Optimization of Processing Conditions of Traditional Cured Tuna Loins–Muxama
Abstract
:1. Introduction
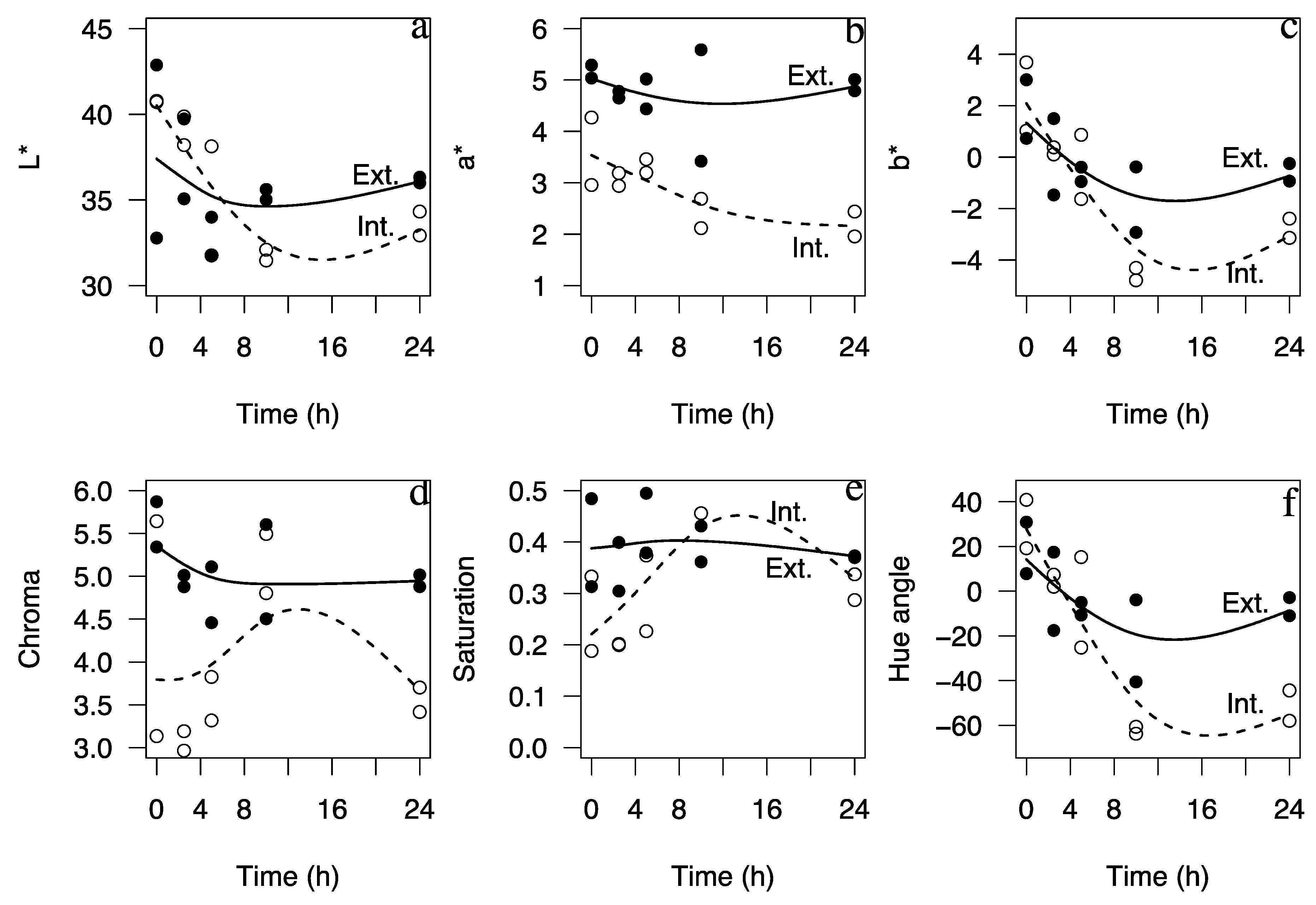
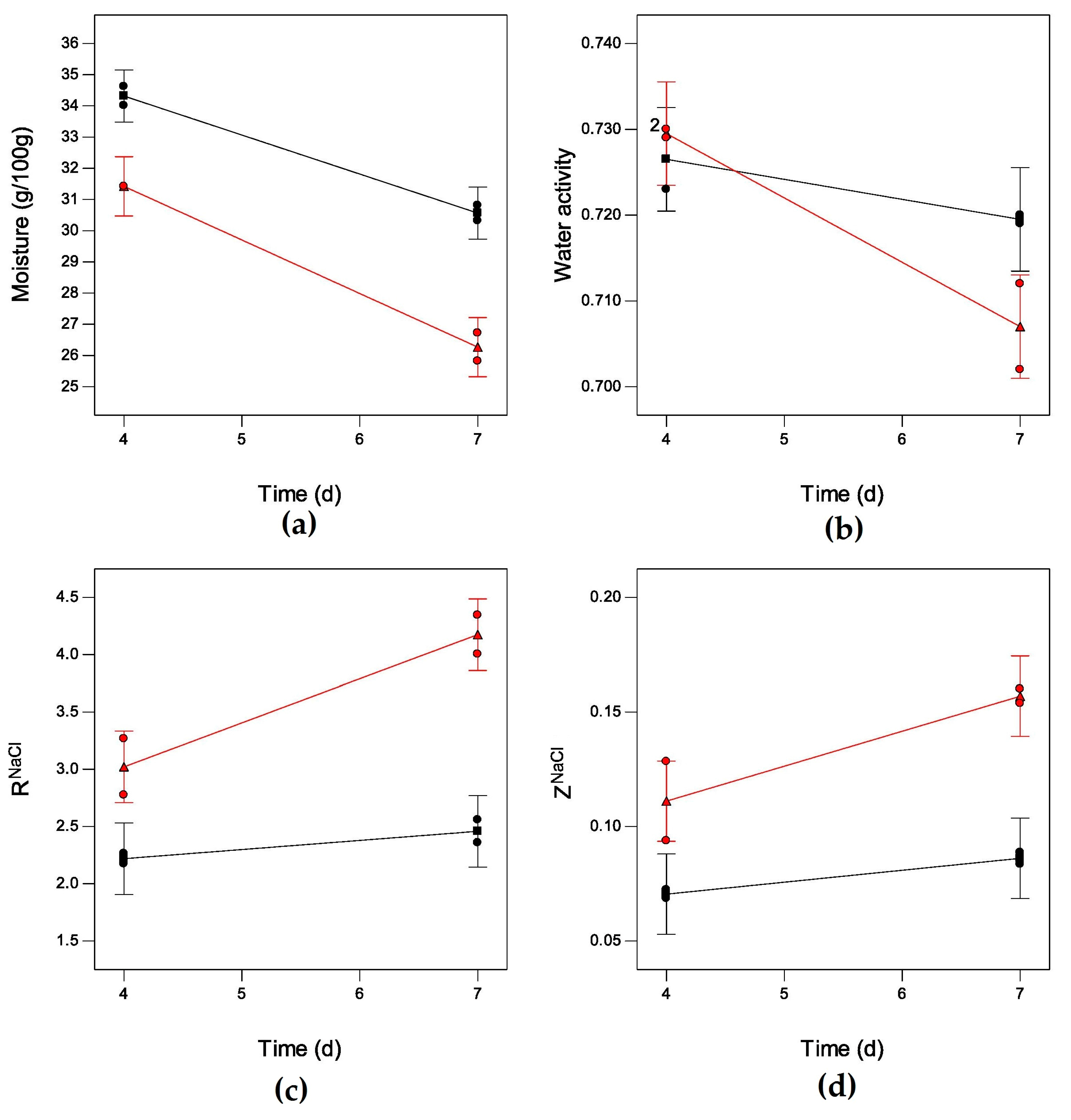
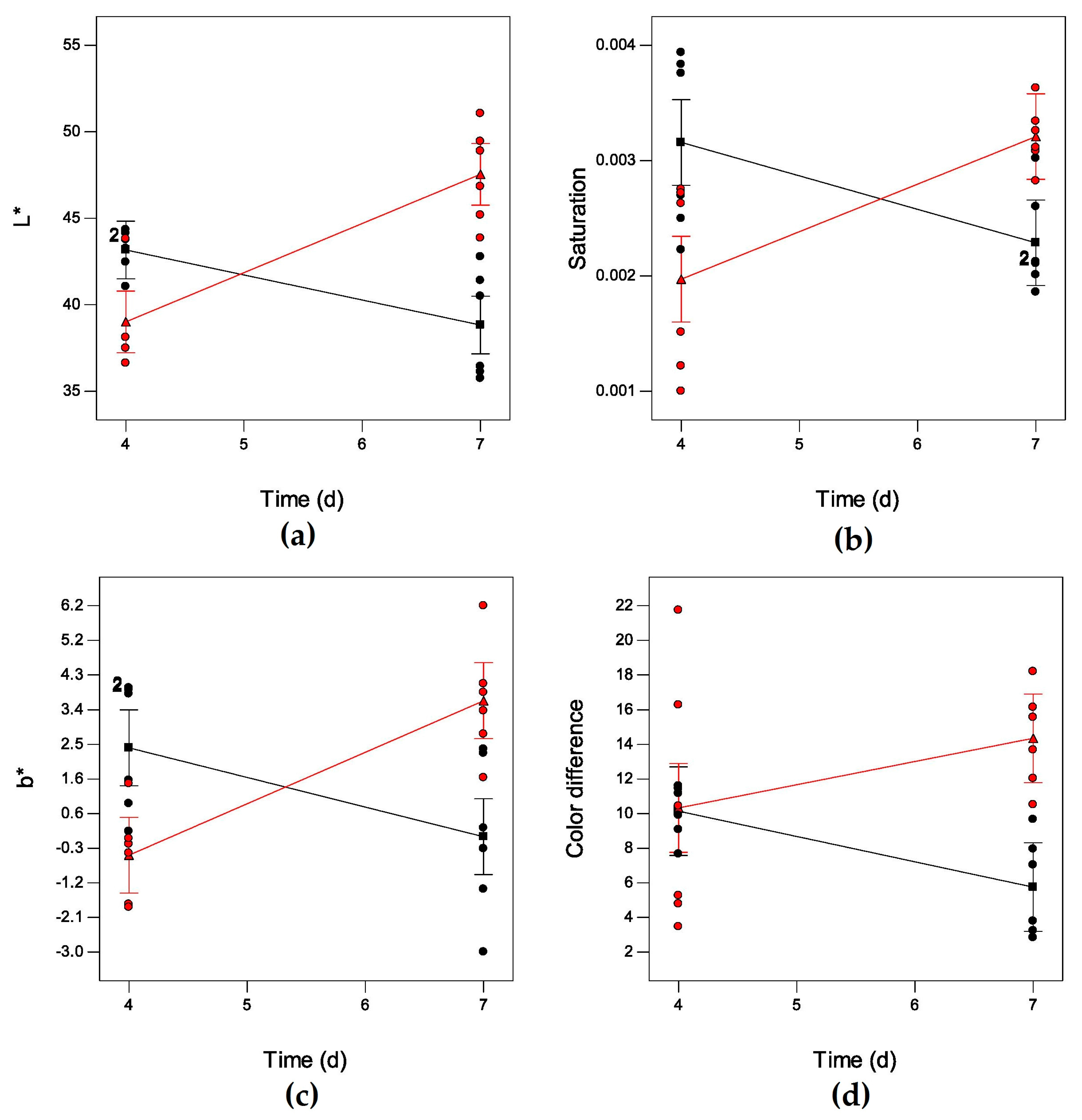
2. Results
3. Discussion
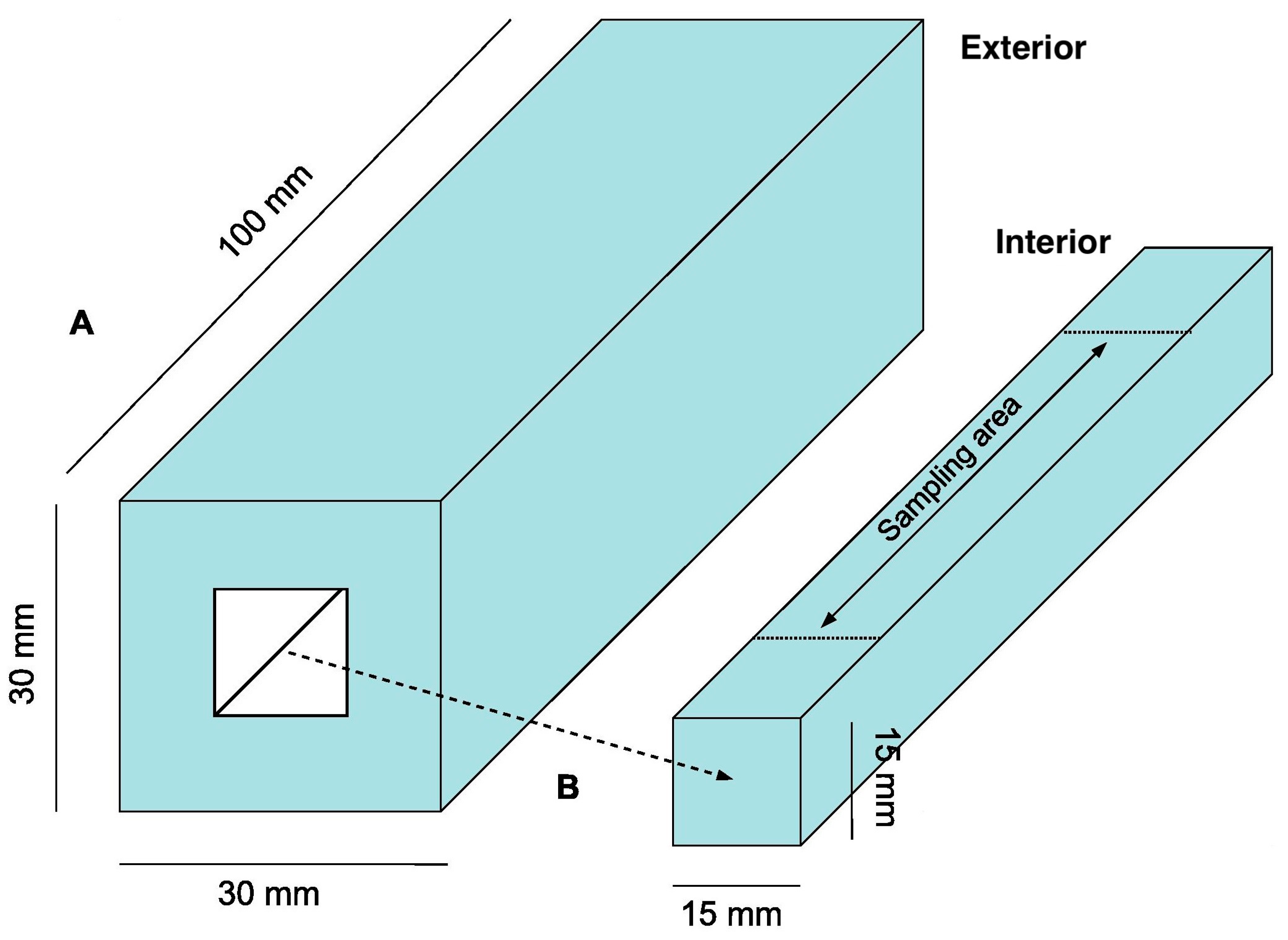
4. Materials and Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Commission. Commission Implementing Regulation (EU) 2015/2110 of 12 November 2015 entering a name in the register of protected designations of origin and protected geographical