Mass Spectrometric Analysis of Bisphenol A Desorption from Titania Nanoparticles: Ammonium Acetate, Fluoride, Formate, and Hydroxide as Chemical Desorption Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Adsorption of Bisphenol A on TiO2 Nanoparticles
2.3. Desorption of Bisphenol A from TiO2 Nanoparticles
2.4. Instrumentation
2.5. Sample Introduction to Mass Spectrometry
3. Results and Discussion
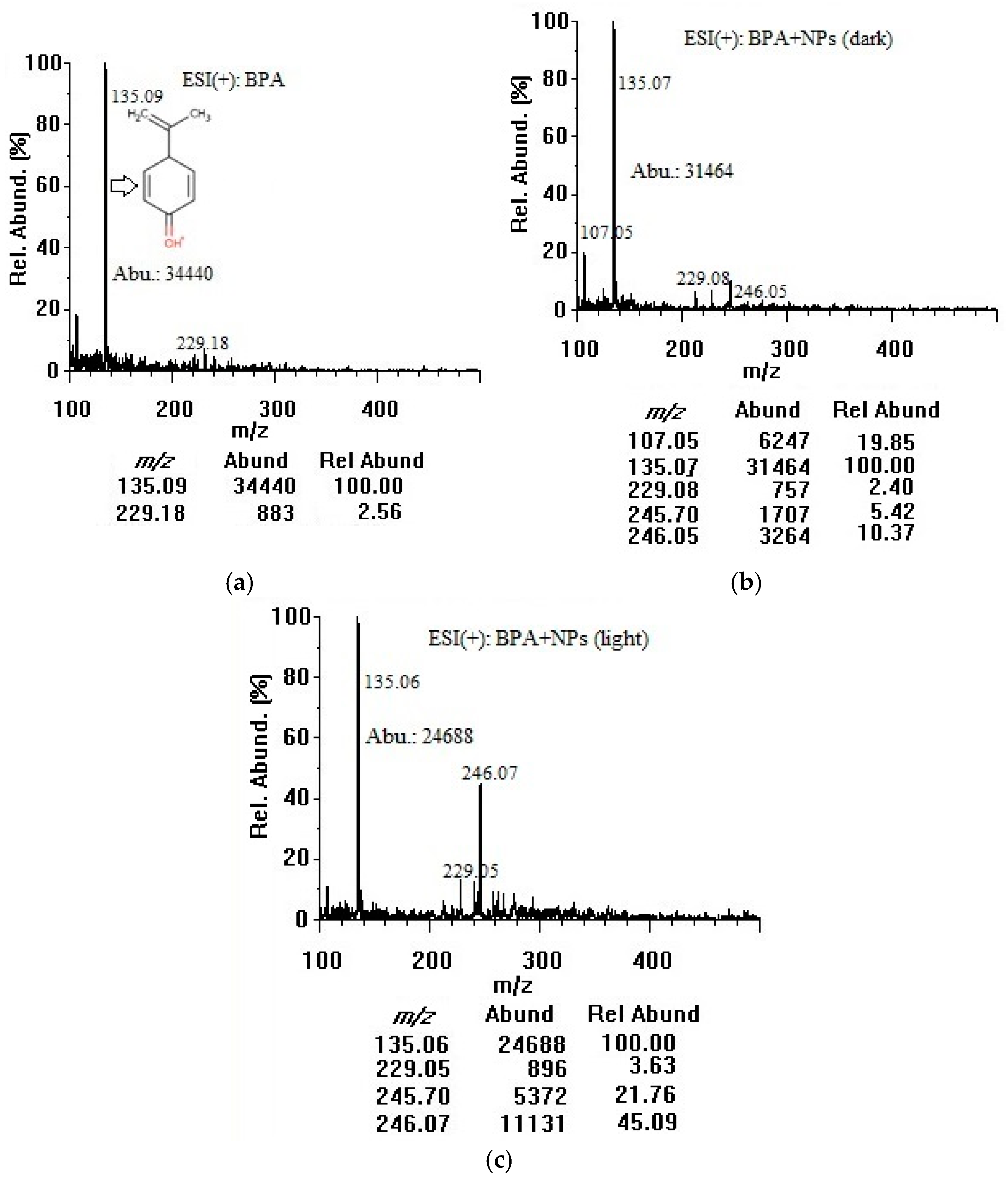
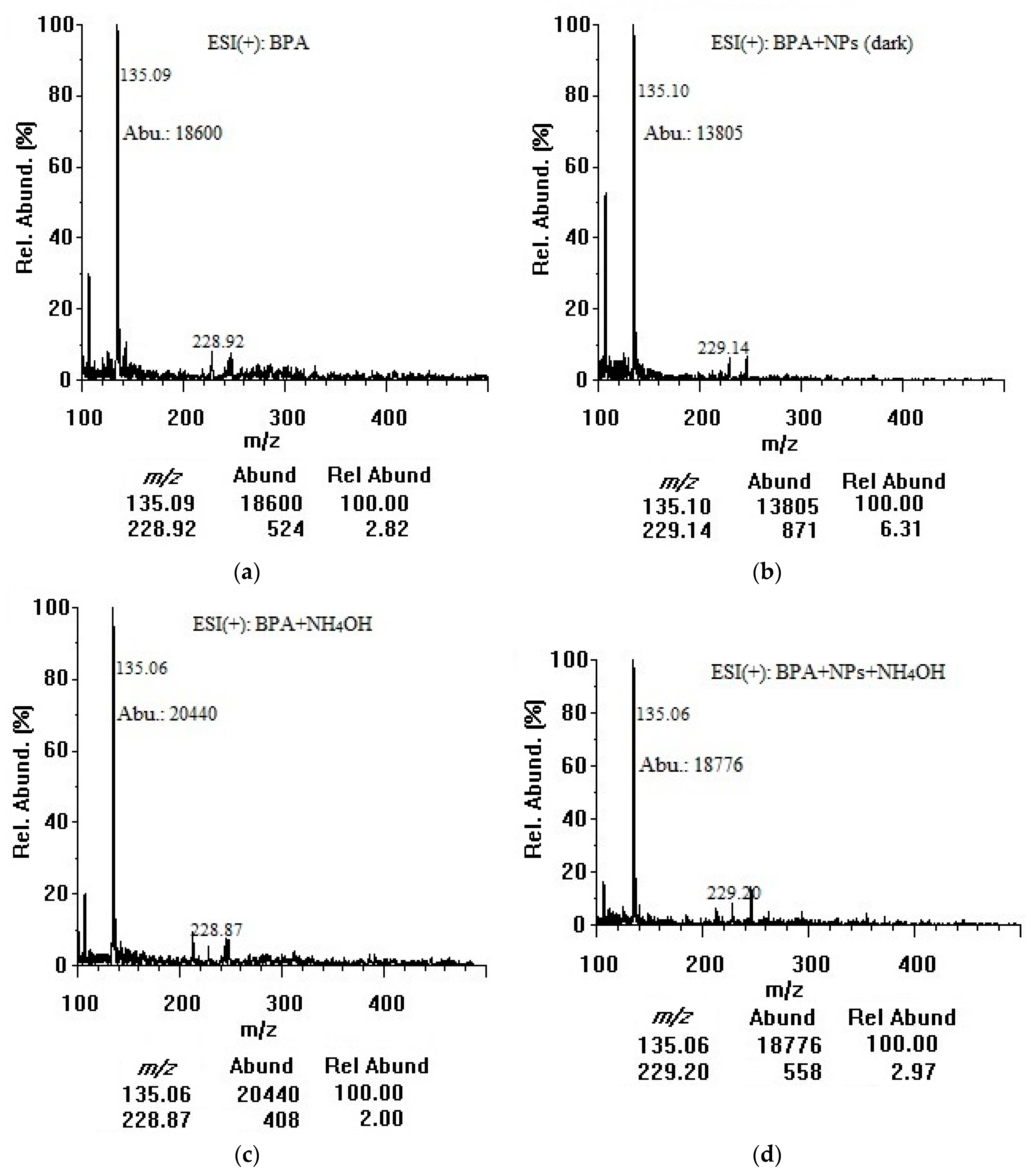
3.1. Adsorption of Bisphenol A on Titania Nanoparticles in Dark and Room Light (Positive-mode Electrospray Ionization Analysis)
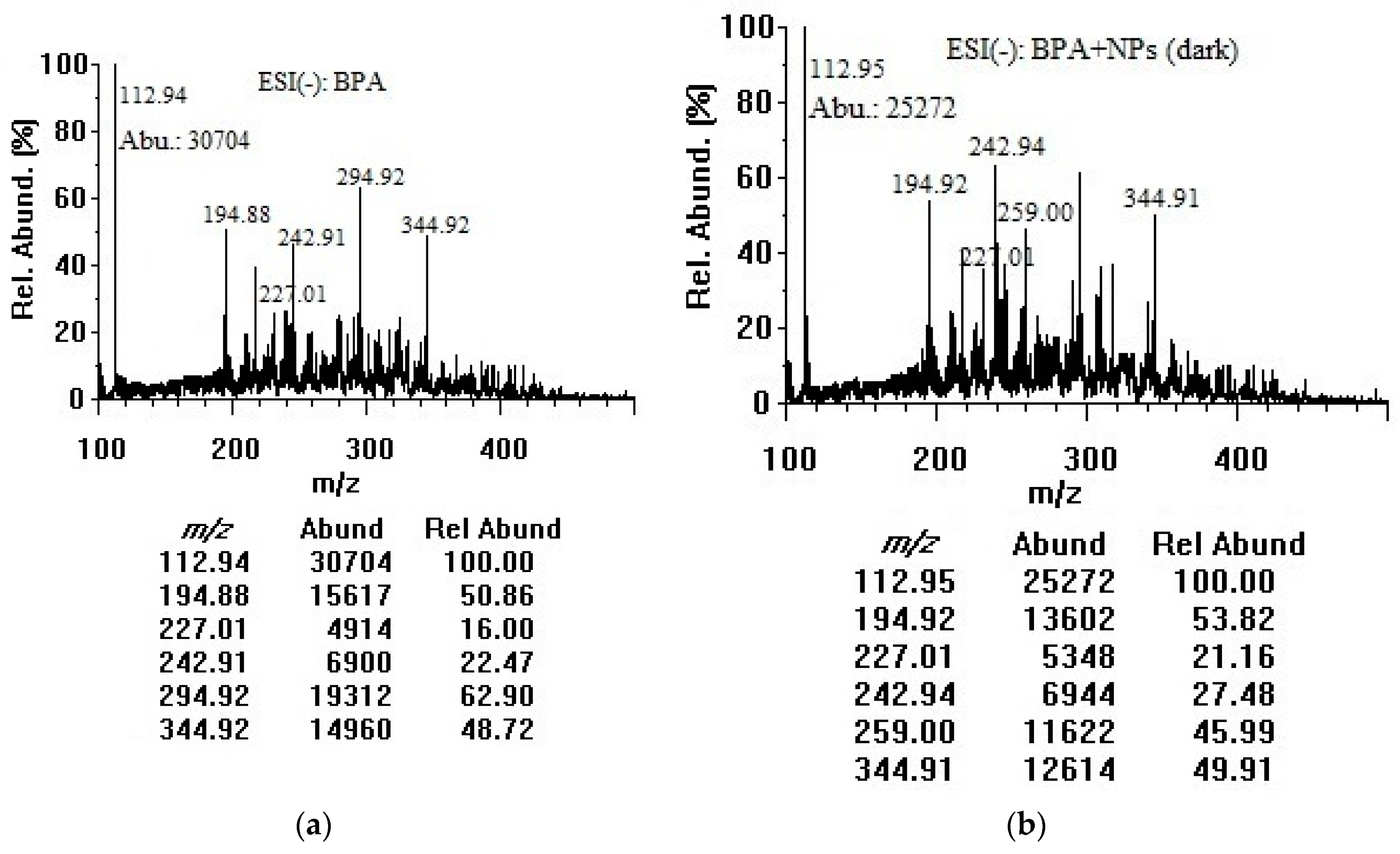
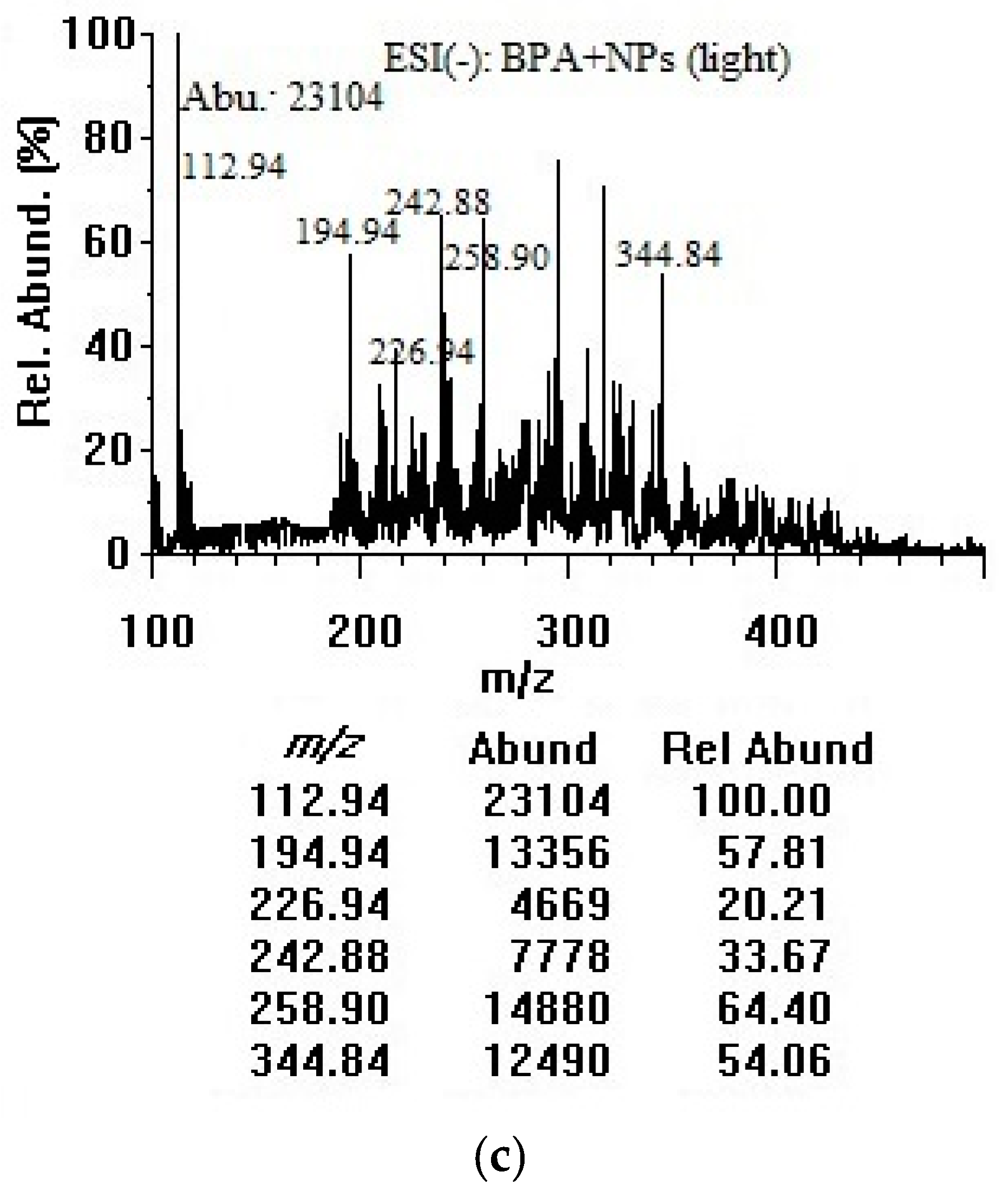
3.2. Adsorption of Bisphenol A on Titania Nanoparticles in Dark and Room Light (Negative-mode Electrospray Ionization Analysis)
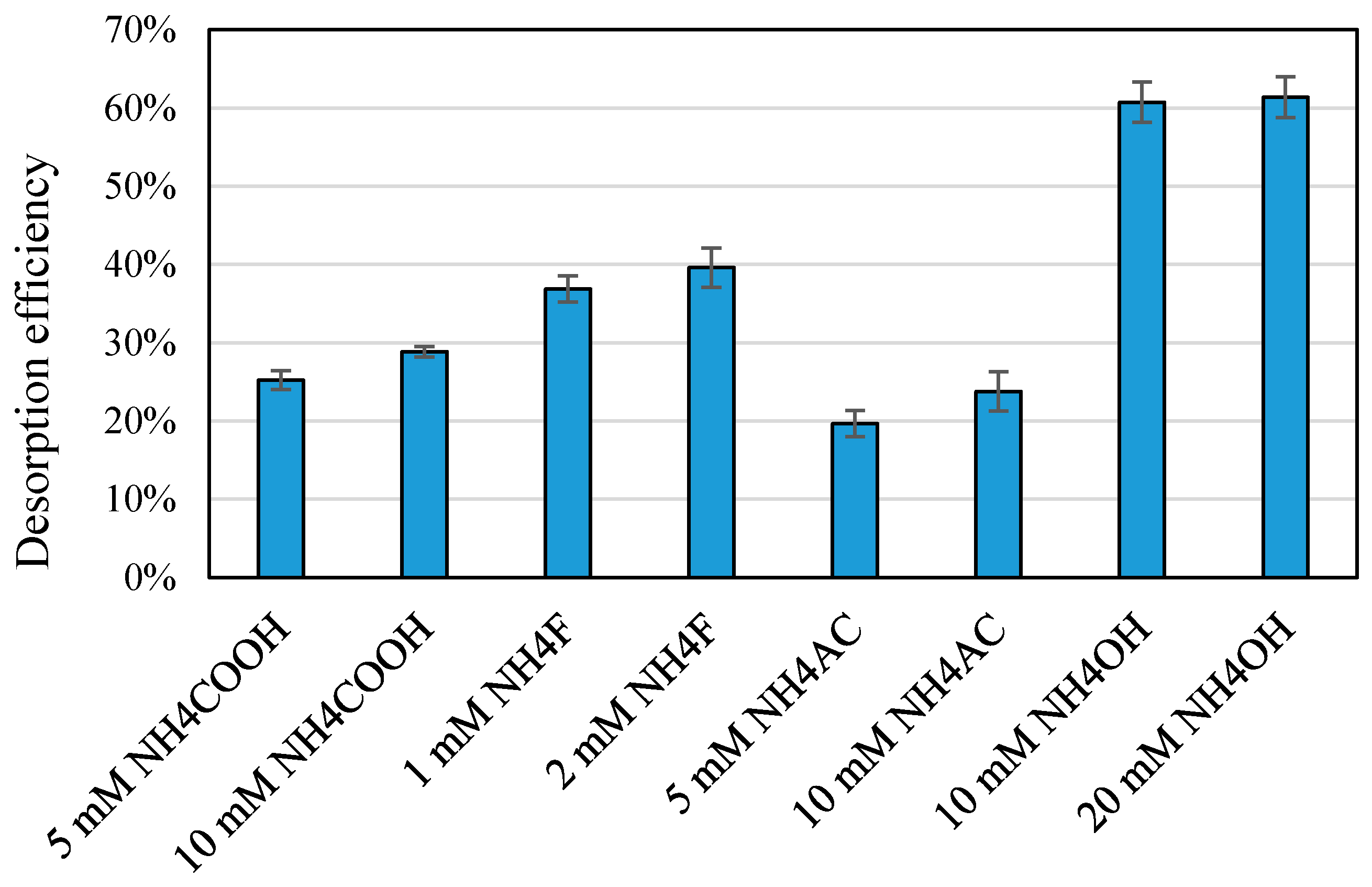
3.3. Application of Mass Spectrometry Modifiers as Chemical Desorption Agents
3.4. Ammonium Hydroxide as a Desorption Agent for Various Nanoparticle Concentrations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adoamnei, E.; Mendiola, J.; Vela-Soria, F.; Fernández, F.; Olea, N.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Environ. Res. 2018, 161, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.A.E.; Trought, K.; Mitchell, C.; Northcott, G.; Tremblay, L.A. Assessment of endocrine disruption and oxidative potential of bisphenol-A, triclosan, nonylphenol, diethylhexyl phthalate, galaxolide, and carbamazepine, common contaminants of municipal