Total Body Water Distribution in Breast Cancer Survivors Following Cancer Rehabilitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population Studied
2.2. Protocol Study
2.3. Bioelectrical Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Haussinger, D.; Roth, E.; Lang, F.; Gerok, W. Cellular hydration state: An important determinant of protein catabolism in health and disease. Lancet 1993, 341, 1330–1332. [Google Scholar] [CrossRef]
- Marken Lichtenbelt, W.D.; Fogelholm, M. Increased extracellular water compartment, relative to intracellular water compartment, after weight reduction. J. Appl. Physiol. 1999, 87, 294–298. [Google Scholar] [PubMed]
- Wareham, N.J.; van Sluijs, E.M.; Ekelund, U. Physical activity and obesity prevention: A review of the current evidence. Proc. Nutr. Soc. 2005, 64, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Manz, F.; Wentz, A. The importance of good hydration for the prevention of chronic disease. Nutr. Rev. 2005, 63, 2–5. [Google Scholar] [CrossRef]
- Stefani, L.; Galanti, G.; Klika, R. Clinical implementation of exercise guidelines for cancer patients: Adaptation of ACSM’s guidelines to the Italian model. J. Funct. Morphol. Kinesiol. 2017, 2, 4. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; ACSM position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Exercise Physiology: Nutrition, Energy, and Human Performance; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2015. [Google Scholar]
