
- Brascamp, J.W.; van Ee, R.; Noest, A.J.; Jacobs, R.H.A.H.; van den Berg, A. The time course of binocular rivalry reveals a fundamental role of noise. J. Vis. 2006, 6, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Jacot-Guillarmod, A.; Wang, Y.; Pedroza, C.; Ogmen, H.; Kilpatrick, Z.; Josic, K. Extending levelt’s propositions to perceptual multistability involving interocular grouping. Vis. Res. 2017, 133, 37–46. [Google Scholar] [CrossRef] [PubMed]
- van Ee, R. Percept-switch nucleation in binocular rivalry reveals local adaptation characteristics of early visual processing. J. Vis. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.; O’Shea, R.P.; Mueller, T.J. Spatial zones of binocular rivalry in central and peripheral vision. Vis. Neurosci. 1992, 8, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Blake, R. A fresh look at interocular grouping during binocular rivalry. Vis. Res. 2004, 44, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Alais, D.; Lorenceau, J.; Arrighi, R.; Cass, J. Contour interactions between pairs of gabors engaged in binocular rivalry reveal a map of the association field. Vis. Res. 2006, 46, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Maruya, K.; Blake, R. Spatial spread of interocular suppression is guided by stimulus configuration. Perception 2009, 38, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, I.; Papathomas, T.V.; Yang, M.; Feher, A. When the brain changes its mind: Interocular grouping during binocular rivalry. Proc. Natl. Acad. Sci. USA 1996, 93, 15508–15511. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Blake, R. Normalization regulates competition for visual awareness. Neuron 2012, 75, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Carrasco, M.; Heeger, D.J. Deconstructing interocular suppression: Attention and divisive normalization. PLOS Comput. Biol. 2016, 11, e1004510–e1004526. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Heeger, D.J. Inter-ocular contrast normalization in human visual cortex. J. Vis. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sperling, G. A gain-control theory of binocular combination. Proc. Natl. Acad. Sci. USA 2006, 103, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, A.; Dorronsoro, C.; Sawides, L.; Webster, M.A.; Marcos, S. A cyclopean neural mechanism compensating for optical differences between the eyes. Curr. Biol. 2015, 25, R188–R189. [Google Scholar] [CrossRef] [PubMed]
- Stromeyer, C.F., III; Lee, J.; Eskew, R.T. Peripheral chromatic sensitivity for flashes: A post-receptoral red-green asymmetry. Vis. Res. 1992, 32, 1865–1873. [Google Scholar] [CrossRef]
- Weitzman, D.O.; Kinney, J.A.S. Effect of stimulus size, duration, and retinal location upon the appearance of color. J. Opt. Soc. Am. 1969, 59, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Dieter, K.C.; Melnick, M.D.; Tadin, D. When can attention influence binocular rivalry? Atten. Percept. Psychophys. 2015. [Google Scholar] [CrossRef] [PubMed]
- Dieter, K.C.; Melnick, M.D.; Tadin, D. Perceptual training profoundly alters binocular rivalry through both sensory and attentional enhancements. Proc. Natl. Acad. Sci. USA 2016. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, N.; Koch, C. Continuous flash suppression reduces negative afterimages. Nature 2005, 8, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.A.; Szinte, M.; Sayim, B.; Cavanagh, P. Variations in crowding, saccadic precision, and spatial localization reveal the shared topology of spatial vision. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef] [PubMed]
- Dieter, K.C.; Blake, R. Sensory eye dominance varies within the visual field. In Proceedings of the Vision Sciences Society, St. Pete Beach, FL, USA, 16 May 2015. [Google Scholar]
- Brainard, D.H. The psychophysics toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Dieter, K.C.; Sy, J.L.; Blake, R. Data and analyses for Experiment 1. Available online: https://figshare.com/s/00e2200bd1af768df6e3 (accessed on 26 June 2017).

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieter, K.C.; Sy, J.L.; Blake, R. Persistent Biases in Binocular Rivalry Dynamics within the Visual Field. Vision 2017, 1, 18. https://doi.org/10.3390/vision1030018
Dieter KC, Sy JL, Blake R. Persistent Biases in Binocular Rivalry Dynamics within the Visual Field. Vision. 2017; 1(3):18. https://doi.org/10.3390/vision1030018
Chicago/Turabian StyleDieter, Kevin C., Jocelyn L. Sy, and Randolph Blake. 2017. "Persistent Biases in Binocular Rivalry Dynamics within the Visual Field" Vision 1, no. 3: 18. https://doi.org/10.3390/vision1030018




