Crystal Growth Techniques for Layered Superconductors
Abstract
:1. Introduction
2. Single-Crystal Growth Techniques for Incongruent Melting Compounds
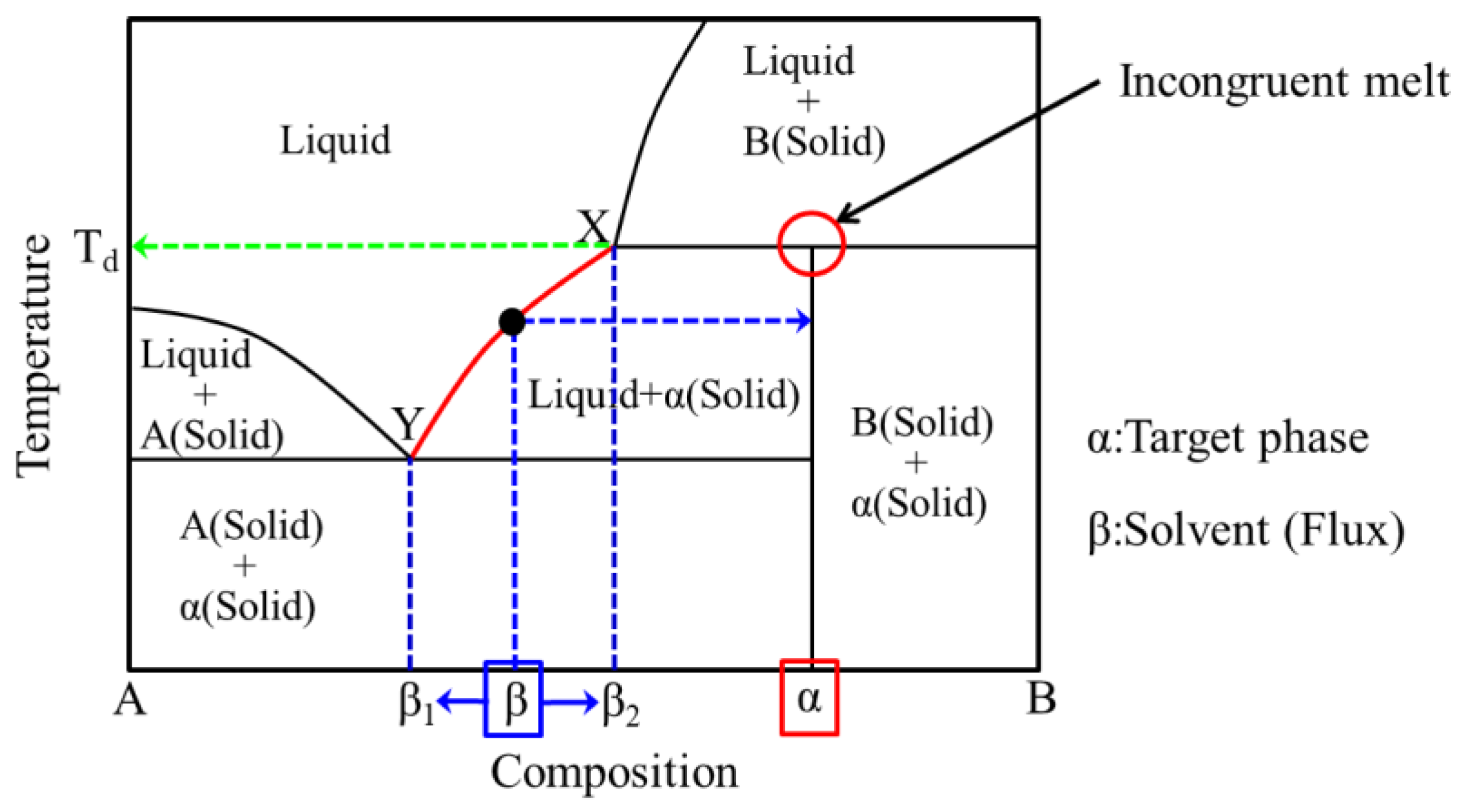
2.1. (Conventional) Flux Method
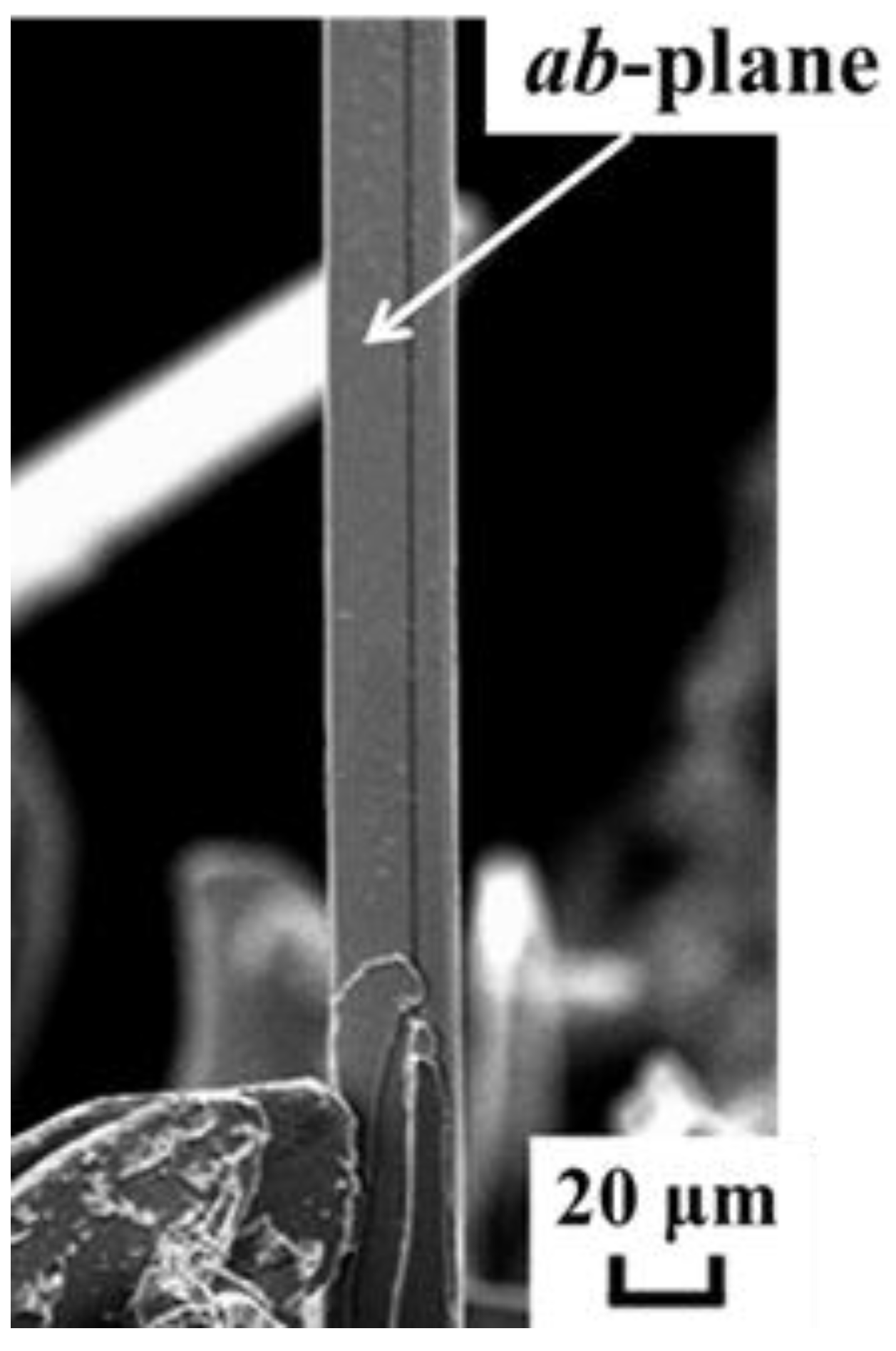
2.1.1. High-Tc Cuprate Superconductor
2.1.2. Fe-Based Superconductors
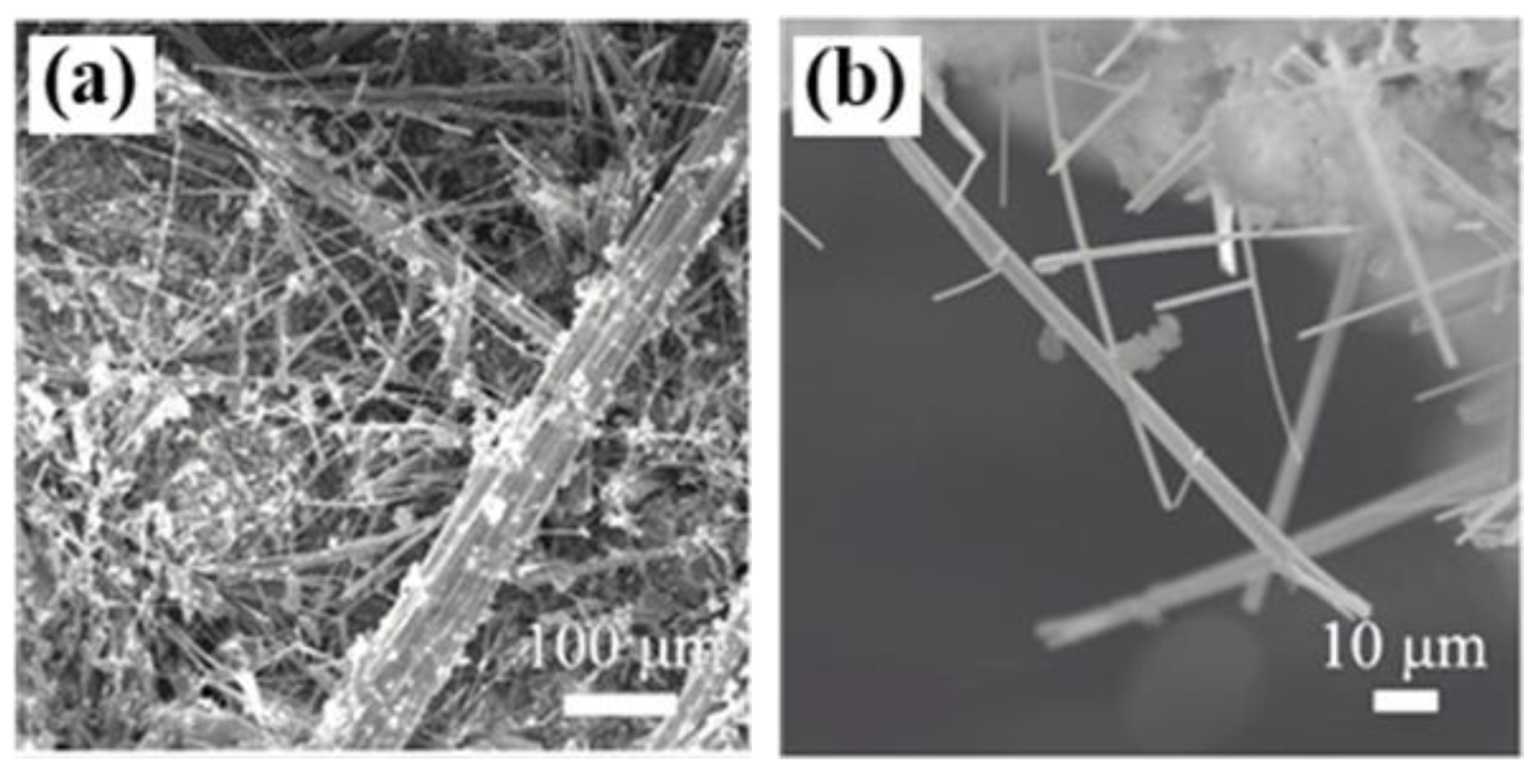
2.1.3. BiS2-Based Superconductors
2.2. Traveling Solvent Floating-Zone (TSFZ) Method
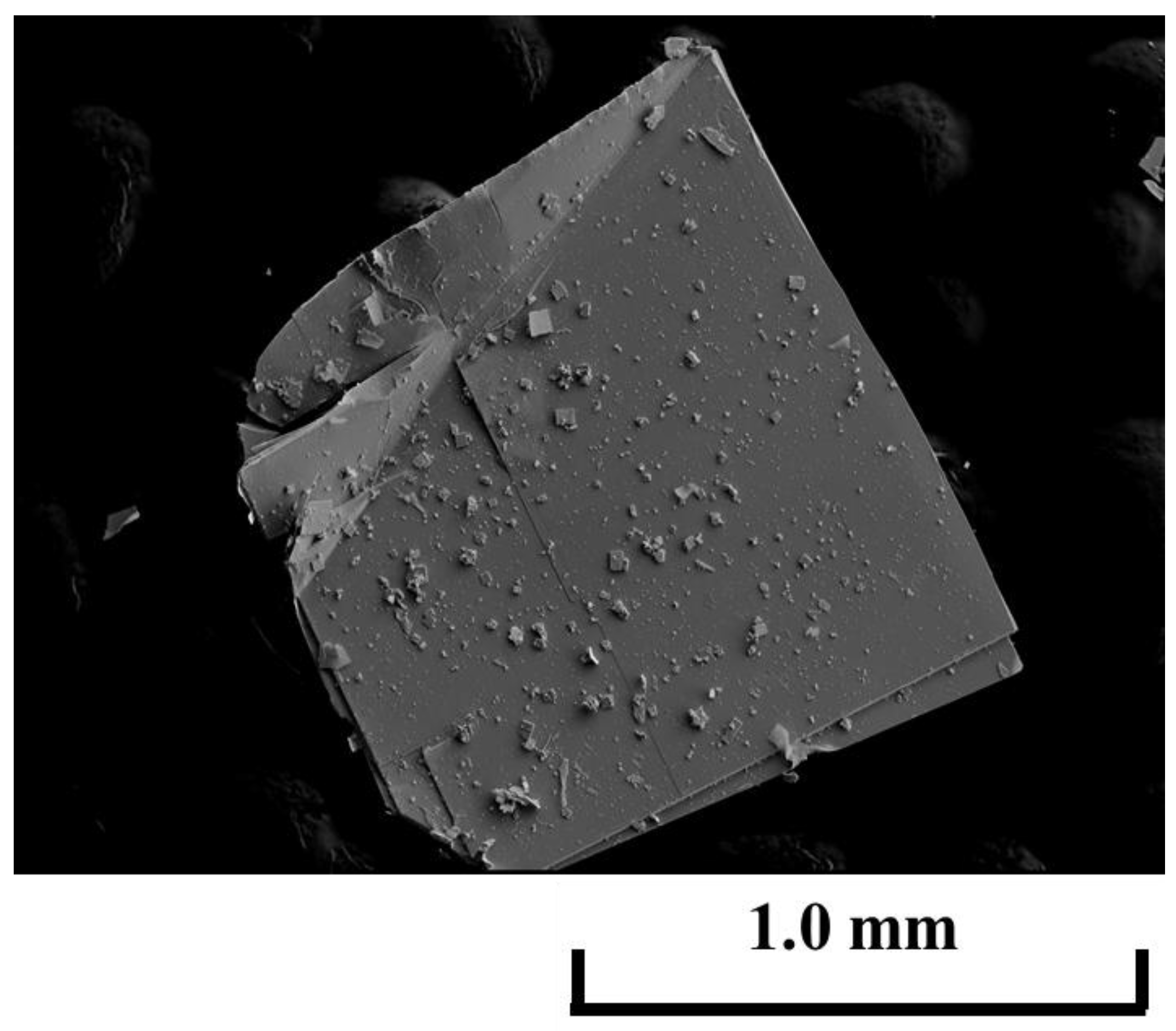
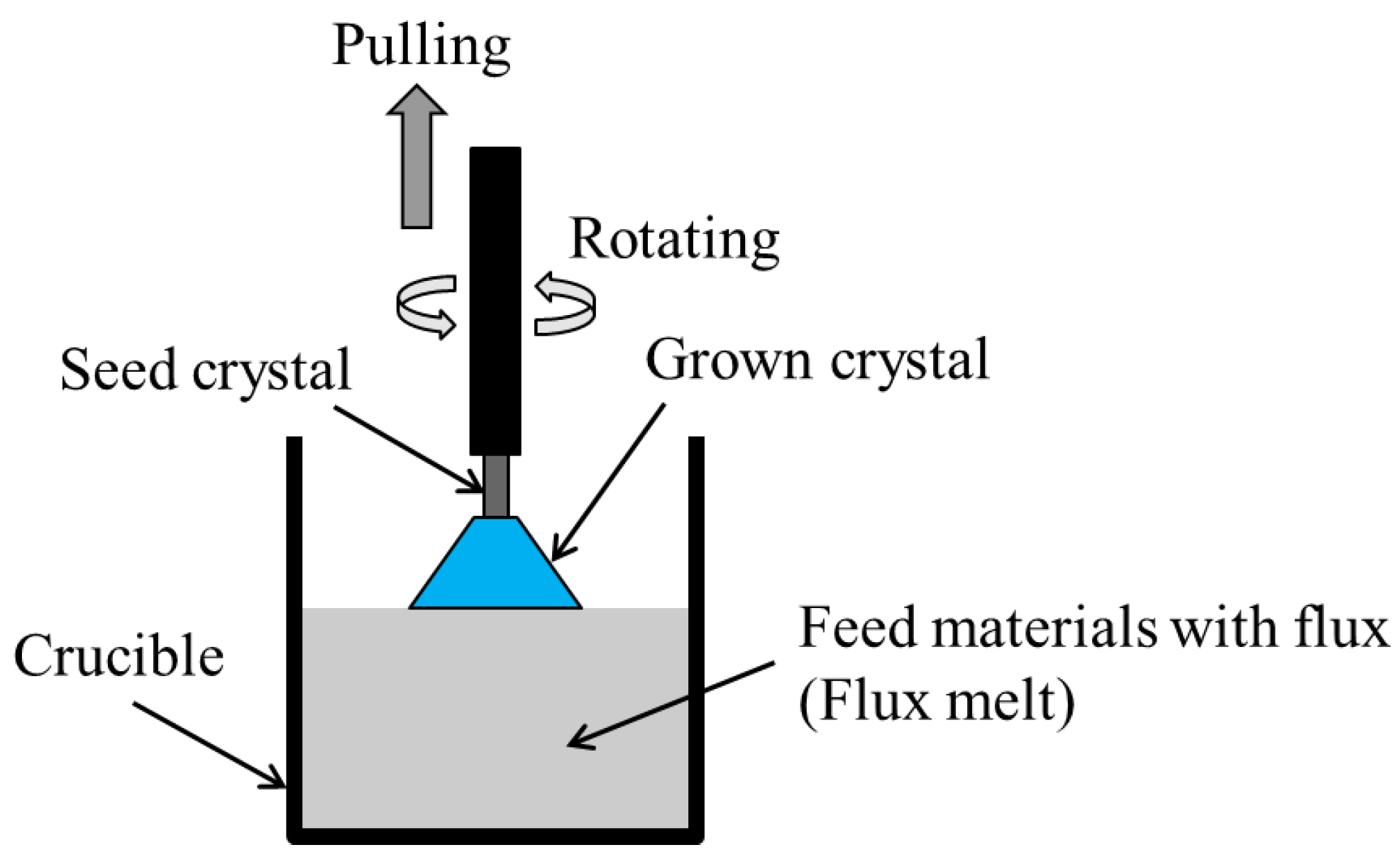
2.3. Top-Seeded Solution Growth (TSSG) Method
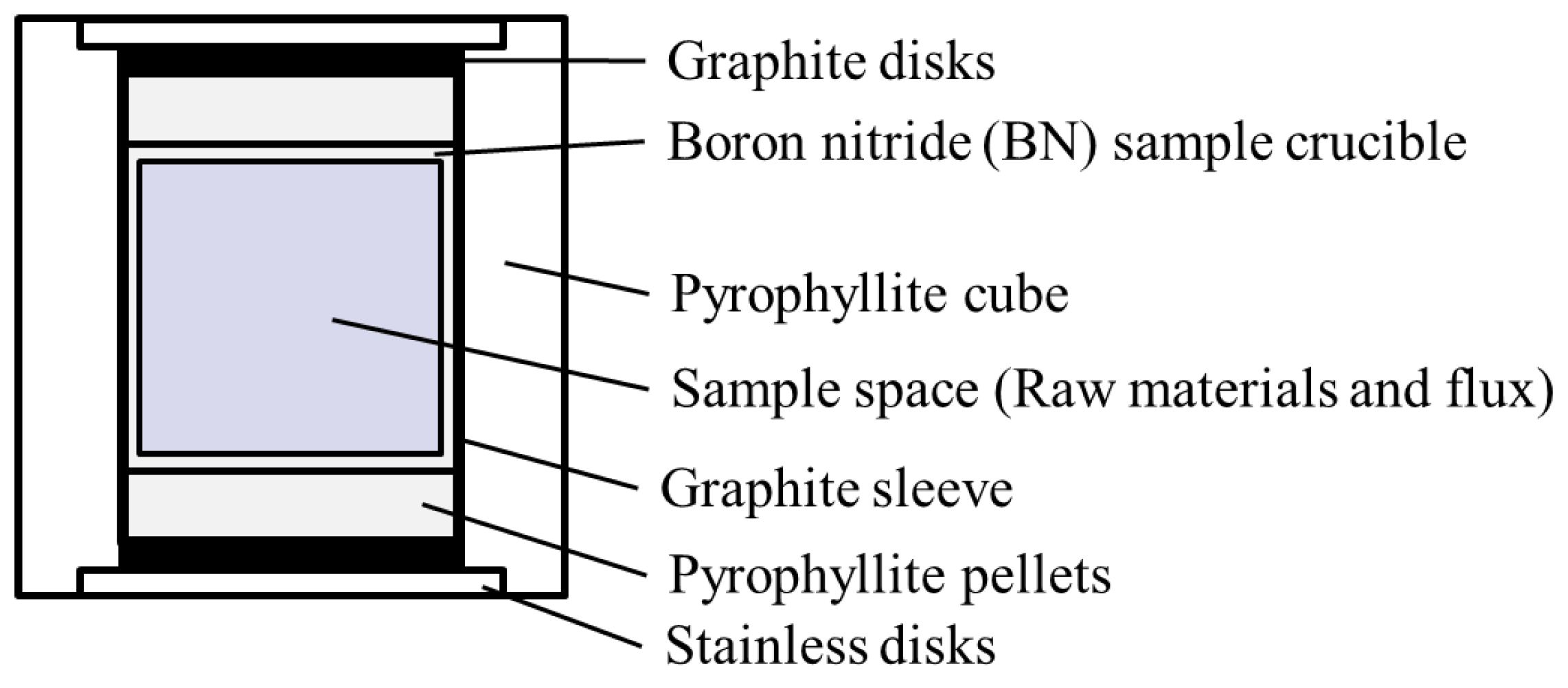
2.4. High-Temperature and High-Pressure (HPHT) Flux Method
3. Summary
Acknowledgments
Conflicts of Interest
References
- Bednorz, J.G.; Müller, K.A. Possible high-Tc superconductivity in the Ba-La-Cu-O system. Z. Phys. B Condens. Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Wu, M.K.; Ashburn, J.R.; Thorng, C.J.; Hor, P.H.; Meng, R.L.; Gao, L.; Huang, Z.J.; Wang, Y.Q.; Chu, C.W. Superconductivity at 93 K in a new mixed-phase Y-Ba-Cu-O compound system at ambient pressure. Phys. Rev. Lett. 1987, 58, 908. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Tanaka, Y.; Fukutomi, M.; Asano, T. A New High-Tc Oxide Superconductor without a Rare Earth Element. Jpn. J. Appl. Phys. 1988, 27, L209. [Google Scholar] [CrossRef]
- Hidaka, Y.; Enomoto, Y.; Suzuki, M.; Oda, M.; Murakami, T. Single-crystal growth of (La1−xAx)2CuO4 (A = Ba or Sr) and Ba2YCu3O7−y. J. Cryst. Growth 1987, 85, 581–584. [Google Scholar] [CrossRef]
- Asaoka, H.; Takei, H.; Iye, Y.; Tamura, M.; Kinoshita, M.; Takeya, H. Growth of Large Isometric YBa2Cu3Ox Single Crystals from Coexisting Region of Solid with Melt in Y2O3 Crucibles. Jpn. J. Appl. Phys. 1993, 32, 1091. [Google Scholar] [CrossRef]
- Funabiki, H.; Okanoue, K.; Takano, C.; Hamasaki, K. Growth conditions and characterization of Bi2Sr2CaCu2O8+δ single crystals. J. Cryst. Growth 2003, 259, 85–89. [Google Scholar] [CrossRef]
- Gencer, F.; Abell, J.S. The growth of YBa2Cu3O7−δ single crystals with the aid of NaCl-KCl flux. J. Cryst. Growth 1991, 112, 337–342. [Google Scholar] [CrossRef]
- Katsui, A. Crystal Growth of Superconducting Bi-Sr-Ca-Cu-O Compounds from KCl Solution. Jpn. J. Appl. Phys. 1988, 27, L844. [Google Scholar] [CrossRef]
- Wang, X.L.; Horvat, J.; Liu, H.K.; Dou, S.X. Spiral growth of Bi2Sr2CaCu2Oy single crystals using KCl flux technique. J. Cryst. Growth 1997, 173, 380–385. [Google Scholar] [CrossRef]
