Intra-MRI Extraction of Diagnostic Electrocardiograms Using Carotidal Magnetohydrodynamic Voltages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Acquisition
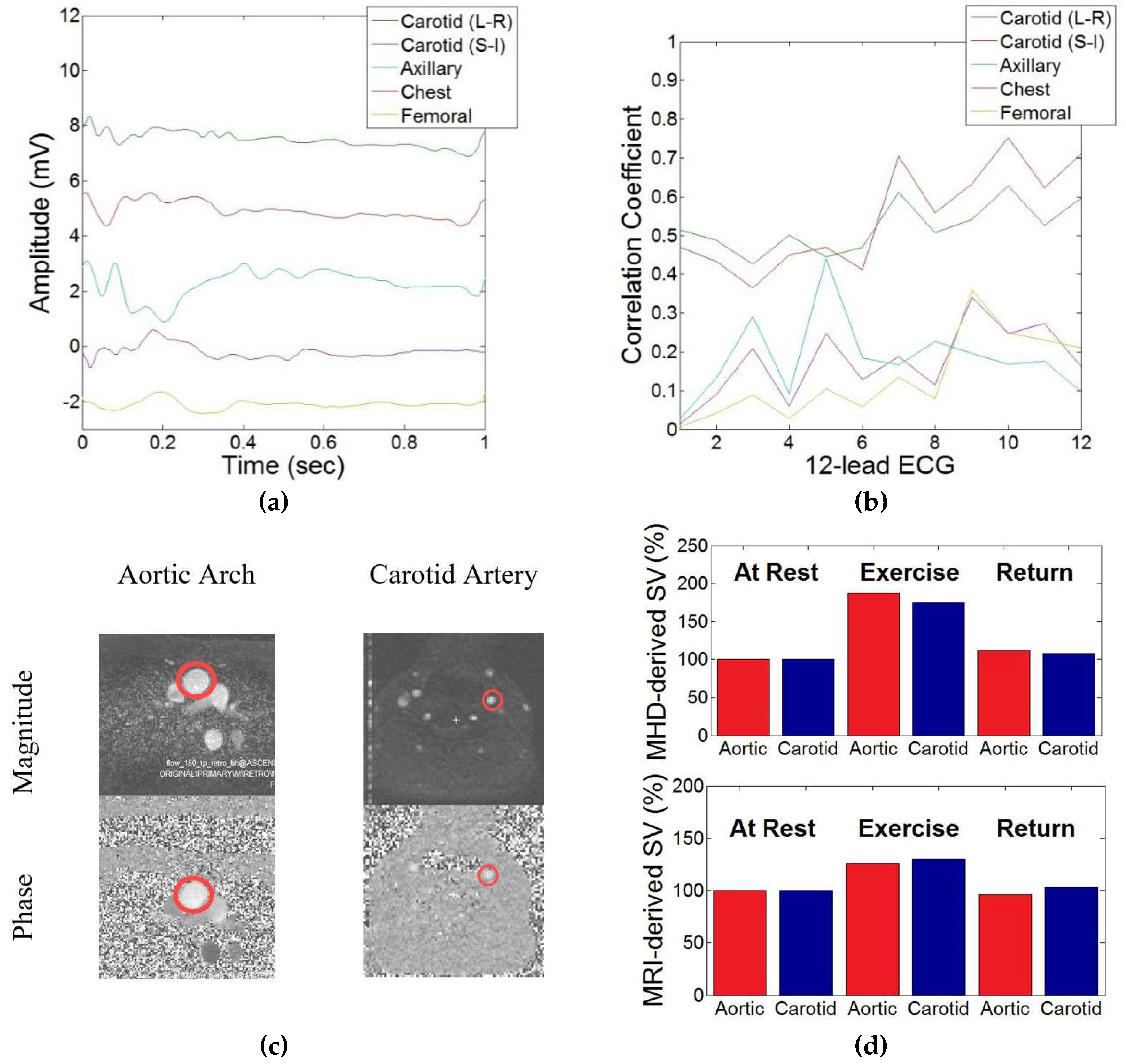
2.2.1. Correlation between Aortic and Carotidal MHD
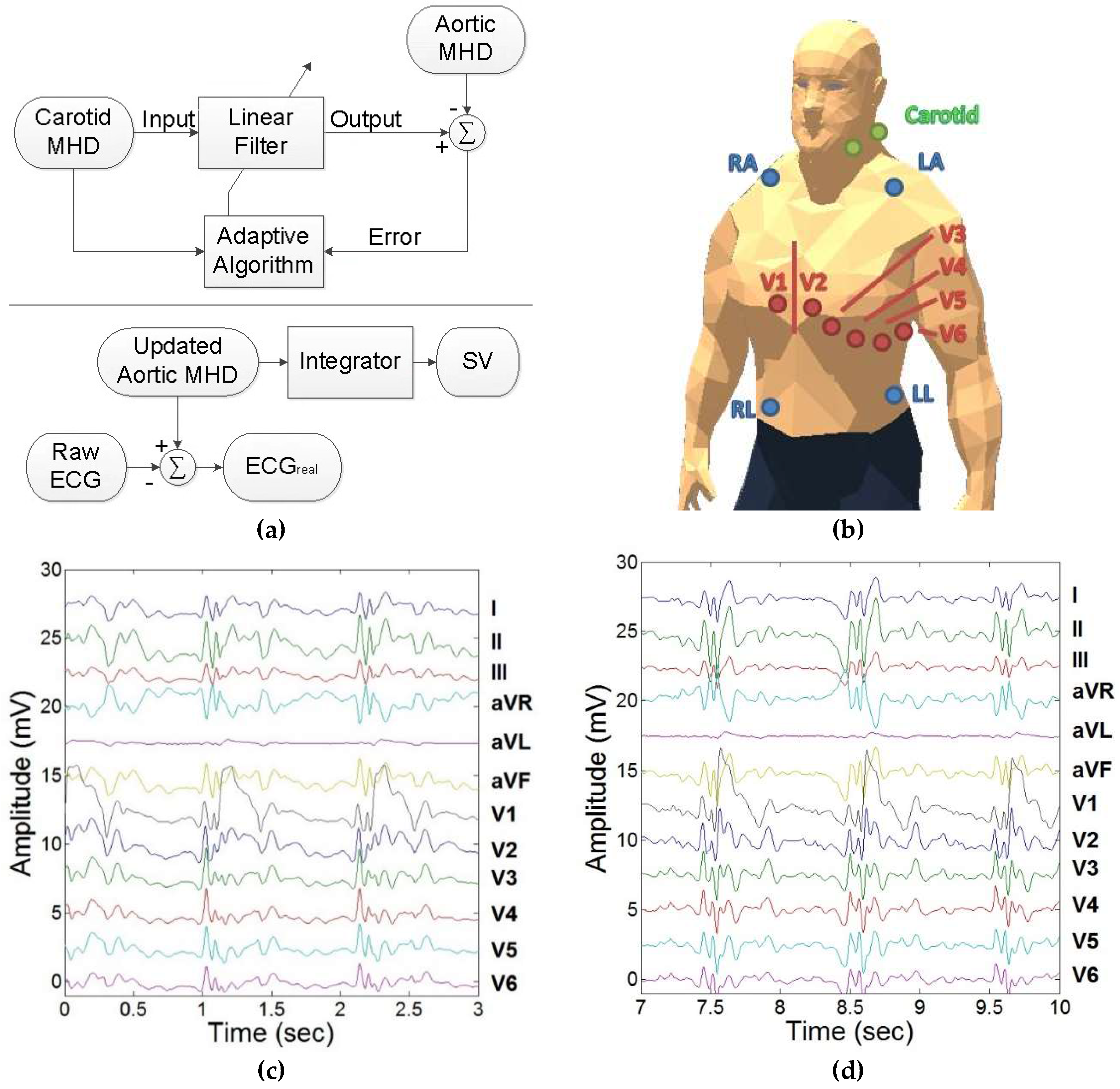
2.2.2. Adaptive Filter Training
2.2.3. Evaluation during Exercise Stress Testing
3. Results
3.1. Correlation between Aortic and Carotidal MHD
3.2. Adaptive Filter Training
3.3. Evaluation during Exercise Stress Testing
4. Discussion
4.1. Discussion
4.2. Limitations
4.3. Future Work
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Birkholz, T.; Schmid, M.; Nimsky, C.; Schuttler, J.; Schmitz, B. ECG artifacts during intraoperative high-field MRI scanning. J. Neurosurg. Anesthesiol. 2004, 16, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.E.; Wickline, S.A.; Lorenz, C.H. Novel real-time R-wave detection algorithm based on the vectorcardiogram for accurate gated magnetic resonance acquisitions. Magn. Reson. Med. 1999, 42, 361–370. [Google Scholar] [CrossRef]
- Gregory, T.S.; Schmidt, E.J.; Zhang, S.H.; Tse, Z.T.H. 3DQRS: A method to obtain reliable QRS complex detection within high field MRI using 12-lead electrocardiogram traces. Magn. Reson. Med. 2014, 71, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.C.; White, R.D.; Laub, G.; McVeigh, E.R.; Li, D.; Simonetti, O.P. Self-gated cardiac cine MRI. Magn. Reson. Med. 2004, 51, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Tse, Z.; Dumoulin, C.L.; Clifford, G.D.; Schweitzer, J.; Qin, L.; Oster, J.; Jerosch-Herold, M.; Kwong, R.Y.; Michaud, G.; Stevenson, W.G.; et al. A 1.5T MRI-conditional 12-lead electrocardiogram for MRI and intra-MR intervention. Magn. Reson. Med. 