Amazon Sailfin Catfish Pterygoplichthys pardalis (Loricariidae) in Bangladesh: A Critical Review of Its Invasive Threat to Native and Endemic Aquatic Species
Abstract
:1. Introduction
2. Recent Data and Update on Bangladesh
3. Remarks on Pterygoplichthys pardalis Identification
4. Invasive Biology and Potential Ecosystem Effects
5. Control, Eradication, and Human Uses.
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nelson, J.S. Fishes of the World, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Weber, C. Révision du genre Pterygoplichthys sensu latu (Pisces, Siluriformes, Loricariidae). Rev. Fr. Aquariol. 1992, 19, 1–36. [Google Scholar]
- Ferraris, C.J. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of Siluriform primary types. Zootaxa 2007, 1418, 1–300. [Google Scholar] [CrossRef]
- Fuller, P.L.; Nico, L.G.; Williams, J.D. Nonindigenous fishes introduced into inland waters of the United States. Am. Fish. Soc. Spec. Pub. 1999, 27, 1–622. [Google Scholar]
- Weber, C. Nouveaux taxa dans Pterygoplichthys sensu lato (Pisces, Siluriformes, Loricariidae). Rev. Suisse Zool. 1991, 98, 637–643. [Google Scholar]
- Montoya-Burgos, J.I.; Weber, C.; Le Bail, P. Phylogenetic relationships within Hypostomus (Siluriformes: Loricariidae) and related genera based on mitochondrial D-loop. Rev. Suisse Zool. 2002, 109, 369–382. [Google Scholar] [CrossRef]
- Armbruster, J.W. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. Zool. J. Linn. Soc. 2004, 141, 1–80. [Google Scholar] [CrossRef]
- CEC (Commission for Environmental Cooperation). Trinational Risk Assessment Guidelines for Aquatic Alien Invasive Species: Test Cases for the Snakeheads (Channidae) and Armored Catfishes (Loricariidae) in North American Inland Waters; CEC Publications: Montreal, Canada, 2009. [Google Scholar]
- Krishnakumar, K.; Raghaban, R.; Prasad, G.; Bijukumar, A.; Sekharan, M.; Pereira, B.; Ali, A. When pets become pests—Exotic aquarium fishes and biological invasions in Kerala, India. Curr. Sci. India 2009, 97, 474–476. [Google Scholar]
- Islam, Md M; Ruhul Amin, A.S.D.; Parker, S.K. Bangladesh. Pallewatta, N., Reaser, J.K., Gutierrez, A.T., Eds.; In Invasive Alien Species in South-Southeast Asia: National Reports & Directory of Resources; Global Invasive Species Programme: Cape Town, South Africa, 2003; pp. 7–20. [Google Scholar]
- Rahman, A.K.A. Freshwater Fishes of Bangladesh, 2nd ed.; Zoological Society of Bangladesh: Dhaka, Bangladesh, 2005. [Google Scholar]
- Biswas, B.C.; Panigrahi, A.K. Diversity of exotic fishes and their ecological importance in southwestern part of Bangladesh. Int. J. Innov. Res. Sci. Tech. 2014, 1, 129–131. [Google Scholar]
- Mohsin, A.B.M.; Galib, S.M. Handbook on Exotic Ornamental Fishes of Bangladesh; Bangladesh Fisheries Information: Rajshahi, Bangladesh, 2013. [Google Scholar]
- Galib, S.M. Exotic Fishes of Bangladesh: Suckermouth Catfish, Hypostomus Plecostomus (Linnaeus, 1958). Available online: http://en.bdfish.org/2011/05/exotic-fishes-of-bangladesh-suckermouth-catfish-hypostomus-plecostomus-linnaeus-1758 (accessed on 20 December 2017).