- Lee, S.; Yamamoto, A.; Tajima, S. Crystal growth of Bi2Sr2Ca2Cu3O10+x and (Bi,Pb)2Sr2Ca2Cu3O10+x by the KCl flux method. J. Mater. Res. 2002, 17, 2286–2293. [Google Scholar] [CrossRef]
- Matsubara, I.; Kageyama, H.; Tanigawa, H.; Okura, T.; Yamashita, H.; Kawai, T. Preparation of Fibrous Bi(Pb)-Sr-Ca-Cu-O Crystals and Their Superconducting Properties in a Bending State. Jpn. J. Appl. Phys. 1989, 28, L1121. [Google Scholar] [CrossRef]
- Nagao, M.; Sato, M.; Maeda, H.; Kim, S.J.; Yamashita, T. Growth and superconducting properties of Bi2Sr2CaCu2O8+δ single-crystal whiskers using tellurium-doped precursors. Appl. Phys. Lett. 2001, 79, 2612–2614. [Google Scholar] [CrossRef]
- Nagao, M.; Sato, M.; Maeda, H.; Kim, S.J.; Yamashita, T. Growth and electrical transport characteristics of Bi2Sr2Ca1Cu2Ox and Bi2Sr2CuOx single-crystal whiskers using tellurium-doped precursors. Phys. C 2002, 377, 260–266. [Google Scholar] [CrossRef]
- Nagao, M.; Sato, M.; Maeda, H.; Kim, S.J.; Yamashita, T. Growth and Superconductivity of (BiPb)2Sr2Ca2Cu3O10+δ Single-Crystal Whiskers. Jpn. J. Appl. Phys. 2002, 41, L43. [Google Scholar] [CrossRef]
- Nagao, M.; Watauchi, S.; Tanaka, I.; Okutsu, T.; Takano, Y.; Hatano, T.; Maeda, H. Growth and Anisotropic Properties of RBa2Cu3Ox Single-Crystal Whiskers. Jpn. J. Appl. Phys. 2010, 49. [Google Scholar] [CrossRef]
- Nagao, M.; Yun, K.S.; Nakane, T.; Wang, H.; Takano, Y.; Hatano, T.; Yamashita, T.; Tachiki, M.; Maeda, H.; Sato, M. Growth of Y1Ba2Cu3Ox Single-Crystal Whisker Using Sb-doped Precursor. Jpn. J. Appl. Phys. 2005, 44, L67. [Google Scholar] [CrossRef]
- Nagao, M.; Kawae, T.; Yun, K.S.; Wang, H.; Takano, Y.; Hatano, T.; Yamashita, T.; Tachiki, M.; Maeda, H.; Sato, M. Intrinsic Josephson junctions in Y1Ba2Cu3Ox single-crystal whiskers grown using Te-doped precursors. J. Appl. Phys. 2005, 98, 073903. [Google Scholar] [CrossRef]
- Nagao, M.; Sato, M.; Maeda, H.; Yun, K.S.; Takano, Y.; Hatano, T.; Kim, S.J. Superconducting properties of single-crystal whiskers of (Y0.86Ca0.14)Ba2Cu3Ox grown from precursors containing calcium and tellurium. Appl. Phys. Lett. 2003, 82, 1899–1901. [Google Scholar] [CrossRef]
- Nagao, M.; Sato, M.; Tachiki, Y.; Miyagawa, K.; Tanaka, M.; Maeda, H.; Yun, K.S.; Takano, Y.; Hatano, T. Growth of R-123 Phase Single Crystal Whiskers. Jpn. J. Appl. Phys. 2004, 43, L324. [Google Scholar] [CrossRef]