2013, 71, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Tse, Z.T.H.; Dumoulin, C.L.; Kwong, R.Y.; Stevenson, W.G.; Watkins, R.; Ward, J.; Wang, W.; Schmidt, E.J. Gradient-induced voltages on 12-lead ECGs during high duty-cycle MRI sequences and a method for their removal considering linear and concomitant gradient terms. Magn. Reson. Med. 2015, 75, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Blandford, R.; Thorne, K. Magnetohydrodynamics. In Applications of Classical Physics; CalTech: Pasadena, CA, USA, 2004. [Google Scholar]
- Gregory, T.S.; Schmidt, E.J.; Zhang, S.H.; Kwong, R.Y.; Stevenson, W.G.; Murrow, J.R.; Tse, Z.T.H. Left-Ventricular Mechanical Activation and Aortic-Arch Orientation Recovered from Magneto-Hydrodynamic Voltages Observed in 12-Lead ECGs Obtained Inside MRIs: A Feasibility Study. Ann. Biomed. Eng. 2014, 42, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Weeks, A.R.; Richie, S.M. Simulation of elevated T-waves of an ECG inside a static magnetic field (MRI). IEEE Trans. Biomed. Eng. 2008, 55, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Tse, Z.T.H.; Dumoulin, C.L.; Clifford, G.; Jerosch-Herold, M.; Kacher, D.; Kwong, R.; Stevenson, W.G.; Schmidt, E.J. Real-ECG extraction and stroke volume from MR-Compatible 12-lead ECGs; testing during stress, in PVC and in AF patients. J. Cardiovasc. Magn. Reson. 2011, 13, P6. [Google Scholar] [CrossRef]
- Gregory, T.S.; Oshinski, J.; Schmidt, E.J.; Kwong, R.Y.; Stevenson, W.G.; Tse, Z.T.H. Continuous Rapid Quantification of Stroke Volume Using Magnetohydrodynamic Voltages in 3T Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2015, 8, e003282. [Google Scholar] [PubMed]
