Optimized Treatment and Recovery of Irradiated [18O]-Water in the Production of [18F]-Fluoride
Abstract
:1. Introduction
2. Materials and Methods
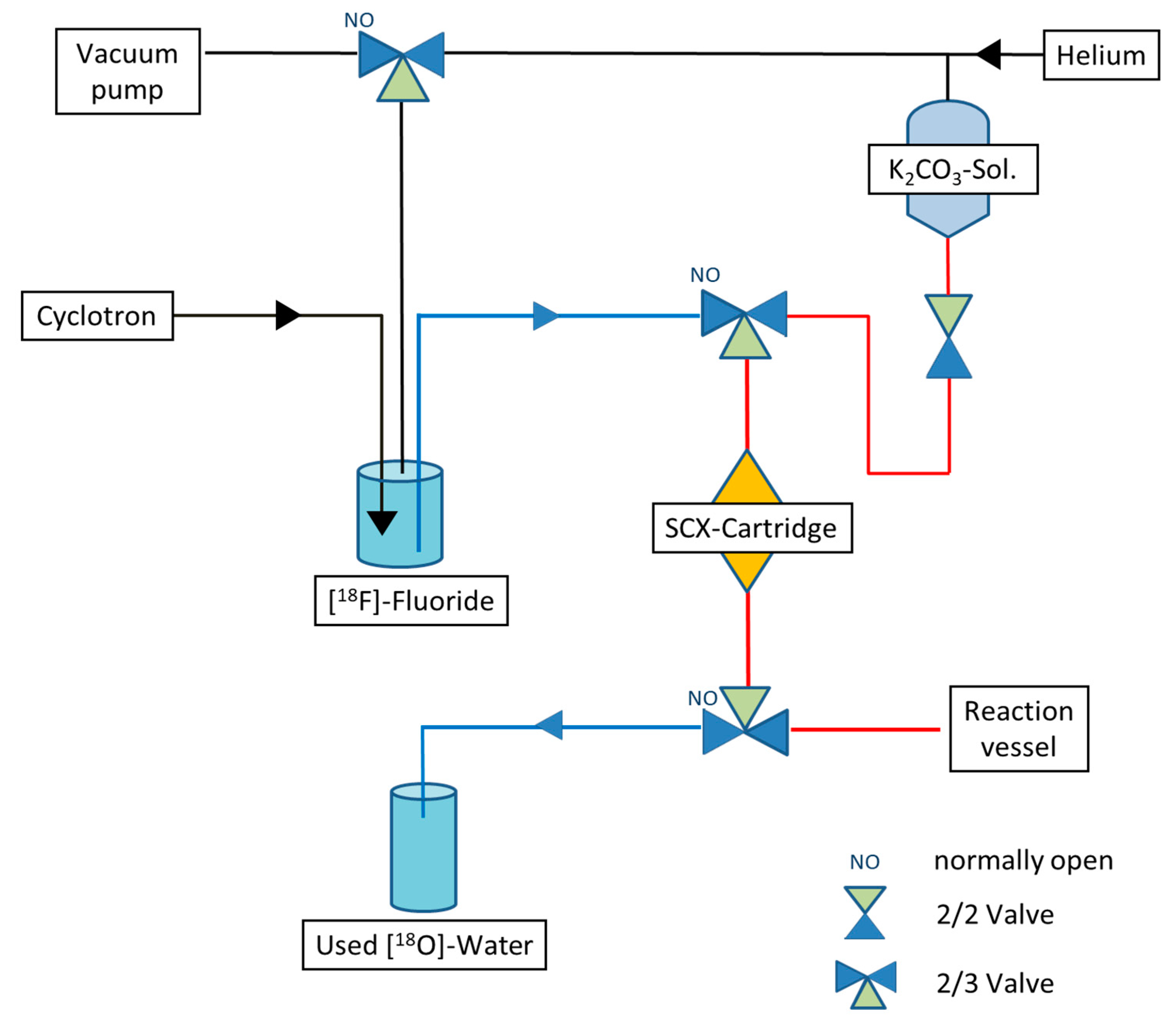
2.1. Overview of the Optimized Recovery Method
2.2. Improved Synthesis Preparation for Maintaining the Enrichment Grade
2.3. Purification by Fractional Distillation
2.4. Separation of the [18O]-Water from the Azeotropic Mixtures by Means of a Molecular Sieve
2.5. Determination of the Degree of Enrichment via Pycnometry
- m [16O]-water (T) = mass of [16O]-water at certain temperature and pipette
- m [18O]-water (T) = mass of [18O]-water sample at certain temperature and pipette
- w: enrichment grade in %
- 9 = 1/K, K = (20.0153/18.0153) − 1;
- K: constant from the difference of the molecular weights.