biosolids. Toxicol. In Vitro 2018, 48, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Eladak, S.; Moison, D.; Guerquin, M.J.; Matilionyte, G.; Kilcoyne, K.; N’Tumba-Byn, T.; Messiaen, S.; Deceuninck, Y.; Pozzi-Gaudin, S.; Benachi, A.; et al. Effects of environmental bisphenol A exposures on germ cell development and Leydig cell function in the human fetal testis. PLoS ONE 2018, 13, e0191934. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Baglin, N.; Lee, B.P.; Kocur, A.L.; Shepherd, M.H.; Steele, A.M.; BPA Schools Study, Consortium; Harries, L.W. An engaged research study to assess the effect of a ‘real-world’ dietary intervention on urinary bisphenol A (BPA) levels in teenagers. BMJ Open 2018, 8, e018742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Shen, X.; Shao, B.; Wu, F. Sensitive analysis of steroid estrogens and bisphenol A in small volumes of water using isotope-dilution ultra-performance liquid chromatography-tandem mass spectrometry. Environ. Pollut. 2018, 235, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Perera, L.; Coons, L.A.; Burns, K.A.; Ramsey, J.T.; Pelch, K.E.; Houtman, R.; van Beuningen, R.; Teng, C.T.; Korach, K.S. Differential in vitro biological action, coregulator interactions, and molecular dynamic analysis of bisphenol A (BPA), BPAF, and BPS ligand–ERα complexes. Environ. Health Perspect. 2018, 126, 017012. [Google Scholar] [CrossRef] [PubMed]