- Grishman, A.; Scherlis, L.; Lasser, R.P. Spatial vectorcardiography. Am. J. Med. 1953, 14, 184–200. [Google Scholar] [CrossRef]
- Krug, J.; Rose, G.; Clifford, G.; Oster, J. Improved ECG based gating in ultra high field cardiac MRI using an independent component analysis approach. J. Cardiovasc. Magn. Reson. 2013, 15, W33. [Google Scholar] [CrossRef]
- Krug, J.; Rose, G.; Stucht, D.; Clifford, G.; Oster, J. Filtering the Magnetohydrodynamic Effect from 12-lead ECG Signals using Independent Component Analysis. Comput. Cardiol. 2012, 589–592. [Google Scholar]
- Oster, J.; Pietquin, O.; Abacherli, R.; Kraemer, M.; Felblinger, J. Independent component analysis-based artefact reduction: Application to the electrocardiogram for improved magnetic resonance imaging triggering. Physiol. Meas. 2009, 30, 1381–1397. [Google Scholar] [CrossRef] [PubMed]
- Mairal, J.; Bach, F.; Ponce, J.; Sapiro, G. Online learning for matrix factorization and sparse coding. J. Mach. Learn. Res. 2010, 11, 19–60. [Google Scholar]
- Yaghoobi, M.; Blumensath, T.; Davies, M.E. Dictionary learning for sparse approximations with the majorization method. IEEE Trans. Signal Process. 2009, 57, 2178–2191. [Google Scholar] [CrossRef]
- Oster, J.; Llinares, R.; Payne, S.; Tse, Z.T.H.; Schmidt, E.J.; Clifford, G.D. Comparison of three artificial models of the magnetohydrodynamic effect on the electrocardiogram. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1400–1417. [Google Scholar] [CrossRef] [PubMed]
- Gregory, T.S.; Schmidt, E.J.; Zhang, S.H.; Kwong, R.Y.; Stevenson, W.G.; Oshinski, J.; Tsz Ho Tse, Z. Rapid Quantification of Stroke Volume using Magnetohydrodynamic Voltages in 3T MRI: A Feasibility Study. J. Cardiovasc. Magn. Reson. 2015, 17, P32. [Google Scholar] [CrossRef]