- Pescatello, L.S.; Arena, R.; Riebe, D.; Thompson, P.D. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; p. 456. [Google Scholar]
- Human Energy Requirements: Energy Requirement of Adults. Report of a Joint FAO/WHO/UNU Expert Consultation. Food and Agriculture Organization of the United Nations, 2004. Available online: http://www.fao.org/docrep/007/y5686e/y5686e00.htm (accessed on 18 April 2017).
- Guyatt, G.H.; Sullivan, M.J.; Thompson, P.J.; Fallen, E.L.; Pugsley, S.O.; Taylor, D.W.; Berman, L.B. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can. Med. Assoc. J. 1985, 132, 919–923. [Google Scholar] [PubMed]
- Robertson, R.J.; Goss, F.L.; Dubé, J.; Rutkowski, J.; Dupain, M.; Brennan, C.; Andreacci, J. Validation of the adult OMNI scale of perceived exertion for cycle ergometer exercise. Med. Sci. Sports Exerc. 2004, 36, 102–108. [Google Scholar] [CrossRef] [PubMed]
- WHO. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Dehghan, M; Merchant, A. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr. J. 2008, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; de Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Pichard, C. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Waki, M.; Kral, J.G.; Mazariegos, M.; Wang, J.; Pierson, R.N.; Heymsfield, S.B. Relative expansion of extracellular fluid in obese vs. nonobese women. Am. J. Physiol. 1991, 261, 199–203. [Google Scholar]