- García-Córcoles, M.T.; Cipa, M.; Rodríguez-Gómez, R.; Rivas, A.; Olea-Serrano, F.; Vílchez, J.L.; Zafra-Gómez, A. Determination of bisphenols with estrogenic activity in plastic packaged baby food samples using solid-liquid extraction and clean-up with dispersive sorbents followed by gas chromatography tandem mass spectrometry analysis. Talanta 2018, 178, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Casero, N.; Lunar, L.; Rubio, S. Analytical methods for the determination of mixtures of bisphenols and derivatives in human and environmental exposure sources and biological fluids. A review. Anal. Chim. Acta 2016, 908, 22–53. [Google Scholar] [CrossRef] [PubMed]
- Acosta, R.; Nabarlatz, D.; Sánchez-Sánchez, A.; Jagiello, J.; Gadonneix, P.; Celzard, A.; Fierro, V. Adsorption of bisphenol A on KOH-activated tire pyrolysis char. J. Environ. Chem. Eng. 2018, 6, 823–833. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Z.; Yang, J. A novel composite hydrogel for adsorption and photocatalytic degradation of bisphenol A by visible light irradiation. Chem. Eng. J. 2018, 334, 1679–1690. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Lee, J.K.; Cho, C.W.; Jhung, S.H. Remarkably efficient adsorbent for the removal of bisphenol A from water: Bio-MOF-1-derived porous carbon. Chem. Eng. J. 2018, 343, 225–234. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Jia, J.; Ma, J.; Jia, Q. Design of a hyper-crosslinked β-cyclodextrin porous polymer for highly efficient removal toward bisphenol A from water. Sep. Purif. Technol. 2018, 195, 130–137. [Google Scholar] [CrossRef]