indications [Mojama de Barbate (PGI)]. In Official Journal of the European Union, L 306; Eur-Lex: Brussels, Belgium, 2015; pp. 1–2. [Google Scholar]
- European Commision. Commission Implementing Regulation (EU) 2016/199 of 9 February 2016 entering a name in the register of protected designations of origin and protected geographical indications (Mojama de Isla Cristina (PGI)). In Official Journal of the European Union, L 39; Eur-Lex: Brussels, Belgium, 2016; p. 1. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EU) No. 1151/2012 of the European Parliament and of the Council of 21 November 2012 on quality schemes for agricultural products and foodstuffs. In Official Journal of the European Union, L 343; Eur-Lex: Brussels, Belgium, 2012; pp. 1–29. [Google Scholar]
- Lã, A.; Vicente, L. O Atum Esquecido; Universidade do Algarve: Algarve, Faro, 1993. [Google Scholar]
- Yubero, I.D. Sabores de Andalucía. Distrib. Consum. 2008, 101, 116–125. [Google Scholar]
- Aníbal, J.; Esteves, E. Muxama and estupeta: Traditional food products obtained from tuna loins in South Portugal and Spain. In Traditional Food Products: General and Consumer Aspects; Kristbergsson, K., Oliveira, J., Eds.; Springer: New York, NY, USA, 2016; pp. 271–274. [Google Scholar]
- Gallart-Jornet, L.; Roberto, I.E.; Maupoei, P.F. La Salazón de Pescado, una Tradición en la Dieta Mediterránea; Editorial de la Universidad Politécnica de Valencia: Valencia, España, 2005; ISBN 8497059182. [Google Scholar]
- Godinho, M. A Muxama. Available online: http://www.projectotasa.com/2011/01/a-muxama/ (accessed on 15 January 2015).