- Galib, S.M.; Mohsin, A.B.M. Exotic ornamental fishes of Bangladesh. Bangladesh J. Prog. Sci. Tech. 2010, 8, 255–258. [Google Scholar]
- Hossain, M.Y.; Rahman, M.M.; Ahmed, Z.F.; Ohtomi, J.; Islam, A.B.M.S. First record of the South American sailfin catfish Pterygoplichthys multiradiatus in Bangladesh. J. Appl. Ichthyol. 2008, 24, 718–720. [Google Scholar]
- Welcomme, R.L. Register of international transfers of inland fish species. FAO Fish. Tech. Pap. 1981, 213, 1–120. [Google Scholar]
- Welcomme, R.L. International introductions of inland aquatic species. FAO Fish. Tech. Pap. 1988, 294, 1–318. [Google Scholar]
- Sinha, R.K.; Sinha, R.K.; Sarkar, U.K.; Lakra, W.S. First record of the southern sailfin catfish, Pterygoplichthys anisitsi Eigenmann & Kennedy, 1903 (Teleostei: Loricariidae), in India. J. Appl. Ichthyol. 2010, 26, 606–608. [Google Scholar]
- Capps, K.A.; Nico, L.G.; Mendoza-Carranza, M.; Arevalo-Frias, W.; Ropicki, A.J.; Hellpern, S.A.; Rodiles-Hernandez, R. Salinity tolerance of non-native suckermouth armoured catfish (Loricariidae: Pterygoplichthys) in south-eastern Mexico: Implications for invasion and dispersal. Aquat. Conserv. 2011, 21, 528–540. [Google Scholar] [CrossRef]
- Nico, L.G. Nocturnal and diurnal activity of armored suckermouth catfish (Loricariidae: Pterygoplichthys) associated with wintering Florida manatees (Trichechus manatus latirostris). Neotrop. Ichthyol. 2010, 6, 893–898. [Google Scholar] [CrossRef]
- Samat, A.; Shukor, M.N.; Mazlan, A.G.; Arshad, A.; Fatimah, M.Y. Length-weight relationship and condition factor of Pterygoplichthys pardalis (Pisces: Loricariidae) in Malaysia Peninsula. Res. J. Fish. Hydrobiol. 2008, 3, 48–53. [Google Scholar]
- Nico, L.G.; Jelks, H.L.; Tuten, T. Non-native suckermouth armored catfishes in Florida: description of nest burrows and burrow colonies with assessment of shoreline conditions. ANSRP Bulletin 2009, 9, 1–30. [Google Scholar]
- Liang, S.H.; Wu, H.P.; Shieh, B.S. Size structure, reproductive phenology, and sex ratio of an exotic armored catfish (Liposarcus multiradiatus) in the Kaoping River of southern Taiwan. Zool. Stud. 2005, 44, 252–259. [Google Scholar]
- Gibbs, M.A.; Shields, J.H.; Lock, D.W.; Talmadge, K.M.; Farrell, T.M. Reproduction in an invasive exotic catfish Pterygoplichthys disjunctivus in Volusia Blue Spring, Florida, USA. J. Fish Biol. 2008, 73, 1562–1572. [Google Scholar] [CrossRef]
- Nico, L.G.; Butt, P.L.; Johnston, G.R.; Jelks, H.L.; Kail, M.; Walsh, S.J. Discovery of South American suckermouth armored catfishes (Loricariidae, Pterygoplichthys spp.) in the Santa Fe River drainage, Suwannee River basin, USA. BioInvasions Rec. 2012, 1, 179–200. [Google Scholar] [CrossRef]
- Gibbs, M.A.; Kurth, B.N.; Bridges, C.D. Age and growth of the loricariid catfish Pterygoplichthys disjunctivus in Volusia Blue Spring, Florida. Aquat. Invasions 2013, 8, 207–218. [Google Scholar] [CrossRef]
- Power, M.E. Habitat quality and the distribution of algae-grazing catfish in a Panamanian stream. J. Anim. Ecol. 1984, 53, 353–374. [Google Scholar] [CrossRef]
- Gestring, K.B.; Shafland, P.L.; Stanford, M.S. Status of the exotic Orinoco sailfin catfish (Pterygoplichthys multiradiatus) in Florida. Fla. Sci. 2010, 73, 122–137. [Google Scholar]
- Gestring, K. Shoreline Erosion Assessment of Loricariid Catfishes in Florida; Non-Native Fish Laboratory Report; Florida Fish & Wildlife Conservation Commission: Boca Raton, FL, USA, 2004. [Google Scholar]