- Choong, C.E.; Ibrahim, S.; Yoon, Y.; Jang, M. Removal of lead and bisphenol a using magnesium silicate impregnated palm-shell waste powdered activated carbon: Comparative studies on single and binary pollutant adsorption. Ecotoxicol. Environ. Saf. 2018, 148, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kusvuran, E.; Yildirim, D. Degradation of bisphenol A by ozonation and determination of degradation intermediates by gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry. Chem. Eng. J. 2013, 220, 6–14. [Google Scholar] [CrossRef]
- Yan, J.; Lin, B.; Hu, C.; Zhang, H.; Lin, Z.; Xi, Z. The combined toxicological effects of titanium dioxide nanoparticles and bisphenol A on zebrafish embryos. Nanoscale Res. Lett. 2014, 9, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehse, S.; Kloas, W.; Zarfl, C. Microplastics reduce short-term effects of environmental contaminants. Part I: Effects of bisphenol a on freshwater zooplankton are lower in presence of polyamide particles. Int. J. Environ. Res. Public Health 2018, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Verdier, T.; Bertron, A.; Erable, B.; Roques, C. Bacterial biofilm characterization and microscopic evaluation of the antibacterial properties of a photocatalytic coating protecting building material. Coatings 2018, 8, 93. [Google Scholar] [CrossRef]
- Dhanasekar, M.; Jenefer, V.; Nambiar, R.B.; Babu, S.G.; Selvam, S.P.; Neppolian, B.; Bhat, S.V. Ambient light antimicrobial activity of reduced graphene oxide supported metal doped TiO2 nanoparticles and their PVA based polymer nanocomposite films. Mater. Res. Bull. 2018, 97, 238–243. [Google Scholar] [CrossRef]