- Esteves, E. Fish products from south Portugal: Dried litão, tuna muxama, and canned mackerel. In Mediterranean Food: Composition and Processing; Cruz, R.M.S., Vieira, M., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 2016; pp. 65–101. [Google Scholar]
- Barat, J.M.; Grau, R. Thawing and salting studies of dry-cured tuna loins. J. Food Eng. 2009, 91, 455–459. [Google Scholar] [CrossRef]
- Rebelo, M.J.F. As Indústrias da Pesca e Conservas de Atum no Algarve do Século XX; Universidade de Algarve: Algarve, Faro, 2010. [Google Scholar]
- Doe, P.E. Fish Drying & Smoking: Production and Quality; Technomic Publishing Company, Inc.: Lancaster, UK, 1998; ISBN 9781566766685. [Google Scholar]
- Van Nguyen, M.; Arason, S.; Eikevik, T.M. Drying of Fish. In Seafood Processing: Technology, Quality and Safety; Boziaris, I.S., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 161–175. [Google Scholar]
- Doe, P.E.; Olley, J. Drying and dried fish products. In Seafood: Resources, Nutritional Composition, and Preservation; Sikorski, Z.E., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1990; pp. 125–145. [Google Scholar]
- Graiver, N.; Pinotti, A.; Califano, A.; Zaritzky, N. Mathematical modeling of the uptake of curing salts in pork meat. J. Food Eng. 2009, 95, 533–540. [Google Scholar] [CrossRef]
- Albarracín, W.; Sánchez, I.C.; Grau, R.; Barat, J.M. Salt in food processing; usage and reduction: A review. Int. J. Food Sci. Technol. 2011, 46, 1329–1336. [Google Scholar] [CrossRef]
- Barat, J.-M.; Pérez-Esteve, E.; Aristoy, M.-C.; Toldrá, F. Partial replacement of sodium in meat and fish products by using magnesium salts. A review. Plant Soil 2013, 368, 179–188. [Google Scholar] [CrossRef]
- Bellagha, S.; Sahli, A.; Farhat, A.; Kechaou, N.; Glenza, A. Studies on salting and drying of sardine (Sardinella aurita): Experimental kinetics and modeling. J. Food Eng. 2007, 78, 947–952. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Djendoubi, N.; Bellagha, S.; Kechaou, N. Study of moisture and salt transfers during salting of sardine fillets. J. Food Eng. 2009, 94, 83–89. [Google Scholar] [CrossRef]
- Jain, D.; Pathare, P.B. Study the drying kinetics of open sun drying of fish. J. Food Eng. 2007, 78, 1315–1319. [Google Scholar] [CrossRef]
- Oliveira, H.; Pedro, S.; Nunes, M.L.; Costa, R.; Vaz-Pires, P. Processing of Salted Cod (Gadus spp.): A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 546–564. [Google Scholar] [CrossRef]