- Human Energy Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation; FAO Food and Nutrition Technical Report Series 1; FAO/WHO/UNU: Rome, Italy, 2001. [Google Scholar]
- Knudsen, N.N.; Kjærulff, T.M.; Ward, L.C.; Sæbye, D.; Holst, C.; Heitmann, B.L. Body water distribution and risk of cardiovascular morbidity and mortality in a healthy population: A prospective cohort study. PLoS ONE 2014, 3, e87466. [Google Scholar] [CrossRef] [PubMed]
- Judelson, D.A.; Maresh, C.M.; Anderson, J.M.; Armstrong, L.E.; Casa, D.J.; Kraemer, W.J.; Volek, J.S. Hydration and muscular performance: Does fluid balance affect strength, power and high-intensity endurance? Sports Med. 2007, 37, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lammersfeld, C.A.; Burrows, J.L.; Dahlk, S.L.; Vashi, P.G.; Grutsch, J.F.; Hoffman, S.; Lis, C.G. Bioelectrical impedance phase angle in clinical practice: Implications for prognosis in advanced colorectal cancer. Am. J. Clin. Nutr. 2004, 80, 1634–1638. [Google Scholar] [PubMed]
- Stefani, L.; Petri, C.; Mascherini, G.; Galanti, G. Lifestyle intervention in surviving cancer patients. J. Funct. Morphol. Kinesiol. 2016, 1, 48–53. [Google Scholar] [CrossRef]