- Deguchi, K.; Ogawara, S.; Okutsu, T.; Nagao, M.; Watanabe, T.; Mizuguchi, Y.; Kubo, Y.; Tomioka, F.; Ishii, S.; Tsuda, S.; et al. Growth of superconducting single-crystalline (Lu,Ca)Ba2Cu3O7−δ whiskers. Phys. C 2009, 469, 965–966. [Google Scholar] [CrossRef]
- Kamihara, Y.; Watanabe, T.; Hirano, M.; Hosono, H. Iron-Based Layered Superconductor La[O1−xFx]FeAs (x = 0.05−0.12) with Tc = 26 K. J. Am. Chem. Soc. 2008, 130, 3296–3297. [Google Scholar] [CrossRef] [PubMed]
- Rotter, M.; Tegel, M.; Johrendt, D. Superconductivity at 38 K in the Iron Arsenide (Ba1−xKx)Fe2As2. Phys. Rev. Lett. 2008, 101, 107006. [Google Scholar] [CrossRef] [PubMed]
- Tapp, J.H.; Tang, Z.; Lv, B.; Sasmal, K.; Lorenz, B.; Chu, P.C.W.; Guloy, A.M. LiFeAs: An intrinsic FeAs-based superconductor with Tc = 18 K. Phys. Rev. B 2008, 78. [Google Scholar] [CrossRef]
- Hsu, F.C.; Luo, J.Y.; Yeh, K.W.; Chen, T.K.; Huang, T.W.; Wu, P.M.; Lee, Y.C.; Huang, Y.L.; Chu, Y.Y.; Yan, D.C.; et al. Superconductivity in the PbO-type structure α-FeSe. Proc. Natl. Acad. Sci. USA 2008, 105, 14262–14264. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jin, S.; Wang, G.; Wang, S.; Zhu, K.; Zhou, T.; He, M.; Chen, X. Superconductivity in the iron selenide KxFe2Se2 (0 ≤ x ≤ 1.0). Phys. Rev. B 2010, 82, 180520. [Google Scholar] [CrossRef]
- Ogino, H.; Matsumura, Y.; Katsura, Y.; Ushiyama, K.; Horii, S.; Kishio, K.; Shimoyama, J. Superconductivity at 17 K in (Fe2P2)(Sr4Sc2O6): A new superconducting layered pnictide oxide with a thick perovskite oxide layer. Supercond. Sci. Technol. 2009, 22. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Z.; Yang, H.; Cheng, P.; Zhu, X.; Wen, H.H. Growth and characterization of A1−xKxFe2As2 (A = Ba, Sr) single crystals with x = 0–0.4. Supercond. Sci. Technol. 2008, 21, 125014. [Google Scholar] [CrossRef]
- Chen, G.F.; Li, Z.; Dong, J.; Li, G.; Hu, W.Z.; Zhang, X.D.; Song, X.H.; Zheng, P.; Wang, N.L.; Luo, J.L. Transport and anisotropy in single-crystalline SrFe2As2 and A0.6K0.4Fe2As2 (A = Sr, Ba) superconductors. Phys. Rev. B 2008, 78, 224512. [Google Scholar] [CrossRef]
- Kihou, K.; Saito, T.; Ishida, S.; Nakajima, M.; Tomioka, Y.; Fukazawa, H.; Kohori, Y.; Ito, T.; Uchida, S.; Iyo, A.; et al. Single-crystal growth and Characterization of the Iron-Based Superconductor KFe2As2 Synthesized by KAs Flux Method. J. Phys. Soc. Jpn. 2010, 79, 124713. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Z.; Yao, C.; Wang, L.; Qi, Y.; Wang, D.; Zhang, X.; Ma, Y. One-step method to grow Ba0.6K0.4Fe2As2 single crystals without fluxing agent. Supercond. Sci. Technol. 2011, 24. [Google Scholar] [CrossRef]