- Yamamoto, M.N.; Tagawa, A.W. Hawaii’s Native and Exotic Freshwater Animals; Mutual Publishing: Honolulu, HI, USA, 2000. [Google Scholar]
- Chavez, J.M.; de la Paz, R.M.; Manohar, S.K.; Pagulayan, R.C.; Vi, J.R.C. New Philippine record of South American sailfin catfishes (Pisces: Loricariidae). Zootaxa 2006, 1109, 57–68. [Google Scholar]
- Ruiz-Carus, R.; Grier, H.J. The exotic armored catfishes (Loricariidae) and Hoplosternum littorale (Callichthyidae) in Florida, particularly in the Hillsborough River. In Proceedings of the 4th Tampa Bay Area Scientific Information Symposium (BASIS 4), Treat, S.E., Ed.;. St. Petersburg, FL, USA, 27–30 October 2003. [Google Scholar]
- Hoover, J.J.; Killgore, K.J.; Cofrancesco, A.F. Suckermouth catfishes: threats to aquatic ecosystems of the United States? ANSRP Bulletin 2004, 4, 1–9. [Google Scholar]
- Golani, D.; Snovsky, G. Occurrence of suckermouth armored catfish (Siluriformes, Loricariidae, Pterygoplichthys) in inland waters of Israel. BioInvasions Rec. 2013, 2, 253–256. [Google Scholar] [CrossRef]
- Levin, B.A.; Phuong, P.H.; Pavlov, D.S. Discovery of the Amazon sailfin catfish Pterygoplichthys pardalis (Castelnau, 1855) (Teleostei: Loricariidae) in Vietnam. J. Appl. Ichthyol. 2008, 24, 715–717. [Google Scholar] [CrossRef]
- Piazzini, S.; Lori, E.; Favilli, L.; Cianfanelli, S.; Vanni, S.; Manganelli, G. A tropical fish community in thermal waters of southern Tuscany. Biol. Invasions 2010, 12, 2959–2965. [Google Scholar] [CrossRef]
- Page, L.M.; Robins, R.H. Identification of sailfin catfishes (Teleostei: Loricariidae) in southeastern Asia. Raffles B. Zool. 2006, 54, 455–457. [Google Scholar]
- Keszka, S.; Panicz, R.; Tański, A. First record of the leopard pleco, Pterygoplichthys gibbiceps (Actinopterygii, Loricariidae) in the Brda River in the centre of Bydgoszcz (northern Poland). Acta Ichthyol. Piscat. 2008, 38, 135–138. [Google Scholar] [CrossRef]
- Özdilek, Ş.Y. Possible threat for Middle East inland water: An exotic and invasive species, Pterygoplichthys disjunctivus (Weber, 1991) in Asi River, Turkey (Pisces: Loricariidae). E.Ü. Su Ürünleri Dergisi 2007, 24, 303–306. [Google Scholar]
- Wakida-Kusunoki, A.T.; Ruiz-Carus, R.; Amador-del-Angel, E. Amazon sailfin catfish, Pterygoplichthys pardalis (Castelnau, 1855) (Loricariidae), another exotic species established in southeastern Mexico. Southwest. Nat. 2007, 52, 141–144. [Google Scholar] [CrossRef]
- Devick, W.S. Patterns of introductions of aquatic organisms to Hawaiian freshwater habitats. In New Directions in Research, Management and Conservation of Hawaiian Freshwater Stream Ecosystems; Devick, W.S., Ed.; Proceedings of the 1990 Symposium of Freshwater Stream Biology and Fisheries Management; Hawaii Department of Land and Natural Resources, Division of Aquatic Resources: Honolulu, HI, USA, 1991; pp. 189–213. [Google Scholar]
- Nico, L.G.; Martin, R.T. The South American suckermouth armored catfish, Pterygoplichthys anisitsi (Pisces: Loricariidae), in Texas, with comments on foreign fish introductions in the American Southwest. Southwest. Nat. 2001, 46, 98–104. [Google Scholar] [CrossRef]
- Armbruster, J.W.; Page, L.M. Redescription of Pterygoplichthys punctatus and description of a new species of Pterygoplichthys (Siluriformes: Loricariidae). Neotrop. Ichthyol. 2006, 4, 401–409. [Google Scholar] [CrossRef]
