Proliferating Cells in Knee Epiphyses of Lizards Allow for Somatic Growth and Regeneration after Damage
Abstract
:1. Introduction
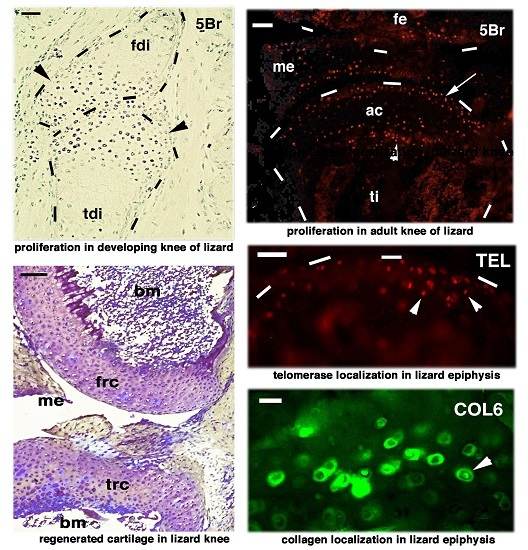
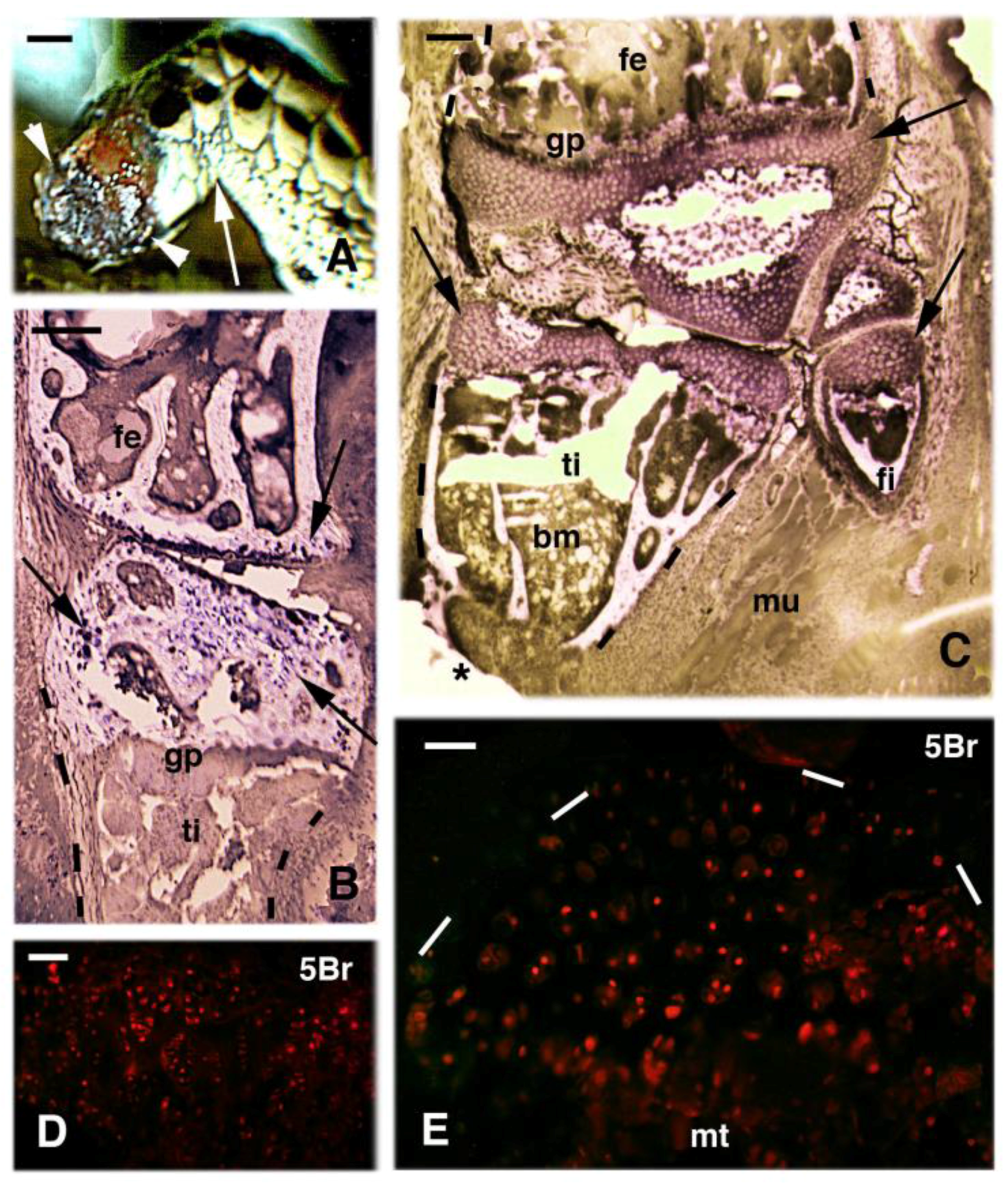
2. Knee Formation and Maturation
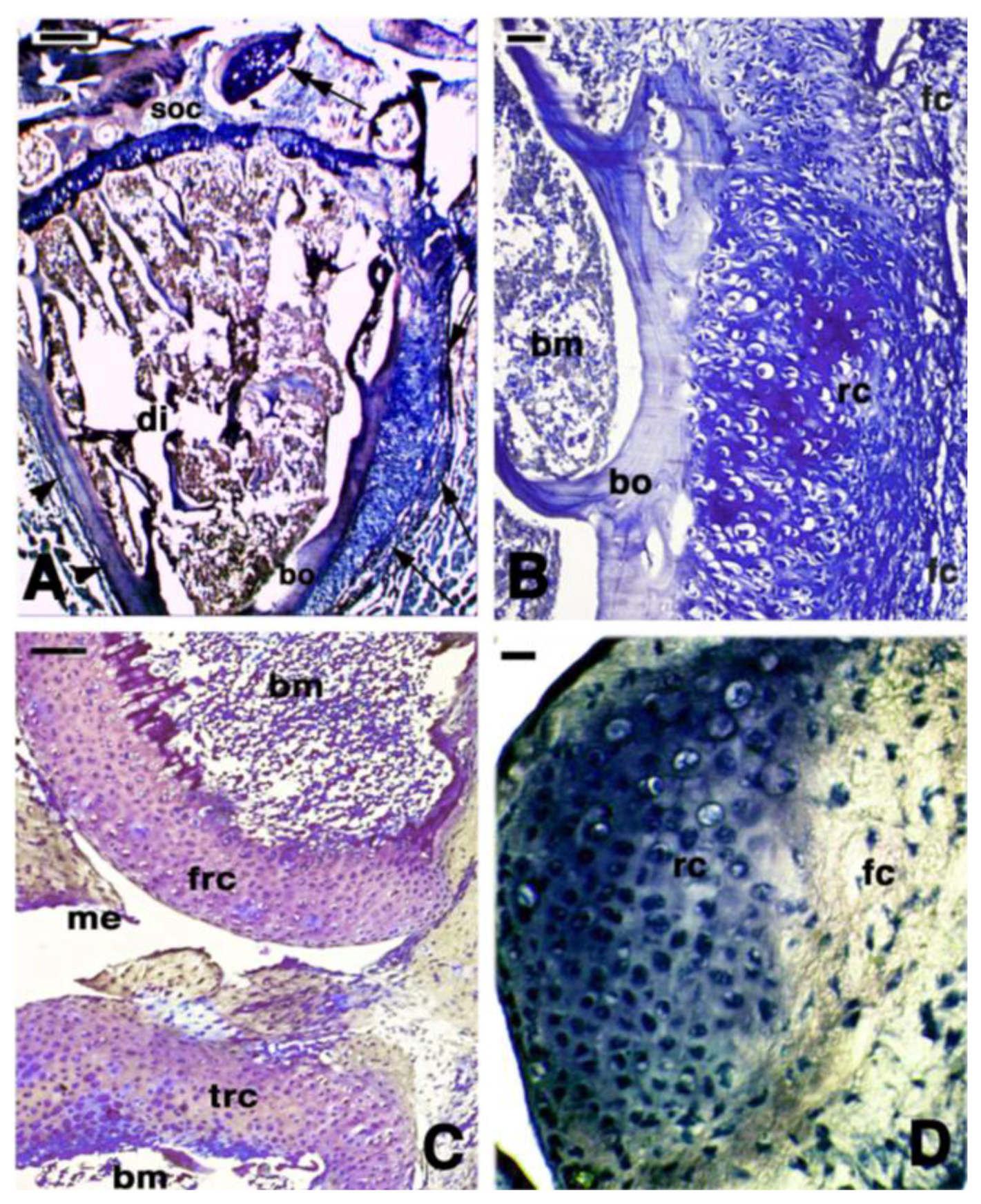
3. Knee Recovery and Regeneration after Injury
4. Knee Regeneration is Supported by Resident Progenitor Chondroblasts
Acknowledgments
Conflicts of Interest
References
- Bellairs, A.d’A.; Bryant, S.V. Autotomy and Regeneration in Reptiles. In Biology of the Reptilia; Gans, C., Billet, F., Maderson, P.F.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1985; Volume 15B, pp. 302–410. [Google Scholar]
- Alibardi, L.; Sala, M. Indagini istochimiche sulla struttura della cartilagine rigenerata nella coda di Lacerta sicula. Arch. Ital. Anat. Embr. 1981, 88, 163–182. [Google Scholar]
- Alibardi, L.; Meyer-Rochow, V.B. Comparative fine structure of the axial skeleton inside the regenerated tail of lizards and the tuatara (Sphenodon punctatus). Gegenbaurs Morphol. Jahrb. 1989, 135, 705–716. [Google Scholar] [PubMed]
- Alibardi, L. Development of the axial cartilaginous skeleton in the regenerating tail of lizards. Bull. Assoc. Anat. 1995, 79, 3–9. [Google Scholar]
- Alibardi, L. Morphological and cellular aspects of tail and limb regeneration in lizard: A model system with implications for tissue regeneration in mammals. Adv. Anat. Embryol. Cell Biol. 2010, 207, 1–112. [Google Scholar]
- Alibardi, L. Original and regenerating lizard tail cartilage contain putative resident stem/progenitor cells. Micron 2015, 78, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.E.; Geiger, L.A.; Stroik, L.K.; Hutchins, E.D.; George, R.M.; DeNardo, D.F.; Kosumi, K.; Rawls, J.A.; Wilson-Rawls, J. A histological comparison of the original and regenerated tail in the green anole, Anolis carolinensis. Anat. Rec. 2012, 295, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.A.B.; Payne, S.L.; Vickaryous, M.K. The anatomy and histology of caudal autotomy and regeneration in lizards. Physiol. Biochem. Zool. 2013, 86, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Lozito, T.P.; Tuan, R.S. Lizard tail regeneration: Regulation of two distinct cartilage regions by Indian hedgehog. Dev. Biol. 2015, 399, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Lozito, T.P.; Tuan, R.S. Lizard tail skeletal regeneration combines aspects of fracture healing and blastema-based regeneration. Development 2016, 143, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.J.; Ruzicka, A.J. Comparison of fracture repair in the frog, lizard and rat. J. Anat. 1950, 84, 236–262. [Google Scholar] [PubMed]
- Alibardi, L. Cell proliferation in the amputated limb of lizard leading to scarring is reduced compared to the regenerating tail. Acta Zool. 2017, 98, 170–180. [Google Scholar] [CrossRef]
- Alibardi, L. Immunolocalization of 5BrdU long retaining labeled cells and macrophage infiltration in the scarring limb of lizard after limb amputation. Tiss. Cell 2016, 48, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lozito, T.P.; Tuan, R.S. Lizard tail regeneration as an instructive model of enchanced healing capabilities in an adult amniote. Conn. Tiss. Res. 2016, 26, 1–10. [Google Scholar]
- Neufield, D.A. Bone healing after amputation of mouse digits and newt limbs: Implications for induced regeneration in mammals. Anat. Rec. 1985, 211, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Stocum, D.L. Regenerative Biology and Medicine; Academic Press-Elsevier: London, UK, 2006. [Google Scholar]
- Han, M.; Yang, X.; Taylor, G.; Bursdal, C.A.; Anderson, R.A.; Muneoka, K. Limb regeneration in higher vertebrates: Developing a roadmap. Anat. Rec. 2005, 287B, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Kapfinger, E.; Geiss, J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr. Cartil. 2007, 15, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Min. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Egawa, S.; Miura, S.; Yokoyama, H.; Endo, T.; Tanura, K. Growth and differentiation of a long bone in limb development, repair and regeneration. Dev. Growth Differ. 