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Uhlending, H.; Burchert, W. Einfluss von FKM oder Nitril-Target-Dichtungen auf radiochemische Ausbeuten. Nuklearmedizin 2017, 56, A84. [Google Scholar]
- Bowden, L.; Vintro, L.L.; Mitchell, P.I.; O’Donnell, R.G.; Seymour, A.M.; Duffy, G.J. Radionuclide impurities in proton-irradiated [18O]H2O for the production of 18F-: Activities and distribution in the [18F]FDG synthesis process. Appl. Radiat. Isot. 2009, 67, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, D.J.; Bastos, M.A.V.; Alexoff, D.; Wolf, A.P. Separation of [18F]fluoride from [18O]water using anion exchange resin. Int. J. Radiat. Appl. Instrum. A 1990, 41, 531–533. [Google Scholar] [CrossRef]
- GE Healthcare. PETtrace OPERATOR GUIDE; DIRECTION 2131768-100 REVISION 15. Unpublished work. 2013; 102. [Google Scholar]
- Schlyer, D.J.; Firouzbakht, M.L.; Wolf, A.P. Impurities in the [18O]water target and their effect on the yield of an aromatic displacement reaction with [18F]fluoride. Appl. Radiat. Isot. 1993, 44, 1459–1465. [Google Scholar] [CrossRef]
- Mangner, T.J.; Mulholland, G.K.; Toorongian, S.A.; Jewett, D.M.; Kilbourn, M.R. Purification of used O-18 target water by photochemical combustion. J. Nucl. Med. 1992, 33, 982–983. [Google Scholar]
- Kitano, H.; Magata, Y.; Tanaka, A.; Mukai, T.; Kuge, Y.; Nagatsu, K.; Konishi, J.; Saji, H. Performance assessment of O-18 water purifier. Ann. Nucl. Med. 2001, 15, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Asti, M.; Grassi, E.; Sghedoni, R.; De Pietri, G.; Fioroni, F.; Versari, A.; Borasi, G.; Salvo, D. Purification by ozonolysis of (18)O enriched water after cyclotron irradiation and the utilization of the purified water for the production of [18F]-FDG (2-deoxy-2-[18F]-fluoro-d-glucose). Appl. Radiat. Isot. 2007, 65, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, M.; Luxen, A.; Nebeling, B.; Argentini, M.; Clark, J.C.; Pike, V.W. Recommendations for fluorine-18 production. Int. J. Radiat. Appl. Instrum. A 1990, 42, 749–762. [Google Scholar] [CrossRef]
- Moon, B.S.; Lee, K.C.; An, G.I.; Chi, D.Y.; Yang, S.D.; Choi, C.W.; Lim, S.M.; Chun, K.S. Preparation of 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT) in ionic liquid, [bmim][OTf]. J. Label. Compd. Radiopharm. 2006, 49, 287–293. [Google Scholar] [CrossRef]
- Uhlending, A.; Henneken, H.; Burchert, W. Abreicherungsarme [18O]-Wasserrückgewinnung durch optimierte Synthesevorbereitung. Nuklearmedizin 2013, 52, A98. [Google Scholar]
- Krasikova, R. Synthesis Modules and Automation in F-18 Labeling. In PET Chemistry; Schubiger, P.A., Lehrmann, L., Friebe, M., Eds.; Ernst Schering Research Foundation Workshop; Springer: Berlin/Heidelberg, Germany, 2007; Volume 64. [Google Scholar]
- Uhlending, A.; Henneken, H.; Burchert, W. Destillation als Reinigungsverfahren zur Mehrfachverwendung von [18O]-angereichertem Wasser bei der 18F-Fluorid-Produktion. Nuklearmedizin 2005, 44, A175. [Google Scholar]
- Gail, R.; Hamacher, K. Verfahren zur Abtrennung von H218O Aus Einem Organischen Lösungsmittel. DE19948427A1, 3 May 2001. [Google Scholar]
- Müller, E.; Bayer, O.; Meerwein, H.; Ziegler, K. Methoden der Organischen Chemie (Houben-Weyl)—Band I/2: Allgemeine Laboratoriumspraxis; Thieme: Stuttgart, Germany, 1959; p. 828. [Google Scholar]