- Morozov, I.; Boltalin, A.; Volkova, O.; Vasiliev, A.; Kataeva, O.; Stockert, U.; Abdel-Hafiez, M.; Bombor, D.; Bachmann, A.; Harnagea, L.; et al. Single-crystal growth and Characterization of Superconducting LiFeAs. Cryst. Growth Des. 2010, 10, 4428–4432. [Google Scholar] [CrossRef]
- Wang, A.F.; Luo, X.G.; Yan, Y.J.; Ying, J.J.; Xiang, Z.J.; Ye, G.J.; Cheng, P.; Li, Z.Y.; Hu, W.J.; Chen, X.H. Phase diagram and calorimetric properties of NaFe1 − xCoxAs. Phys. Rev. B 2012, 85, 224521. [Google Scholar] [CrossRef]
- Moll, P.J.W.; Zhu, X.; Cheng, P.; Wen, H.H.; Batlogg, B. Intrinsic Josephson junctions in the iron-based multi-band superconductor (V2Sr4O6)Fe2As2. Nat. Phys. 2014, 10, 644–647. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J.; Tang, D.M.; Zhang, S.B.; Li, M.Y.; Guo, Y.F.; Tsujimoto, Y.; Hatano, T.; Arisawa, S.; Golberg, D.; et al. Growth of Single-Crystal Ca10(Pt4As8)(Fe1.8Pt0.2As2)5 Nanowhiskers with Superconductivity up to 33 K. J. Am. Chem. Soc. 2012, 134, 4068–4071. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Bud’ko, S.L.; Kreyssig, A.; Nandi, S.; Rustan, G.E.; Goldman, A.I.; Gupta, S.; Corbett, J.D.; Kracher, A.; Canfield, P.C. Anisotropic thermodynamic and transport properties of single-crystalline Ba1−xKxFe2As2 (x = 0 and 0.45). Phys. Rev. B 2008, 78. [Google Scholar] [CrossRef]
- Bukowski, Z.; Weyeneth, S.; Puzniak, R.; Moll, P.; Katrych, S.; Zhigadlo, N.D.; Karpinski, J.; Keller, H.; Batlogg, B. Superconductivity at 23 K and low anisotropy in Rb-substituted BaFe2As2 single crystals. Phys. Rev. B 2009, 79, 104521. [Google Scholar] [CrossRef]
- Hu, R.; Lei, H.; Abeykoon, M.; Bozin, E.S.; Billinge, S.J.L.; Warren, J.B.; Siegrist, T.; Petrovic, C. Synthesis, crystal structure, and magnetism of β-Fe1.00(2)Se1.00(3) single crystals. Phys. Rev. B 2011, 83, 224502. [Google Scholar] [CrossRef]
- Böhmer, A.E.; Hardy, F.; Eilers, F.; Ernst, D.; Adelmann, P.; Schweiss, P.; Wolf, T.; Meingast, C. Lack of coupling between superconductivity and orthorhombic distortion in stoichiometric single-crystalline FeSe. Phys. Rev. B 2013, 87, 180505. [Google Scholar] [CrossRef]