- Hubbs, C.; Lucier, T.; Garrett, G.P.; Edwards, R.J.; Dean, S.M.; Marsh, E. Survival and abundance of introduced fishes near San Antonio, Texas. Tex. J. Sci. 1978, 30, 369–376. [Google Scholar]
- Power, M.E. Grazing responses of tropical freshwater fishes to different scales of variation in their food. Environ. Biol. Fish. 1983, 9, 103–115. [Google Scholar] [CrossRef]
- Power, M.E. The importance of sediment in the grazing ecology and size class interactions of an armored catfish, Ancistrus spinosus. Environ. Biol. Fish. 1984, 10, 173–181. [Google Scholar] [CrossRef]
- Power, M.E. Predator avoidance by grazing fishes in temperate and tropical streams: Importance of stream depth and prey size. In Predation: Direct and Indirect Impacts in Aquatic Communities; Kerfoot, W.C., Sih, A., Eds.; University Press of New England: Dartmouth, NH, USA, 1987; pp. 333–351. [Google Scholar]
- Power, M.E.; Dudley, T.L.; Cooper, S.D. Grazing catfish, fishing birds, and attached algae in a Panamanian stream. Environ. Biol. Fish. 1989, 26, 285–294. [Google Scholar] [CrossRef]
- Power, M.E. Resource enhancement by indirect effects of grazers: Armored catfish, algae, and sediment. Ecology 1990, 71, 897–904. [Google Scholar] [CrossRef]
- Pfeiffer, E. Armored Catfish Wreaking Havoc in South Florida Lakes. Yahoo News. Available online: https://www.yahoo.com/news/blogs/sideshow/armored-catfish-wreaking-havoc-south-florida-lakes-182812663.html (accessed on 20 December 2017).
- Ludlow, M.E.; Walsh, S.J. Occurrence of a South American armored catfish in the Hillsborough River, Florida. Fla. Sci. 2014, 54, 48–50. [Google Scholar]
- Gibbs, M.; Futral, T.; Mallinger, M.; Martin, D.; Ross, M. Disturbance of the Florida manatee by an invasive catfish. Southeast. Nat. 2010, 9, 635–648. [Google Scholar] [CrossRef]
- Nico, L.G.; Loftus, W.F.; Reid, J.P. Interactions between non-native armored suckermouth catfish (Loricariidae: Pterygoplichthys) and native Florida manatee (Trichechus manatus latirostris) in artesian springs. Aquat. Invasions 2009, 4, 511–519. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance, 5th ed.; Harper & Row: New York, NY, USA, 2010. [Google Scholar]
- Glick, P.; Stein, B.A.; Edelson, N.A. (Eds.) Scanning the Conservation Horizon: A Guide to Climate Change Vulnerability Assessment; National Wildlife Federation: Washington, DC, USA, 2011. [Google Scholar]
- Bunkley-Williams, L.; Williams, E.H., Jr.; Lilystrom, C.G.; Corujo-Flores, I.; Zerbi, A.J.; Aliaume, C. The South American sailfin catfish, Liposarcus multiradiatus (Hancock), a new exotic established in Puerto Rican fresh waters. Caribb. J. Sci. 1994, 30, 90–94. [Google Scholar]
- Soares, M.G.M.; Almeida, R.G.; Junk, W.J. The trophic status of the fish fauna in Lago Camaleão, a macrophyte dominated floodplain lake in the middle Amazon. Amazoniana 1986, 9, 511–526. [Google Scholar]
- Nonogaki, H.; Nelson, J.A.; Patterson, W.P. Dietary histories of herbivorous loricariid catfishes: Evidence from δ13C values of otoliths. Environ. Biol. Fish. 2006, 78, 13–21. [Google Scholar] [CrossRef]
- Walker, B. The fish with the folded mouth. Aquarium Series II 1968, 1, 36–43. [Google Scholar]
- CBIF (Canadian Biodiversity Information Facility). Integrated Taxonomic Information System (ITIS). Available online: http://www.cbif.gc.ca/eng/integrated-taxonomic-information-system-itis/?id=1381347793621 (accessed on 20 December 2017).