| n = 28 Cancer Patients | T0 | T6 | PΔt0–t6 | T12 | PΔt0–t12 | ANOVA Test |
|---|---|---|---|---|---|---|
| Weight (kg) | 70.2 ± 9.9 | 69.9 ± 14.9 | NS | 70.5 ± 15.8 | NS | NS |
| BMI (kg·m−2) | 26.7 ± 5.4 | 26.6 ± 5.6 | NS | 26.8 ± 5.8 | NS | NS |
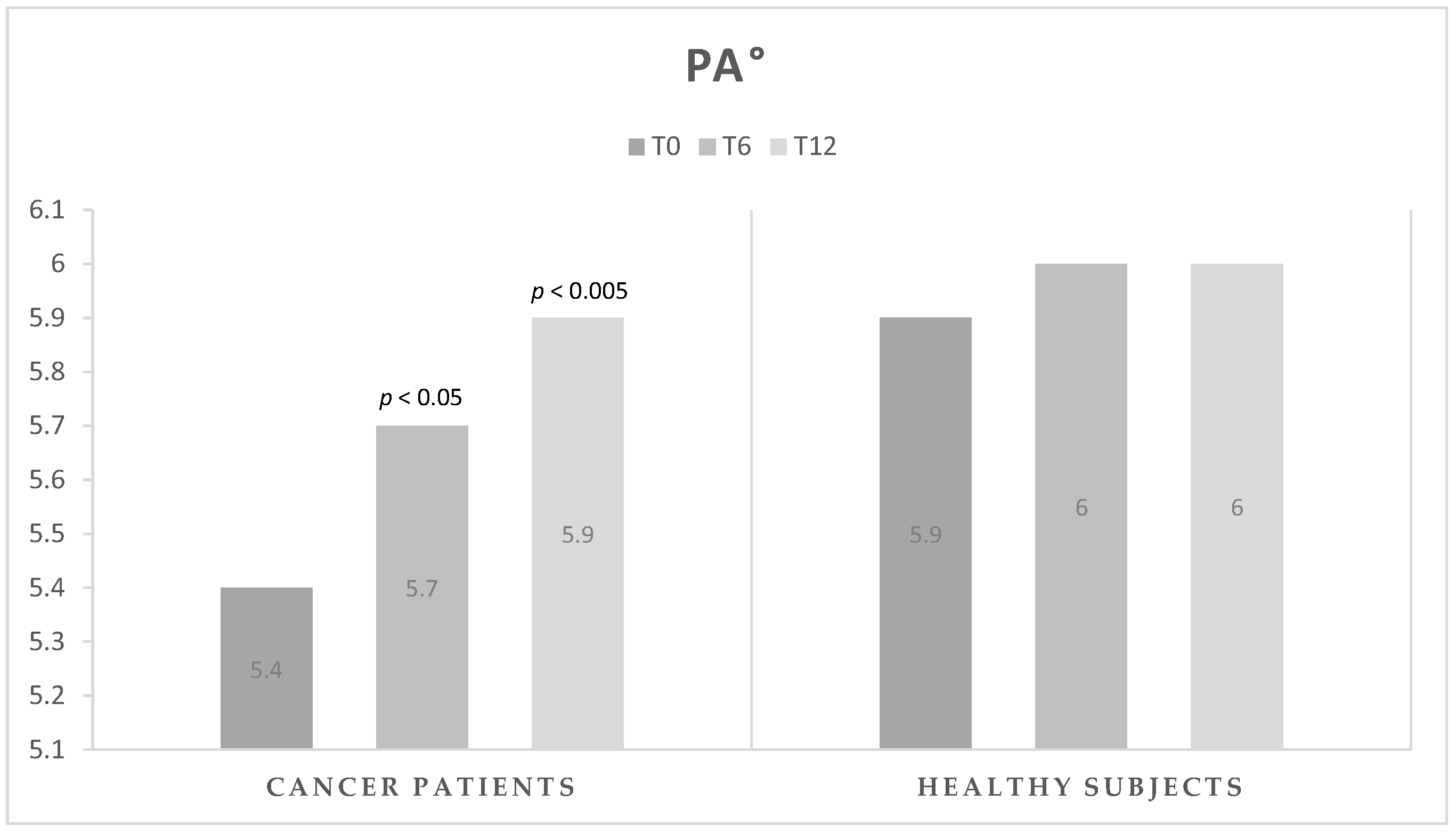
| PA (°) | 5.4 ± 0.7 | 5.7 ± 0.8 | <0.05 | 5.9 ± 0.7 | <0.005 | <0.05 |
| FM (%) | 34.6 ± 8.3 | 34.4 ± 8.5 | NS | 33.7 ± 10.3 | NS | NS |
| FFM (%) | 65.4 ± 8.3 | 65.6 ± 8.5 | NS | 66.3 ± 10.3 | NS | NS |
| TBW (%) | 49.2 ± 5.6 | 49.5 ± 6.0 | NS | 50.0 ± 7.0 | NS | NS |
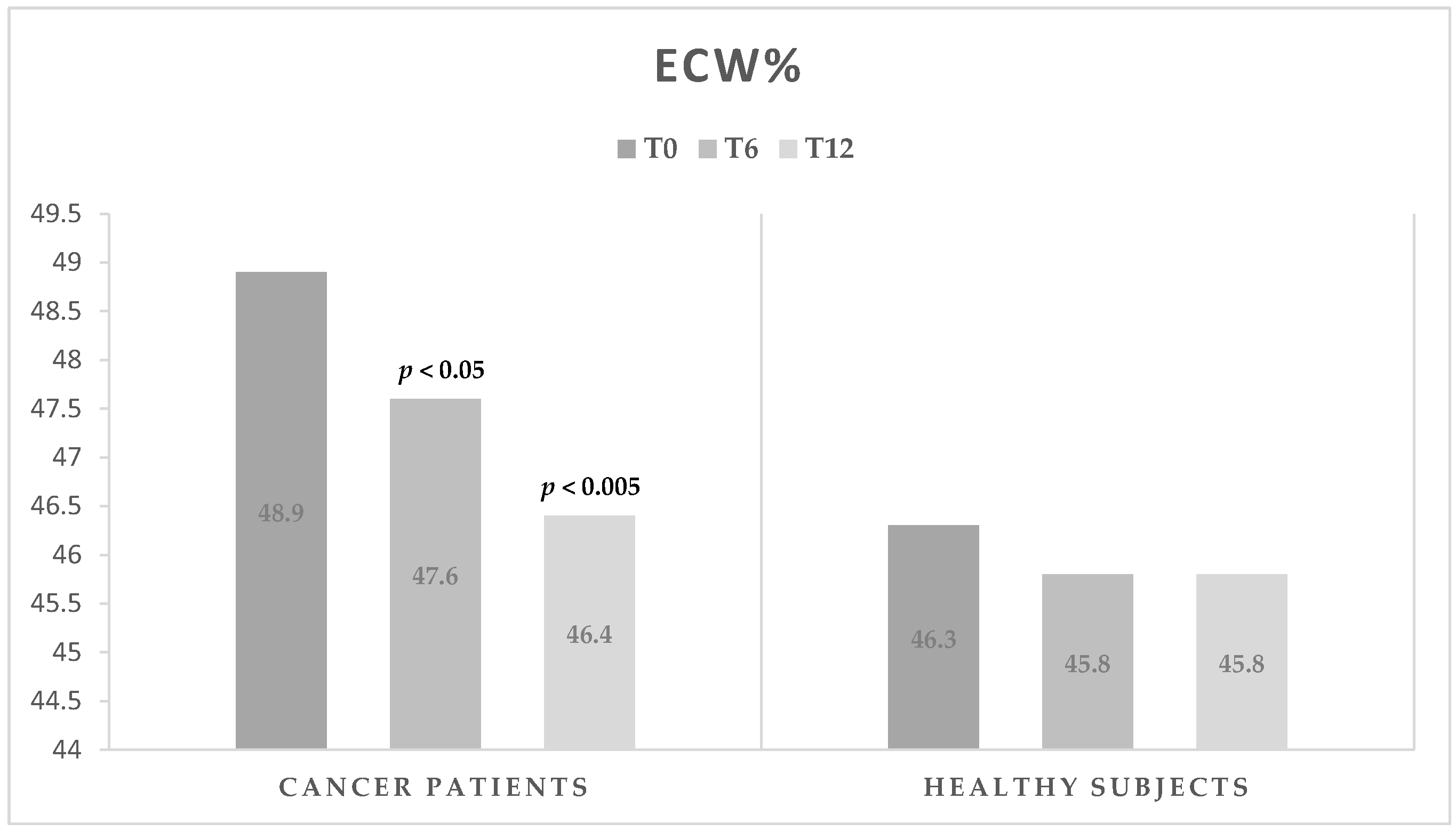
| ECW (%) | 48.9 ± 3.9 | 47.6 ± 4.1 | <0.05 | 46.4 ± 3.1 | <0.005 | <0.05 |
| ICW (%) | 51.1 ± 3.9 | 52.4 ± 4.1 | <0.05 | 53.6 ± 3.1 | <0.005 | <0.05 |
| BCM (%) | 50.3 ± 4.2 | 51.7 ± 4.4 | <0.05 | 53.0 ± 3.4 | <0.005 | <0.05 |
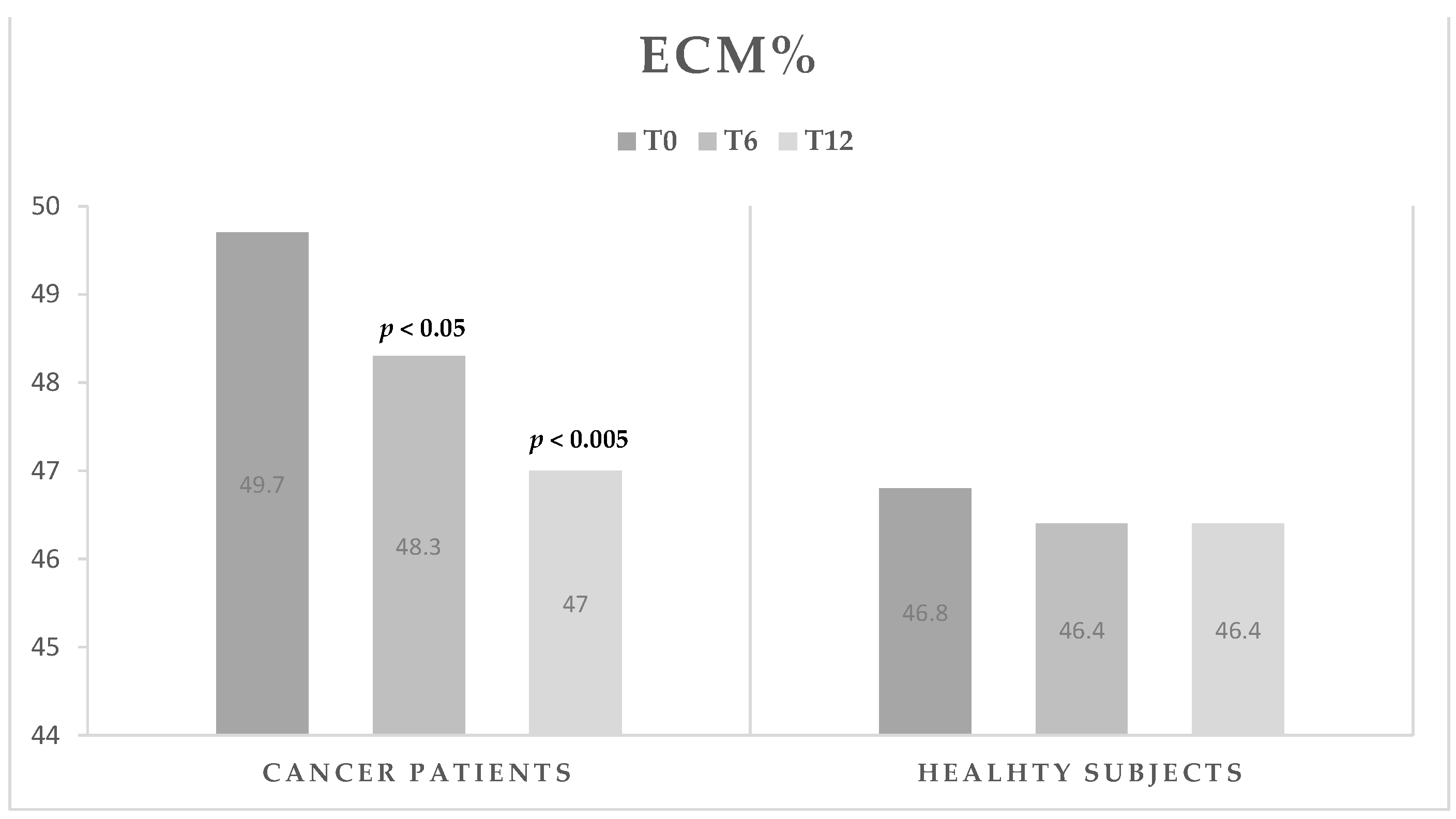
| ECM (%) | 49.7 ± 4.2 | 48.3 ± 4.4 | <0.05 | 47.0 ± 3.4 | <0.005 | <0.05 |
| n = 23 Healthy Subjects | T0 | T6 | PΔt0–t6 | T12 | PΔt0–t12 | ANOVA Test |
|---|---|---|---|---|---|---|
| Weight (kg) | 76.6 ± 12.3 | 76.0 ± 11.6 | NS | 75.6 ± 11.4 | NS | NS |
| BMI | 26.1 ± 2.8 | 25.8 ± 2.4 | NS | 25.6 ± 2.5 | NS | NS |
| PA (°) | 5.9 ± 0.9 | 6.0 ± 0.9 | NS | 6.0 ± 0.9 | NS | NS |
| FM (%) | 28.8 ± 5.4 | 27.5 ± 6.4 | <0.05 | 28.0 ± 5.4 | NS | NS |