- Zhang, S.B.; Sun, Y.P.; Zhu, X.D.; Zhu, X.B.; Wang, B.S.; Li, G.; Lei, H.C.; Luo, X.; Yang, Z.R.; Song, W.H.; et al. Crystal growth and superconductivity of FeSex. Supercond. Sci. Technol. 2009, 22. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Fujihisa, H.; Gotoh, Y.; Suzuki, K.; Usui, H.; Kuroki, K.; Demura, S.; Takano, Y.; Izawa, H.; Miura, O. BiS2-based layered superconductor Bi4O4S3. Phys. Rev. B 2012, 86, 220510. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Demura, S.; Deguchi, K.; Takano, Y.; Fujihisa, H.; Gotoh, Y.; Izawa, H.; Miura, O. Superconductivity in Novel BiS2-Based Layered Superconductor LaO1−xFxBiS2. J. Phys. Soc. Jpn. 2012, 81, 114725. [Google Scholar] [CrossRef]
- Xing, J.; Li, S.; Ding, X.; Yang, H.; Wen, H.H. Superconductivity appears in the vicinity of semiconducting-like behavior in CeO1−xFxBiS2. Phys. Rev. B 2012, 86, 214518. [Google Scholar] [CrossRef]
- Jha, R.; Kumar, A.; Singh, S.K.; Awana, V.P.S. Synthesis and Superconductivity of New BiS2 Based Superconductor PrO0.5F0.5BiS2. J. Supercond. Novel Magn. 2013, 26, 499–502. [Google Scholar] [CrossRef]
- Demura, S.; Mizuguchi, Y.; Deguchi, K.; Okazaki, H.; Hara, H.; Watanabe, T.; Denholme, S.J.; Fujioka, M.; Ozaki, T.; Fujihisa, H.; et al. New Member of BiS2-Based Superconductor NdO1−xFxBiS2. J. Phys. Soc. Jpn. 2013, 82. [Google Scholar] [CrossRef]
- Yazici, D.; Huang, K.; White, B.D.; Chang, A.H.; Friedman, A.J.; Maple, M.B. Superconductivity of F-substituted LnOBiS2 (Ln = La, Ce, Pr, Nd, Yb) compounds. Philos. Mag. 2013, 93, 673–680. [Google Scholar] [CrossRef]
- Lin, X.; Ni, X.; Chen, B.; Xu, X.; Yang, X.; Dai, J.; Li, Y.; Yang, X.; Luo, Y.; Tao, Q.; et al. Superconductivity induced by La doping in Sr1−xLaxFBiS2. Phys. Rev. B 2013, 87. [Google Scholar] [CrossRef]
- Lei, H.C.; Wang, K.F.; Abeykoon, M.; Bozin, E.S.; Petrovic, C. New Layered Fluorosulfide SrFBiS2. Inorg. Chem. 2013, 52, 10685–10689. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.F.; Tang, Z.T.; Jiang, H.; Xu, K.; Zhang, K.; Zhang, P.; Bao, J.K.; Sun, Y.L.; Jiao, W.H.; Nowik, I.; et al. Possible charge-density wave, superconductivity, and f-electron valence instability in EuBiS2F. Phys. Rev. B 2014, 90. [Google Scholar] [CrossRef]
- Yazici, D.; Huang, K.; White, B.D.; Jeon, I.; Burnett, V.W.; Friedman, A.J.; Lum, I.K.; Nallaiyan, M.; Spagna, S.; Maple, M.B. Superconductivity induced by electron doping in La1 − xMxOBiS2 (M = Ti, Zr, Hf, Th). Phys. Rev. B 2013, 87, 174512. [Google Scholar] [CrossRef]