2014, 56, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Cosden, R.S.; Lattermann, C.; Romine, S.; Gao, J.; Voss, S.R.; MacLeod, J.N. Intrinsic repair of full-thickness articular cartilage defects in the axolotl salamander. Osteoarthr. Cartil. 2011, 19, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Gardiner, D.M. Regeneration of limb joints in the axolotl (Ambystoma mexicanum). PLoS ONE 2012, 7, e50615. [Google Scholar] [CrossRef] [PubMed]
- Mescher, A.L.; Neff, A.W.; King, M.W. Inflammation and immunity in organ regeneration. Dev. Comp. Immunol. 2017, 66, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Rhosenthal, N. Scar-free healing and regeneration in amphibians: Immunological influence on regenerative success. Differentiation 2014, 87, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Microscopic observations show invasion of inflammatory cells in the limb blastema and epidermis in pre-metamorphic frog tadpoles which destroy the apical epidermal cap and impede regeneration. Ann. Anat. 2016, 210, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. FGFs treatment on amputated lizard limbs stimulate the regeneration of long bones, opening new avenues for limb regeneration in amniotes. J. Funct. Morphol. Kinesiol. 2017. under review. [Google Scholar]
- Alibardi, L. Permanence of proliferating cells in developing, juvenile and adult knee epiphyses of lizards in relation to bone growth and regeneration. Acta Zool. 2017, 98, 278–284. [Google Scholar] [CrossRef]
- Mankin, H.J. Localization of tritiated thymidine in articular cartilage of rabbits: I. Growth in immature cartilage. J. Bone Joint. Surg. 1962, 44, 682–688. [Google Scholar] [CrossRef]
- Archer, C.W.; Morrison, H.; Pitsillides, A.A. Cellular aspects of the development of diarthrodial joints and articular cartilage. J. Anat. 2009, 184, 447–456. [Google Scholar]
- Reno, P.L.; McBurney, D.L.; Lovejoy, C.O.; Horton, W.E. Ossification of the mouse metatarsal: Differentiation and proliferation in the presence/absence of a defined growth plate. Anat. Rec. 2006, 288A, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Haines, R.W. The evolution of epiphyses and of endochondral bone. Biol. Rev. 1942, 17, 267–292. [Google Scholar] [CrossRef]