| FFM (%) | 71.2 ± 5.4 | 72.5 ± 6.4 | <0.05 | 72.0 ± 5.4 | NS | NS |
| TBW (%) | 53.0 ± 4.4 | 54.1 ± 5.0 | <0.05 | 53.7 ± 4.3 | NS | NS |
| ECW (%) | 46.3 ± 4.2 | 45.8 ± 3.9 | NS | 45.8 ± 4.6 | NS | NS |
| ICW (%) | 53.7 ± 4.2 | 54.2 ± 3.9 | NS | 54.2 ± 4.6 | NS | NS |
| BCM (%) | 53.2 ± 4.5 | 53.6 ± 4.3 | NS | 53.6 ± 5.0 | NS | NS |
| ECM (%) | 46.8 ± 4.5 | 46.4 ± 4.3 | NS | 46.4 ± 5.0 | NS | NS |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefani, L.; Palmerini, D.; Corezzi, M.; Mascherini, G.; Petri, C.; Klika, R.J.; Galanti, G. Total Body Water Distribution in Breast Cancer Survivors Following Cancer Rehabilitation. J. Funct. Morphol. Kinesiol. 2017, 2, 12. https://doi.org/10.3390/jfmk2020012
Stefani L, Palmerini D, Corezzi M, Mascherini G, Petri C, Klika RJ, Galanti G. Total Body Water Distribution in Breast Cancer Survivors Following Cancer Rehabilitation. Journal of Functional Morphology and Kinesiology. 2017; 2(2):12. https://doi.org/10.3390/jfmk2020012
Chicago/Turabian StyleStefani, Laura, Davide Palmerini, Michele Corezzi, Gabriele Mascherini, Cristian Petri, Riggs J. Klika, and Giorgio Galanti. 2017. "Total Body Water Distribution in Breast Cancer Survivors Following Cancer Rehabilitation" Journal of Functional Morphology and Kinesiology 2, no. 2: 12. https://doi.org/10.3390/jfmk2020012