- Zhai, H.F.; Zhang, P.; Wu, S.Q.; He, C.Y.; Tang, Z.T.; Jiang, H.; Sun, Y.L.; Bao, J.K.; Nowik, I.; Felner, I.; et al. Anomalous Eu Valence State and Superconductivity in Undoped Eu3Bi2S4F4. J. Am. Chem. Soc. 2014, 136, 15386–15393. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Yao, X.; Liu, Z.; Pi, L.; Tan, S.; Zhang, C.; Zhang, Y. Superconductivity in BiO1−xFxBiS2 and possible parent phase of Bi4O4S3 superconductor. Supercond. Sci. Technol. 2015, 28. [Google Scholar] [CrossRef]
- Okada, T.; Ogino, H.; Shimoyama, J.; Kishio, K. Topotactic synthesis of a new BiS2-based superconductor Bi2(O,F)S2. Appl. Phys. Express 2015, 8. [Google Scholar] [CrossRef]
- Krzton-Maziopa, A.; Guguchia, Z.; Pomjakushina, E.; Pomjakushin, V.; Khasanov, R.; Luetkens, H.; Biswas, P.; Amato, A.; Keller, H.; Conder, K. Superconductivity in a new layered bismuth oxyselenide: LaO0.5F0.5BiSe2. J. Phys. Condens. Matter 2014, 26, 215702. [Google Scholar] [CrossRef] [PubMed]
- FTsalt-FACT Salt Phase Diagrams (CsCl-KCl). Available online: http://www.crct.polymtl.ca/fact/phase_diagram.php?file=CsCl-KCl.jpg&dir=FTsalt (accessed on 16 October 2017).
- Nagao, M.; Demura, S.; Deguchi, K.; Miura, A.; Watauchi, S.; Takei, T.; Takano, Y.; Kumada, N.; Tanaka, I. Structural Analysis and Superconducting Properties of F-Substituted NdOBiS2 Single Crystals. J. Phys. Soc. Jpn. 2013, 82, 113701. [Google Scholar] [CrossRef]
- Nagao, M.; Miura, A.; Demura, S.; Deguchi, K.; Watauchi, S.; Takei, T.; Takano, Y.; Kumada, N.; Tanaka, I. Growth and superconducting properties of F-substituted ROBiS2 (R = La, Ce, Nd) single crystals. Solid State Commun. 2014, 178, 33–36. [Google Scholar] [CrossRef]
- Higashinaka, R.; Asano, T.; Nakashima, T.; Fushiya, K.; Mizuguchi, Y.; Miura, O.; Matsuda, T.D.; Aoki, Y. Pronounced—Log T Divergence in Specific Heat of Nonmetallic CeOBiS2: A Mother Phase of BiS2-Based Superconductor. J. Phys. Soc. Jpn. 2015, 84. [Google Scholar] [CrossRef]
- Nagao, M.; Miura, A.; Ueta, I.; Watauchi, S.; Tanaka, I. Superconductivity in CeOBiS2 with cerium valence fluctuation. Solid State Commun. 2016, 245, 11–14. [Google Scholar] [CrossRef]
- Nagao, M. Growth and characterization of R(O,F)BiS2 (R = La, Ce, Pr, Nd) superconducting single crystals. Nov. Supercond. Mater. 2015, 1, 64–74. [Google Scholar] [CrossRef]
- Liu, J.; Fang, D.; Wang, Z.; Xing, J.; Du, Z.; Li, S.; Zhu, X.; Yang, H.; Wen, H.H. Giant superconducting fluctuation and anomalous semiconducting normal state in NdO1−xFxBi1−yS2 single crystals. Europhys. Lett. 2014, 106, 67002. [Google Scholar] [CrossRef]