- Patil, S.R.; Patil, S.S.; Sathe, T.V. Preliminary analysis of diversity status with reference to Pisces from major wetlands of Ajara tahsil of Kolhapur district, Maharashtra, India. Biolife 2015, 3, 12–15. [Google Scholar]
- Sagouis, A.; Cucherousset, J.; Villéger, S.; Santoul, F.; Boulêtreau, S. Non-native species modify the isotopic structure of freshwater fish communities across the globe. Ecography 2015, 38, 979–985. [Google Scholar] [CrossRef]
- Cagauan, A.G. Exotic aquatic species introduction in the Philippines for aquaculture—A threat to biodiversity or a boom to the economy? J. Environ. Sci. Manage. 2007, 10, 48–62. [Google Scholar]
- Page, L.M.; Espinosa-Pérez, H.; Findley, L.T.; Gilbert, C.R.; Lea, R.N.; Mandrak, N.E.; Mayden, R.L.; Nelson, J.S. Common and Scientific Names of Fishes from the United States, Canada, and Mexico, 7th ed.; American Fishes Society: Bethesda, MD, USA, 2013. [Google Scholar]
- Val, A.L.; de Almeida-Val, V.M.F. Fishes of the Amazon and their environment: physiological and biochemical aspects. Zoophysiology 1995, 32, 1–245. [Google Scholar]
- Burgess, W.E. An Atlas of Freshwater and Marine Catfishes—A Preliminary Survey of the Siluriformes; T.F.H. Publications: Neptune City, NJ, USA, 1989. [Google Scholar]
- Sato, M.; Kawaguchi, Y.; Nakajima, J.; Mukai, T.; Shimatani, Y.; Onikura, N. A review of the research on introduced freshwater fishes: new perspectives, the need for research, and management implications. Landsc. Ecol. Eng. 2010, 6, 99–108. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase. 2017. Available online: http://www.fishbase.org (accessed on 20 December 2017).
- USGS (U.S. Geological Survey). Species List of Nonindigenous Fish. Available online: https://nas.er.usgs.gov/taxgroup/fish/default.aspx (accessed on 20 December 2017).