- Haines, R.W. Epiphyses and Sesamoids. In Biology of the Reptilia; Gans, C., Ed.; Academic Press: London, UK, 1969; Volume 1 Morphology A, pp. 81–115. [Google Scholar]
- Alibardi, L. Ultrastructural features of the process of wound healing after tail and limb amputation in lizard. Acta Zool. 2010, 91, 306–318. [Google Scholar] [CrossRef]
- Alibardi, L. Regeneration of the articular cartilage in lizard knee from resident stem/progenitor cells. Int. J. Mol. Sci. 2015, 16, 20731–20747. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Localization of proliferating cells in the inter-vertebral region of the developing and adult vertebrae of lizards in relation to growth and regeneration. Anat. Rec. 2016, 299, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Regeneration of the epiphyses including the articular cartilage in the injured knees of the lizard Podarcis muralis. J. Dev. Biol. 2015, 3, 71–89. [Google Scholar] [CrossRef]
- Shapiro, F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cells Mater. 2008, 15, 53–76. [Google Scholar] [CrossRef]
- Hutchinson, C.; Pilote, M.; Roy, S. The axolotl limb: A model for bone development, regeneration and fracture healing. Bone 2007, 40, 45–56. [Google Scholar] [CrossRef] [PubMed]
- King, M.W.; Neff, A.W.; Mescher, A.L. The developing Xenopus limb as a model for studies on the balance between inflammation and regeneration. Anat. Rec. 2012, 295B, 1552–1561. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alibardi, L. Proliferating Cells in Knee Epiphyses of Lizards Allow for Somatic Growth and Regeneration after Damage. J. Funct. Morphol. Kinesiol. 2017, 2, 23. https://doi.org/10.3390/jfmk2030023
Alibardi L. Proliferating Cells in Knee Epiphyses of Lizards Allow for Somatic Growth and Regeneration after Damage. Journal of Functional Morphology and Kinesiology. 2017; 2(3):23. https://doi.org/10.3390/jfmk2030023
Chicago/Turabian StyleAlibardi, Lorenzo. 2017. "Proliferating Cells in Knee Epiphyses of Lizards Allow for Somatic Growth and Regeneration after Damage" Journal of Functional Morphology and Kinesiology 2, no. 3: 23. https://doi.org/10.3390/jfmk2030023