- Nagao, M.; Tanaka, M.; Watauchi, S.; Tanaka, I.; Takano, Y. Superconducting Anisotropies of F-Substituted LaOBiSe2 Single Crystals. J. Phys. Soc. Jpn. 2014, 83, 114709. [Google Scholar] [CrossRef]
- Nagao, M.; Tanaka, M.; Matsumoto, R.; Tanaka, H.; Watauchi, S.; Takano, Y.; Tanaka, I. Growth and Structure of Ce(O,F)SbS2 Single Crystals. Cryst. Growth Des. 2016, 16, 3037–3042. [Google Scholar] [CrossRef]
- Kimura, S.; Shindo, I. Single-crystal growth of YIG by the floating zone method. J. Cryst. Growth 1977, 41, 192–198. [Google Scholar] [CrossRef]
- Koohpayeh, S.M. Single-crystal growth by the traveling solvent technique: A review. Prog. Cryst. Growth Charact. Mater. 2016, 62, 22–34. [Google Scholar] [CrossRef]
- Tanaka, I.; Kojima, H. Superconducting single crystals. Nature 1988, 337, 21–22. [Google Scholar] [CrossRef]
- Tanaka, I.; Yamane, K.; Kojima, H. Single-crystal growth of superconducting La2−xSrxCuO4 by the TSFZ method. J. Cryst. Growth 1989, 96, 711–715. [Google Scholar] [CrossRef]
- Tanaka, I.; Watanabe, T.; Komai, N.; Kojima, H. Growth and superconductivity of Nd2−xCexCuO4 single crystals. Physica C 1991, 185–189, 437–438. [Google Scholar] [CrossRef]
- Takekawa, S.; Nozaki, H.; Umezono, A.; Kosuda, K.; Kobayashi, M. Single-crystal growth of the superconductor Bi2.0(Bi0.2Sr1.8Ca1.0)Cu2.0O8. J. Cryst. Growth 1988, 92, 687–690. [Google Scholar] [CrossRef]
- Fujii, T.; Watanabe, T.; Matsuda, A. Single-crystal growth of Bi2Sr2Ca2Cu3O10+δ (Bi-2223) by TSFZ method. J. Cryst. Growth 2001, 223, 175–180. [Google Scholar] [CrossRef]
- Yamada, Y.; Shiohara, Y. Continuous crystal growth of YBa2Cu3O7−x by the modified top-seeded crystal pulling method. Physica C 1993, 217, 182–188. [Google Scholar] [CrossRef]
- Rytz, D.; Wechsler, B.A.; Nelson, C.C.; Kirby, K.W. Top-seeded solution growth of BaTiO3, KNbO3, SrTiO3, Bi12TiO20 and La2−x, BaxCuO4. J. Cryst. Growth 1990, 99, 864–868. [Google Scholar] [CrossRef]
- Zhokhov, A.A.; Emel’chenko, G.A. Growth of YBa2Cu3O7−δ single crystals on seeds by a modified top seeded solution growth (TSSG) technique. J. Cryst. Growth 1993, 129, 786–788. [Google Scholar] [CrossRef]
- Oka, K.; Unoki, H. Primary crystallization fields and crystal growth of YBa2Cu3O7−y and NdBa2Cu3O7 − y. J. Cryst. Growth 1990, 99, 922–924. [Google Scholar] [CrossRef]