| Lead Number | Lead Name | Carotid (L-R) | Carotid (S-I) | Axillary | Chest | Femoral |
|---|---|---|---|---|---|---|
| 1 | I | 0.51 | 0.47 | 0.03 | 0.01 | 0.01 |
| 2 | II | 0.49 | 0.43 | 0.13 | 0.09 | 0.04 |
| 3 | III | 0.43 | 0.37 | 0.29 | 0.21 | 0.09 |
| 4 | aVR | 0.50 | 0.45 | 0.09 | 0.06 | 0.03 |
| 5 | aVL | 0.45 | 0.47 | 0.44 | 0.25 | 0.10 |
| 6 | aVF | 0.47 | 0.41 | 0.18 | 0.13 | 0.06 |
| 7 | V1 | 0.61 | 0.70 | 0.17 | 0.19 | 0.14 |
| 8 | V2 | 0.51 | 0.56 | 0.23 | 0.11 | 0.08 |
| 9 | V3 | 0.54 | 0.63 | 0.20 | 0.34 | 0.36 |
| 10 | V4 | 0.63 | 0.75 | 0.17 | 0.25 | 0.25 |
| 11 | V5 | 0.53 | 0.62 | 0.18 | 0.27 | 0.23 |
| 12 | V6 | 0.60 | 0.71 | 0.10 | 0.16 | 0.21 |
| Mean | 0.52 | 0.55 | 0.18 | 0.17 | 0.13 | |
| Standard Deviation | 0.06 | 0.13 | 0.11 | 0.10 | 0.11 |
| Lead | I | II | III | aVR | aVL | aVF | V1 | V2 | V3 | V4 | V5 | V6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation Coefficient | 0.79 | 0.77 | 0.72 | 0.78 | 0.78 | 0.76 | 0.87 | 0.91 | 0.89 | 0.86 | 0.85 | 0.76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregory, T.S.; Wu, K.J.; Oshinski, J.N.; Tse, Z.T.H. Intra-MRI Extraction of Diagnostic Electrocardiograms Using Carotidal Magnetohydrodynamic Voltages. J. Imaging 2018, 4, 66. https://doi.org/10.3390/jimaging4050066
Gregory TS, Wu KJ, Oshinski JN, Tse ZTH. Intra-MRI Extraction of Diagnostic Electrocardiograms Using Carotidal Magnetohydrodynamic Voltages. Journal of Imaging. 2018; 4(5):66. https://doi.org/10.3390/jimaging4050066
Chicago/Turabian StyleGregory, T. Stan, Kevin James Wu, John N. Oshinski, and Zion Tsz Ho Tse. 2018. "Intra-MRI Extraction of Diagnostic Electrocardiograms Using Carotidal Magnetohydrodynamic Voltages" Journal of Imaging 4, no. 5: 66. https://doi.org/10.3390/jimaging4050066