- Crossman, E.J. Introduced freshwater fishes: a review of the North American perspective with emphasis on Canada. Can. J. Fish. Aquat. Sci. 1991, 48, 46–57. [Google Scholar] [CrossRef]
- Dill, W.A.; Cardone, A.J. History and status of introduced fishes in California, 1871–1996. Fish Bull. Calif. Dep. Fish Game 1997, 178, 1–414. [Google Scholar]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters, 2nd ed.; Chapman & Hall: New York, NY, USA, 2007. [Google Scholar]
- Reisner, M. Cadillac Desert: The American West and its Disappearing Water; Viking Press: New York, NY, USA, 1986. [Google Scholar]
- Vadas, R.L., Jr. The springtime phytoplankton of two calcareous ponds in Ohio. J. Freshwater Ecol. 1992, 7, 407–418. [Google Scholar] [CrossRef]
- Vadas, R.L., Jr. Instream-flow needs for anadromous salmonids and lamprey on the Pacific coast, with special reference to the Pacific Southwest. Environ. Monit. Assess. 2000, 64, 331–358. [Google Scholar] [CrossRef]
- Nichols, S.A. The use of overwinter drawdown for aquatic vegetation management. Water Res. Bull. 1975, 11, 1137–1148. [Google Scholar] [CrossRef]