- Uhlending, A.; Henneken, H.; Burchert, W. Routinetaugliche Abtrennung von [18O]-Wasser aus azeotropen [18O]-Wasser/Acetonitril-Mischungen unter Einsatz von Molsieb. Nuklearmedizin 2010, 49, A112. [Google Scholar]
- Fawdry, R.M. A simple effective method for estimating the [18O] enrichment of water mixtures. Appl. Radiat. Isot. 2004, 60, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Uhlending, A.; Henneken, H.; Burchert, W. Pykonmetirsche Bestimmung des 18O-Isotpengehaltes von 18O-angrereicherten Wasser: Validierung und Anwendung in der Routine. Nuklearmedizin 2004, 43, A155. [Google Scholar]



| Enrichment Grade | Common Synthesis Preparation | Drying Module + Cartridge with Helium Flow | Drying Module + Cartridge with Acetonitrile |
|---|---|---|---|
| [%] | 74.0–80.0 | 84.0–88.0 | 95.0–96.5 |
| Parameter | Method | Device |
|---|---|---|
| [18O]-Enrichment grade | Relative pycnometry | Libra, pipette |
| Solvent content | Gas chromatography | Trace 1310 Thermo Fischer, FID |
| Aromatic compounds | UV-Spectroscopy | Genesys 10 S Thermo Fisher |
| Ions | Conductivity measurement | Vario Cond, WTW, 0.001-200 µS/cm |
| Radioactive nuclide | Gamma measurement, multi-channel-analyzer | Germanium Detector GC 1200-7500 SL Canberra |
| Parameter | Unit | Collected- [18O]-H2O | Main Fraction | First Fraction | Residue | Specification |
|---|---|---|---|---|---|---|
| Volume | % | 100 | 80 | 15 | 5 | - |
| Conductivity | µS/cm | 1200 | 0.9 | 133 | 3000 | <10 |
| Acetonitrile | % | 8–10 | <0.00001 | 40–80 | <0.1 | <0.0001 |
| Ethanol | µg/mL | 126 | <0.1 | 1600 | <0.1 | <100 |
| Acetone | µg/mL | 19.2 | <0.1 | 260 | <0.1 | <100 |
| UV-Spectrum | 220 nm | 0.120 | 0.0005 | 0.052 | 2.095 | <0.2 |
| 280 nm | 0.040 | 0.0014 | 0.010 | 0.400 | <0.1 | |
| MCA | keV | 810; 846; 1238; 1460; 1770 | not detectable | 136; 1460 | 122; 320; 744; 810; 846; 1037; 1238; 1770 | not detectable |
| [18O]-Enrichment | % | 100 | <0.1 | 0.3 | 99.6 | - |
| % | 95.0 | 94.5 | - | - | 95.5 |
| Enrichment Grade | [18O]-Water Original | [18O]-Water after Distillation | [18O]-Water after Molecular Sieve |
|---|---|---|---|
| [%] | 97.0 | 96.5 | 96.5 |
| Molecular Sieve | Amount | Equates to X mL H2O |
| 150 g | 200 mL | |
| Absorbed water | 0.2 mL/g | 30 mL |
| Substituted O-Functions | 3.3 mmol/g | 7.6 mL |
| Separation Temp. for Acetonitrile | 40 °C | Desorption Temp. [18O]-H2O | 200 °C |
|---|---|---|---|
| Recovery rate | >95% | time | 30 min |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhlending, A.; Henneken, H.; Hugenberg, V.; Burchert, W. Optimized Treatment and Recovery of Irradiated [18O]-Water in the Production of [18F]-Fluoride. Instruments 2018, 2, 12. https://doi.org/10.3390/instruments2030012
Uhlending A, Henneken H, Hugenberg V, Burchert W. Optimized Treatment and Recovery of Irradiated [18O]-Water in the Production of [18F]-Fluoride. Instruments. 2018; 2(3):12. https://doi.org/10.3390/instruments2030012
Chicago/Turabian StyleUhlending, Antje, Harald Henneken, Verena Hugenberg, and Wolfgang Burchert. 2018. "Optimized Treatment and Recovery of Irradiated [18O]-Water in the Production of [18F]-Fluoride" Instruments 2, no. 3: 12. https://doi.org/10.3390/instruments2030012