- Sobukola, O.P.; Olatunde, S.O. Effect of salting techniques on salt uptake and drying kinetics of African catfish (Clarias gariepinus). Food Bioprod. Process. 2011, 89, 170–177. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Mujumdar, A.S. Trends in Processing Technologies for Dried Aquatic Products. Dry. Technol. 2011, 29, 382–394. [Google Scholar] [CrossRef]
- Rahman, M.S. Osmotic dehydration of foods. In Handbook of Food Processing; Rahman, M.S., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 2007; pp. 433–446. ISBN 9781845697587. [Google Scholar]
- Arason, S.; Van Nguyen, M.; Thorarinsdottir, K.A.; Thorkelsson, G. Preservation of Fish by Curing. In Seafood Processing: Technology, Quality and Safety; Boziaris, I.S., Ed.; John Wiley & Sons, Ltd.: Oxford, UK, 2014; pp. 129–160. [Google Scholar]
- Zugarramurdi, A.; Lupín, H. A model to explain observed behavior on fish salting. J. Food Sci. 1980, 45, 1305–1311. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; ISBN 9780470973929. [Google Scholar]
- Hall, G.M. Fish. Processing; Wiley-Blackwell Publishing Ltd.: Chichester, UK, 2011; ISBN 9781405190473. [Google Scholar]
- Monteiro, M.L.G.; Mársico, E.T.; Vital, H.C. Avaliação físico-química dos efeitos da irradiação e da evisceração na conservação de atum (Thunnus atlanticus) refrigerado. [Physicochemical evaluations of the effects of irradiation and evisceration on the conservation of refrigerated blackfin tuna (Thunnus atlanticus)]. Rev. Port. Ciênc. Vet. 2010, 105, 45–48. [Google Scholar]
- Bernardi, D.C.; Mársico, E.T.; Freitas, M.Q. De Quality Index Method (QIM) to Assess the Freshness and Shelf Life of Fish. Braz. Arch. Biol. Technol. 2013, 56, 587–598. [Google Scholar] [CrossRef]
- Gómez, R.; Carmona, M.; Fernández-Salguero, J. Estudio de los alimentos de humedad intermedia españoles. I. Actividad del agua y pH. In II Jornadas Científicas Sobre “Alimentación Española”; RACVAO: Jaen, Spain, 1991; pp. 124–130. [Google Scholar]
- Sharma, G. Digital Imaging Handbook; CRC Press Inc.: Boca Raton, FL, USA, 2003; ISBN 978-1-4200-4148-4. [Google Scholar]
- Gallart-Jornet, L.; Barat, J.M.M.; Rustad, T.; Erikson, U.; Escriche, I.; Fito, P. A comparative study of brine salting of Atlantic cod (Gadus morhua) and Atlantic salmon (Salmo salar). J. Food Eng. 2007, 79, 261–270. [Google Scholar] [CrossRef]