- Siver, P.A.; Coleman, A.M.; Benson, G.A.; Simpson, J.T. The effects of winter drawdown on macrophytes in Candlewood Lake, Connecticut. Lake Reserv. Manage. 1986, 2, 69–73. [Google Scholar] [CrossRef]
- Payne, B.S.; Miller, A.C. Risk assessment, decision analysis, and invasive species. ANSRP Bulletin 2004, 4, 10–13. [Google Scholar]
- IUCN (International Union of Conservation of Nature) Bangladesh. Red List of Bangladesh, vol. 5, Freshwater Fishes; IUCN, Bangladesh Country Office: Dhaka, Bangladesh, 2015. [Google Scholar]
- Chang, A.L.; Grossman, J.D.; Spezio, T.S.; Weiskel, H.W.; Blum, J.C.; Burt, J.W.; Muir, A.A.; Piovia-Scott, J.; Veblem, K.E.; Grosholz, E.D. Tackling aquatic invasions: risks and opportunities for the aquarium fish industry. Biol. Invasions 2009, 11, 773–785. [Google Scholar] [CrossRef]
- Greenwood, M.F.D. Distribution, spread, and habitat predictability of a small, invasive, piscivorous fish in an important estuarine fish nursery. Fishes 2017, 2, 6. [Google Scholar] [CrossRef]
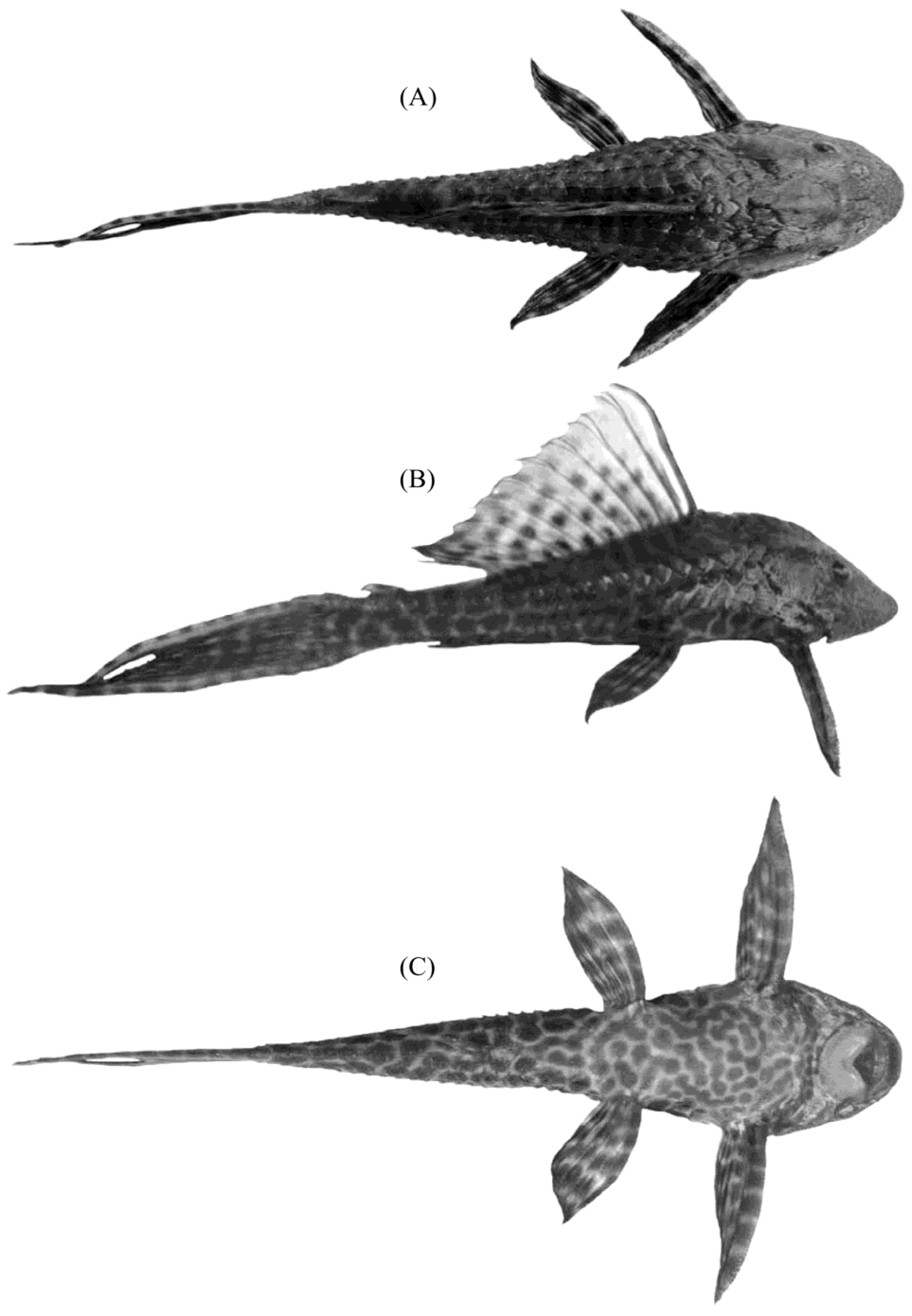
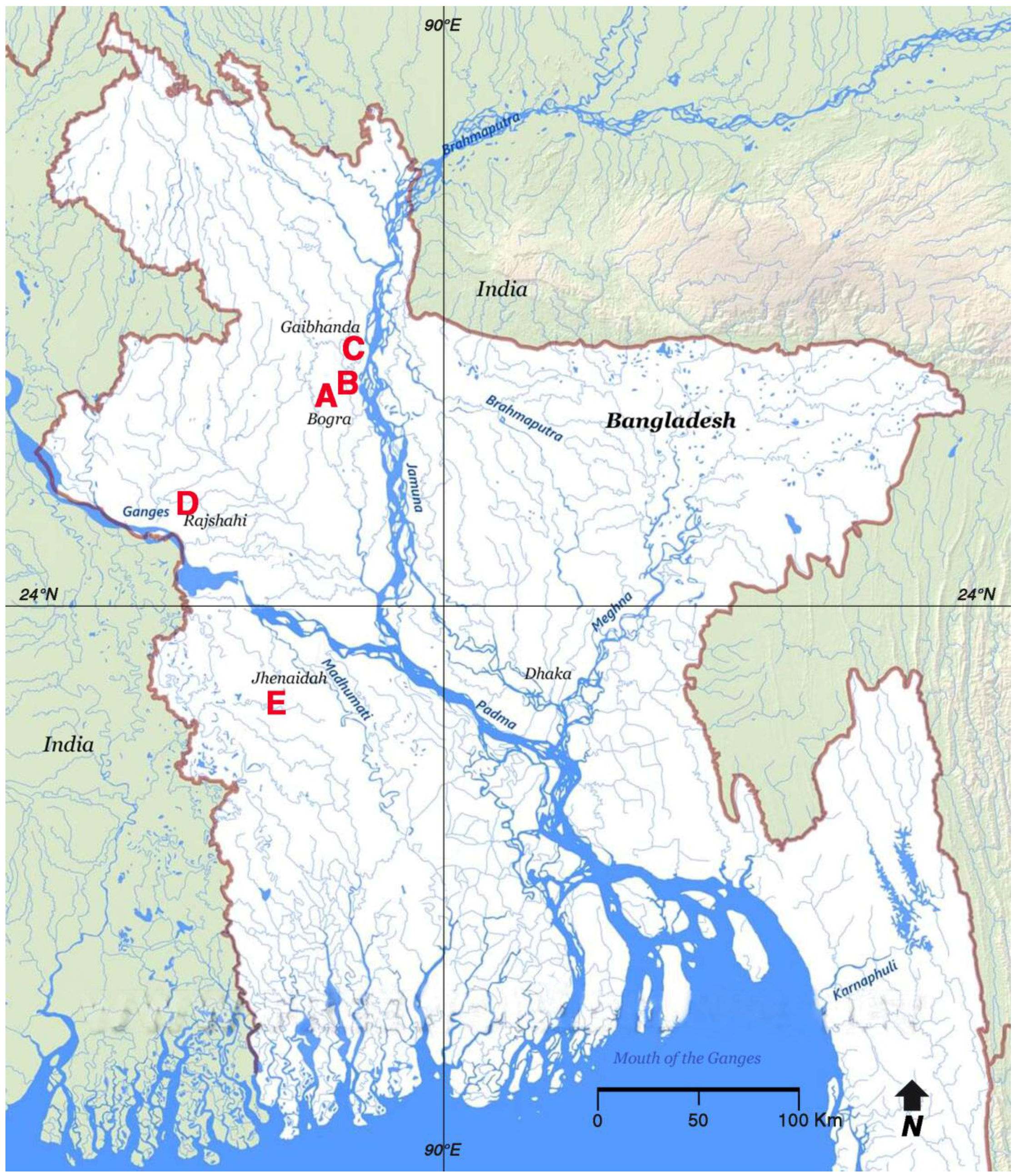
| Date | Division | Fishery | Gear | Habitat | Number | Preservation |
|---|---|---|---|---|---|---|
| 16 Jun 2007 | Rajshahi A | Commercial | Lift-net | Irrigation canal | 3 | Yes |