- Hosseini-Zori, M. Co-doped TiO2 nanostructures as a strong antibacterial agent and self-cleaning cover: Synthesis, characterization and investigation of photocatalytic activity under UV irradiation. J. Photochem. Photobiol. B Biol. 2018, 178, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Žerjav, G.; Kaplan, R.; Pintar, A. Catalytic wet air oxidation of bisphenol A aqueous solution in trickle-bed reactor over single TiO2 polymorphs and their mixtures. J. Environ. Chem. Eng. 2018, 6, 2148–2158. [Google Scholar] [CrossRef]
- Žerjav, G.; Djinović, P.; Pintar, A. TiO2-Bi2O3/(BiO)2CO3-reduced graphene oxide composite as an effective visible light photocatalyst for degradation of aqueous bisphenol A solutions. Catal. Today 2018, 315, 237–246. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Williams, M.; Kookana, R.; de Marchi, M.R.R. Predicting bioaccessibility of contaminants of emerging concern in marine sediments using chemical methods. J. Soil Sediment 2018, 18, 1720–1728. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, N.; Wang, X.; Tang, Y.; Zhu, L.; Huang, Z.; Tang, H.; Shi, Y.; Wu, Y.; Zhang, M.; et al. Effects of the interaction of TiO2 nanoparticles with bisphenol A on their physicochemical properties and in vitro toxicity. J. Hazard. Mater. 