- Chen, X.; Koiwasaki, T.; Yamanaka, S. High-Pressure Synthesis and Crystal Structures of β-MNCl (M = Zr and Hf). J. Solid State Chem. 2001, 159, 80–86. [Google Scholar] [CrossRef]
- Karpinski, J.; Angst, M.; Jun, J.; Kazakov, S.M.; Puzniak, R.; Wisniewski, A.; Roos, J.; Keller, H.; Perucchi, A.; Degiorgi, L.; et al. MgB2 single crystals: High pressure growth and physical properties. Supercond. Sci. Technol. 2003, 16, 221. [Google Scholar] [CrossRef]
- Karpinski, J.; Zhigadlo, N.D.; Katrych, S.; Puzniak, R.; Rogacki, K.; Gonnelli, R. Single crystals of MgB2: Synthesis, substitutions and properties. Physica C 2007, 456, 3–13. [Google Scholar] [CrossRef]
- Zhigadlo, N.D.; Weyeneth, S.; Katrych, S.; Moll, P.J.W.; Rogacki, K.; Bosma, S.; Puzniak, R.; Karpinski, J.; Batlogg, B. High-pressure flux growth, structural, and superconducting properties of LnFeAsO (Ln = Pr, Nd, Sm) single crystals. Phys. Rev. B 2012, 86, 214509. [Google Scholar] [CrossRef]
- Zhigadlo, N.D.; Katrych, S.; Bukowski, Z.; Weyeneth, S.; Puzniak, R.; Karpinski, J. Single crystals of superconducting SmFeAsO1 − xFy grown at high pressure. J. Phys. Condens. Matter 2008, 20, 342202. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, J.H.; Lee, J.Y.; Kim, J.Y.; Sung, N.H.; Koo, T.Y.; Cho, B.K.; Jung, C.U.; Saini, S.; Kim, S.J.; et al. High-pressure growth of fluorine-free SmFeAsO1−x superconducting single crystals. Supercond. Sci. Technol. 2009, 22. [Google Scholar] [CrossRef]
- Zhigadlo, N.D. Growth of whisker-like and bulk single crystals of PrFeAs(O,F) under high pressure. J. Cryst. Growth 2013, 382, 75–79. [Google Scholar] [CrossRef]
- Karpinski, J.; Zhigadlo, N.D.; Katrych, S.; Bukowski, Z.; Moll, P.; Weyeneth, S.; Keller, H.; Puzniak, R.; Tortello, M.; Daghero, D.; et al. Single crystals of LnFeAsO1−xFx (Ln = La, Pr, Nd, Sm, Gd) and Ba1−xRbxFe2As2: Growth, structure and superconducting properties. Phys. C 2009, 469, 370–380. [Google Scholar] [CrossRef] [Green Version]




© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagao, M. Crystal Growth Techniques for Layered Superconductors. Condens. Matter 2017, 2, 32. https://doi.org/10.3390/condmat2040032
Nagao M. Crystal Growth Techniques for Layered Superconductors. Condensed Matter. 2017; 2(4):32. https://doi.org/10.3390/condmat2040032
Chicago/Turabian StyleNagao, Masanori. 2017. "Crystal Growth Techniques for Layered Superconductors" Condensed Matter 2, no. 4: 32. https://doi.org/10.3390/condmat2040032