| 28 Jun 2007 | Rajshahi B | Commercial | Lift-net | Irrigation canal | 2 | Yes |
| 3 Jul 2007 | Rangpur C | Commercial | Lift-net | Irrigation canal | 5 | No |
| 22 Sep 2009 | Rajshahi D | Private | Cast net | Urban fish pond | 6 | Yes |
| 1 Apr 2012 | Khulna E | Private | Cast net | Rural fish pond | 2 | No |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.Y.; Vadas, R.L., Jr.; Ruiz-Carus, R.; Galib, S.M. Amazon Sailfin Catfish Pterygoplichthys pardalis (Loricariidae) in Bangladesh: A Critical Review of Its Invasive Threat to Native and Endemic Aquatic Species. Fishes 2018, 3, 14. https://doi.org/10.3390/fishes3010014
Hossain MY, Vadas RL Jr., Ruiz-Carus R, Galib SM. Amazon Sailfin Catfish Pterygoplichthys pardalis (Loricariidae) in Bangladesh: A Critical Review of Its Invasive Threat to Native and Endemic Aquatic Species. Fishes. 2018; 3(1):14. https://doi.org/10.3390/fishes3010014
Chicago/Turabian StyleHossain, Mohammad Y., Robert L. Vadas, Jr., Ramon Ruiz-Carus, and Shams M. Galib. 2018. "Amazon Sailfin Catfish Pterygoplichthys pardalis (Loricariidae) in Bangladesh: A Critical Review of Its Invasive Threat to Native and Endemic Aquatic Species" Fishes 3, no. 1: 14. https://doi.org/10.3390/fishes3010014