2012, 199, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.C.; Reis Teodoro, J.A.; de Cássia Franco Afonso, R.J.; Aquino, S.F.; Augusti, R. Photodegradation of bisphenol A in aqueous medium: Monitoring and identification of by-products by liquid chromatography coupled to high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Linden, K.G.; Hinton, D.E.; Kashiwada, S.; Rosenfeldt, E.J.; Kullman, S.W. Biological assessment of bisphenol A degradation in water following direct photolysis and UV advanced oxidation. Chemosphere 2006, 65, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ge, M.; Liu, L.; Gao, G.; Feng, Y.; Wang, Y. Directed synthesis of mesoporous TiO2 microspheres: Catalysts and their photocatalysis for bisphenol A degradation. Environ. Sci. Technol. 2010, 44, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.A.; Pétrier, C.; Combet, E.; Carrier, M.; Pulgarin, C. Ultrasonic cavitation applied to the treatment of bisphenol A. Effect of sonochemical parameters and analysis of BPA by-products. Ultrason. Sonochem. 2008, 15, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Alfaro, P.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction: An innovative sample preparation approach applied to the analysis of specific migration from food packaging. Anal. Chim. Acta 2016, 936, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Konermann, L.; Ahadi, E.; Rodriguez, A.D.; Vahidi, S. Unraveling the mechanism of electrospray ionization. Anal. Chem. 2013, 85, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Ayala, H.; Moyano, E.; Galceran, M.T. Liquid chromatography/multi-stage mass spectrometry of bisphenol A and its halogenated derivatives. Rapid Commun. Mass Spectrom. 2007, 21, 4039–4048. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xiang, L.; Li, J.; Yang, Z.; Fang, J.; Zhao, C.; Xu, S.; Cai, Z. Investigation on fragmentation pathways of bisphenols by using electrospray ionization Orbitrap mass spectrometry. Rapid Commun. Mass. Spectrom. 2016, 30, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Maragou, N.C.; Lampi, E.N.; Thomaidis, N.S.; Koupparis, M.A. Determination of bisphenol A in milk by solid phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A 2006, 1129, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Konermann, L. Addressing a common misconception: Ammonium acetate as neutral pH “buffer” for native electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Nieves, A.; de las Nieves, F.J.; Richter, C. Point of zero charge estimation for a TiO2/water interface. In Trends in Colloid and Interface Science XII. Progress in Colloid and Polymer Science; Koper, G.J.M., Bedeaux, D., Cavaco, C., Sager, W.F.C., Eds.; Steinkopff Verlag: Heidelberg, Germany, 1998; Volume 110, pp. 21–24. ISBN 978-3-7985-1117-0. [Google Scholar]


| Parameter | Value |
|---|---|
| Voltage (polarity) | −3.5 kV (negative) or +4.0 kV (positive) |
| Nebulizer gas (N2) pressure | 15 psi |
| Drying gas flow rate | 7.0 L/min |
| Drying gas temperature | 300 °C |
| Mass-to-charge scan range | m/z 100–500 |
| TiO2 Concentration (µg/mL) Available for BPA Adsorption | Abundance of m/z 135.1 Peak (arb. Unit) | % Adsorbed = (Ainitial − Aexp)/Ainitial | % Recovery = A’exp/A’initial | % Desorbed = % Recovery-(100% − % Adsorbed) | Desorption Efficiency = % Desorbed/% Adsorbed | |