- Macdougall, D.B. Colour in Food Improving Quality; CRC Press Inc.; Woodhead Publ. Limited: Cambridge, UK, 2002; ISBN 978-1-85573-590-3. [Google Scholar]
- Ritz, C.; Streibig, J.C. Nonlinear Regression with R; Springer: Berlin, Germany, 2008; ISBN 9780387096162. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Burnham, K.K.P.; Anderson, D.R.D. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: Berlin, Germany, 2002; Volume 172, ISBN 978-0-387-22456-5. [Google Scholar]
- Baty, F.; Ritz, C.; Charles, S.; Brutsche, M.; Flandrois, J.-P.; Delignette-Muller, M.-L. A Toolbox for Nonlinear Regression in R: The Package nlstools. J. Stat. Softw. 2015, 66, 1–21. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; ISBN 3-900051-07-0. [Google Scholar]
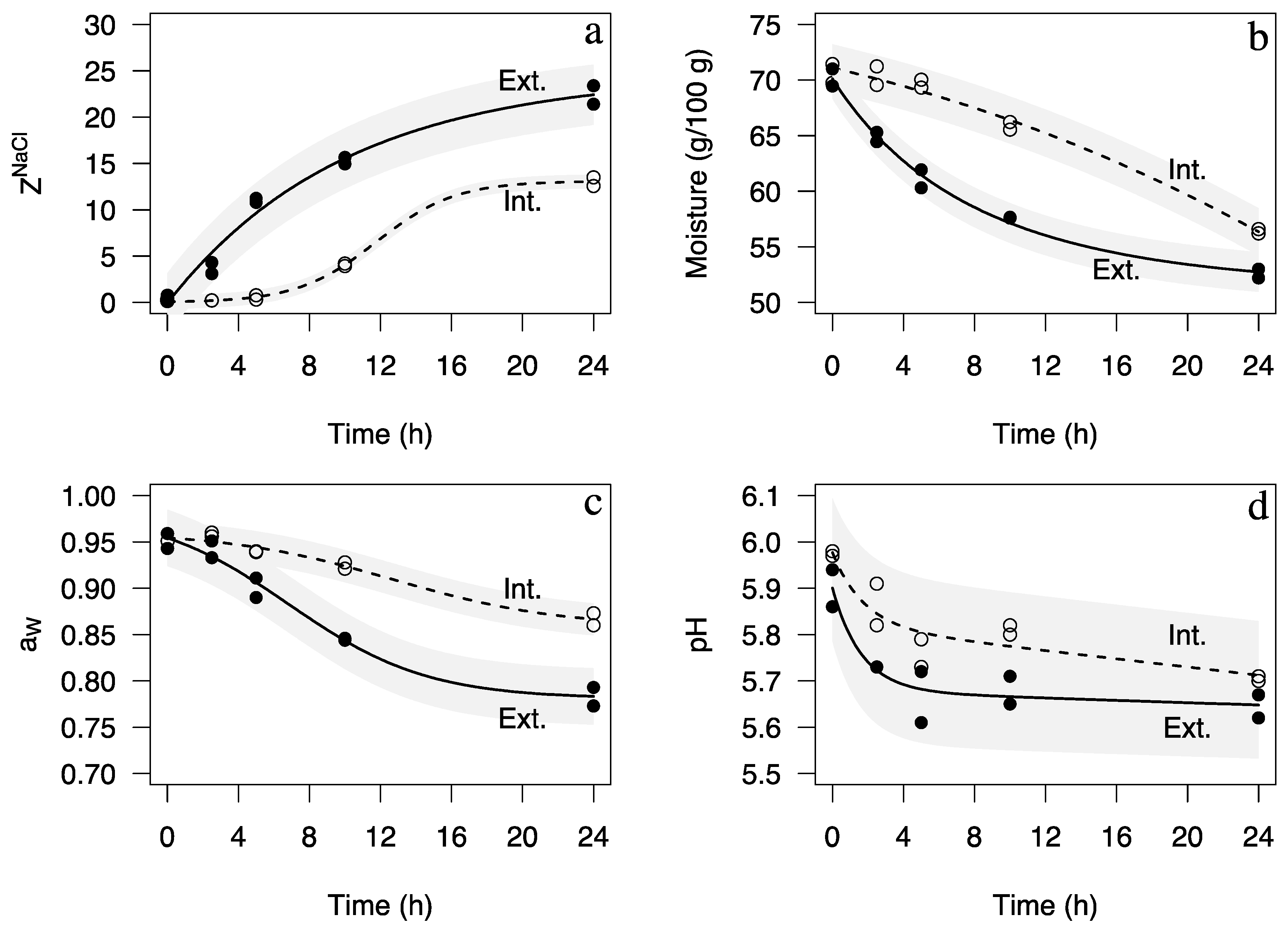
| Parameter | Portion of Loin | Model Name and Equation [Reference] | Estimated Model Parameters (±SE) and Significance a | Pseudo-R2 | Assumptions b |
|---|---|---|---|---|---|
| ZNaCl | Exterior | Zugarramurdi & Lupín [26]: | = −0.009 (0.009) p = 0.9186 = 0.246 (0.019) p < 0.0001 0.100 (0.018) p = 0.0009 | 0.988 | W = 0.952, p = 0.6863 Z = 0.1405, p = 0.8883 |
| Interior | Three-parameter logistic [27]: | a = 0.131 (0.002) p < 0.0001 b = 11.778 (0.372) p < 0.0001 c = 2.229 (0.344) p = 0.0003 | 0.999 | W = 0.979, p = 0.9643 Z = −0.671, p = 0.5023 | |
| Moisture | Exterior | Three-parameter logistic [27]: | a = 51.389 (0.92) p < 0.0001 b = −0.267 (0.013) p < 0.0001 c = 0.097 (0.014) p = 0.0002 | 0.995 | W = 0.906, p = 0.2553 Z = 0.141, p = 0.8883 |
| Interior | Three-parameter logistic [27]: | a = 78.92 (5.96) p < 0.0001 b = 0.11 (0.08) p = 0.2022 c = −0.054 (0.019) p = 0.0256 | 0.993 | W = 0.9637, p = 0.8273 Z = 0, p = 0.9999 | |
| aW | Exterior | Four-parameter logistic [27]: | a = 98.41 (3.66) p < 0.0001 b = 78.09 (1.05) p < 0.0001 m = 6.856 (1.645) p = 0.0059 c = 3.884 (1.373) p = 0.0300 | 0.985 | W = 0.964, p = 0.8317 Z = 1.545, p = 0.1223 |
| Interior | Four-parameter logistic [27]: | a = 96.11 (1.90) p < 0.0001 b = 85.79 (2.68) p < 0.0001 m = 12.696 (2.988) p = 0.0054 c = 4.716 (4.482) p = 0.3333 | 0.995 | W = 0.863, p = 0.0821 Z = −0.562, p = 0.5741 | |
| pH | Exterior | Biexponential [27]: | A1 = 0.223 (0.062) p = 0.0113 lrc1 = −0.491 (0.731) p = 0.5267 A2 = 5.678 (0.053) p < 0.0001 lrc2 = −8.417 (2.407) p = 0.0129 | 0.944 | W = 0.932, p = 0.4702 Z = 0.140, p = 0.8883 |
| Interior | Biexponential [27]: | A1 = 0.158 (0.064) p = 0.0491 lrc1 = −0.554 (1.020) p = 0.6063 A2 = 5.819 (0.056) p < 0.0001 lrc2 = −7.170 (0.707) p < 0.001 | 0.943 | W = 0.958, p = 0.7608 Z = −0.562, p = 0.5741 |
| Parameter | Factor a (X) | F | p | Model b (Adjusted R2 vs. Predicted R2) |
|---|---|---|---|---|
| Moisture | Time (t) | 4.95 | 0.0901 | Moisture = 49.67 − 1.03t− 0.83T |
| (g·100 g−1) | Temperature (T) | 12.70 | 0.0235 * | (0.7296 vs. 0.5056) |
| t × T | 0.22 | 0.6608 | ||
| Water activity | t | 23.05 | 0.0086 ** | aW = 0.68 + 97 × 10−3t + 3.9 × 10−3T − 8.6 × 10−4t × T |
| (aw) | T | 2.39 | 0.1970 | (0.8045 vs. 0.5532) |
| t × T | 6.36 | 0.0652 | ||
| ZNaCl | t | 11.84 | 0.0263 * | ZNaCl = −0.108 − 0.010t + 9.27 × 10−5T |
| T | 38.92 | 0.0034 ** | (0.8335 vs. 0.6956) | |
| t × T | 2.85 | 0.1666 | ||
| RNaCl | t | 19.13 | 0.0119 * | RNaCl = 2.87 − 0.63t − 0.07T + 0.05t × T |
| T | 62.56 | 0.0014 ** | (0.9255 vs. 0.8296) | |
| t × T | 8.23 | 0.0455 * | ||
| L* | t | 3.38 | 0.0824 | L* = 98.71 − 11.46t − 3.55T + 0.72t × T |
| T | 4.00 | 0.0609 | (0.6450 vs. 0.5355) | |
| t × T | 31.95 | <0.0001 *** | ||
| a* | t | 0.09 | 0.7742 | a* = 4.93 |
| T | <0.01 | 0.9524 | ||
| t × T | 2.97 | 0.1001 | ||
| b* | t | 1.63 | 0.2166 | b* = 32.36 − 5.83t − 1.92T + 0.36t × T |
| T | 0.31 | 0.5842 | (0.4821 vs. 0.3515) | |
| t × T | 22.47 | <0.0001 *** | ||
| Chroma | t | 1.53 | 0.2300 | C = 25.621 − 4.30t − 1.35T + 0.26t × T |
| T | 2.01 | 0.1715 | (0.5862 vs. 0.4819)) | |
| t × T | 32.04 | <0.0001 *** | ||
| Saturation (Sab) | t | 0.53 | 0.4757 | Sab = 0.01 − 2.0 × 10−3t − 6.8 × 10−4T + 1.2 × 10−4t × T |
| T | 0.28 | 0.6039 | (0.4006 vs. 0.2494) | |
| t × T | 17.56 | 0.0005 *** | ||
| Hue angle (Hab) | t | 0.01 | 0.9443 | Hab = 60.36 |
| T | 0.002 | 0.9656 | ||
| t × T | 0.65 | 0.4654 | ||
| Color difference (ΔE) | t | 0.01 | 0.9170 | ΔE = 41.76 − 8.01t − 1.84T + 0.47t × T |
| T | 6.41 | 0.0199 * | (0.3807 vs. 0.2878) | |
| t × T | 5.88 | 0.0249 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, E.; Aníbal, J. Optimization of Processing Conditions of Traditional Cured Tuna Loins–Muxama. Fishes 2018, 3, 3. https://doi.org/10.3390/fishes3010003
Esteves E, Aníbal J. Optimization of Processing Conditions of Traditional Cured Tuna Loins–Muxama. Fishes. 2018; 3(1):3. https://doi.org/10.3390/fishes3010003
Chicago/Turabian StyleEsteves, Eduardo, and Jaime Aníbal. 2018. "Optimization of Processing Conditions of Traditional Cured Tuna Loins–Muxama" Fishes 3, no. 1: 3. https://doi.org/10.3390/fishes3010003