|---|---|---|---|---|---|---|
| A before Adding 10 mM NH4OH | A’ after Adding 10 mM NH4OH | before Adding 10 mM NH4OH | after Adding 10 mM NH4OH | by Adding 10 mM NH4OH | of 10 mM NH4OH | |
| 0 | 18,600 | 20,400 | 0.0% | 100.0% | - | - |
| 50 | 15,100 | 19,300 | 18.8% | 94.6% | 13.4% | 71.3% |
| 100 | 13,800 | 18,800 | 25.8% | 92.2% | 18.0% | 69.6% |
| 200 | 12,400 | 17,800 | 33.3% | 86.9% | 20.1% | 60.6% |
| 300 | 12,100 | 17,400 | 34.9% | 85.3% | 20.2% | 57.9% |
| 400 | 11,800 | 16,800 | 36.6% | 82.4% | 18.9% | 51.7% |
| 500 | 10,700 | 15,500 | 42.5% | 76.0% | 18.5% | 43.4% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majedi, S.M.; Lai, E.P.C. Mass Spectrometric Analysis of Bisphenol A Desorption from Titania Nanoparticles: Ammonium Acetate, Fluoride, Formate, and Hydroxide as Chemical Desorption Agents. Methods Protoc. 2018, 1, 26. https://doi.org/10.3390/mps1030026
Majedi SM, Lai EPC. Mass Spectrometric Analysis of Bisphenol A Desorption from Titania Nanoparticles: Ammonium Acetate, Fluoride, Formate, and Hydroxide as Chemical Desorption Agents. Methods and Protocols. 2018; 1(3):26. https://doi.org/10.3390/mps1030026
Chicago/Turabian StyleMajedi, Seyed Mohammad, and Edward P. C. Lai. 2018. "Mass Spectrometric Analysis of Bisphenol A Desorption from Titania Nanoparticles: Ammonium Acetate, Fluoride, Formate, and Hydroxide as Chemical Desorption Agents" Methods and Protocols 1, no. 3: 26. https://doi.org/10.3390/mps1030026





