Reference Tracts and Generative Models for Brain White Matter Tractography †
Abstract
:1. Introduction
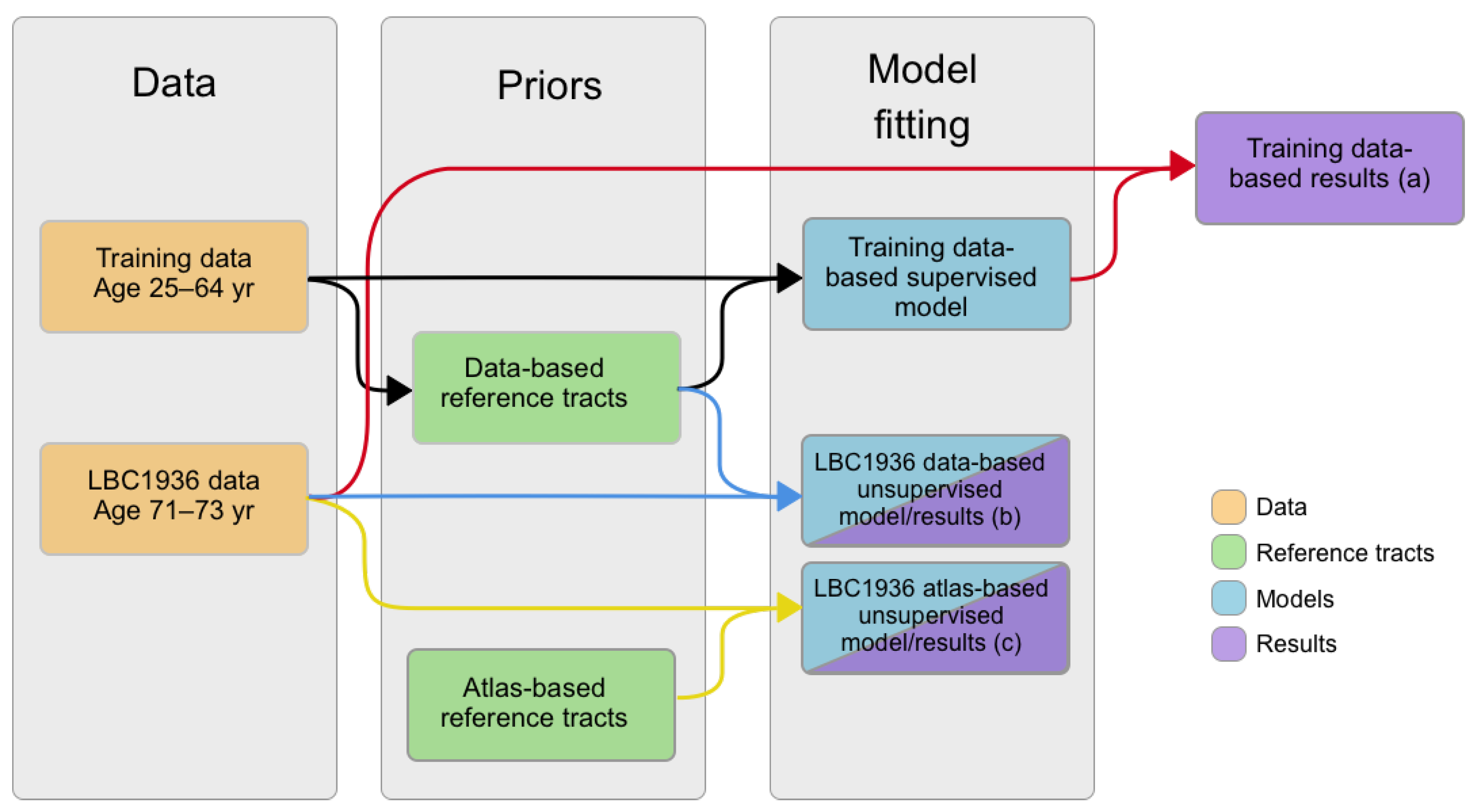
2. Materials and Methods
2.1. Participants
2.1.1. Training Data
2.1.2. Testing Data
2.2. MRI
2.3. Image Analysis
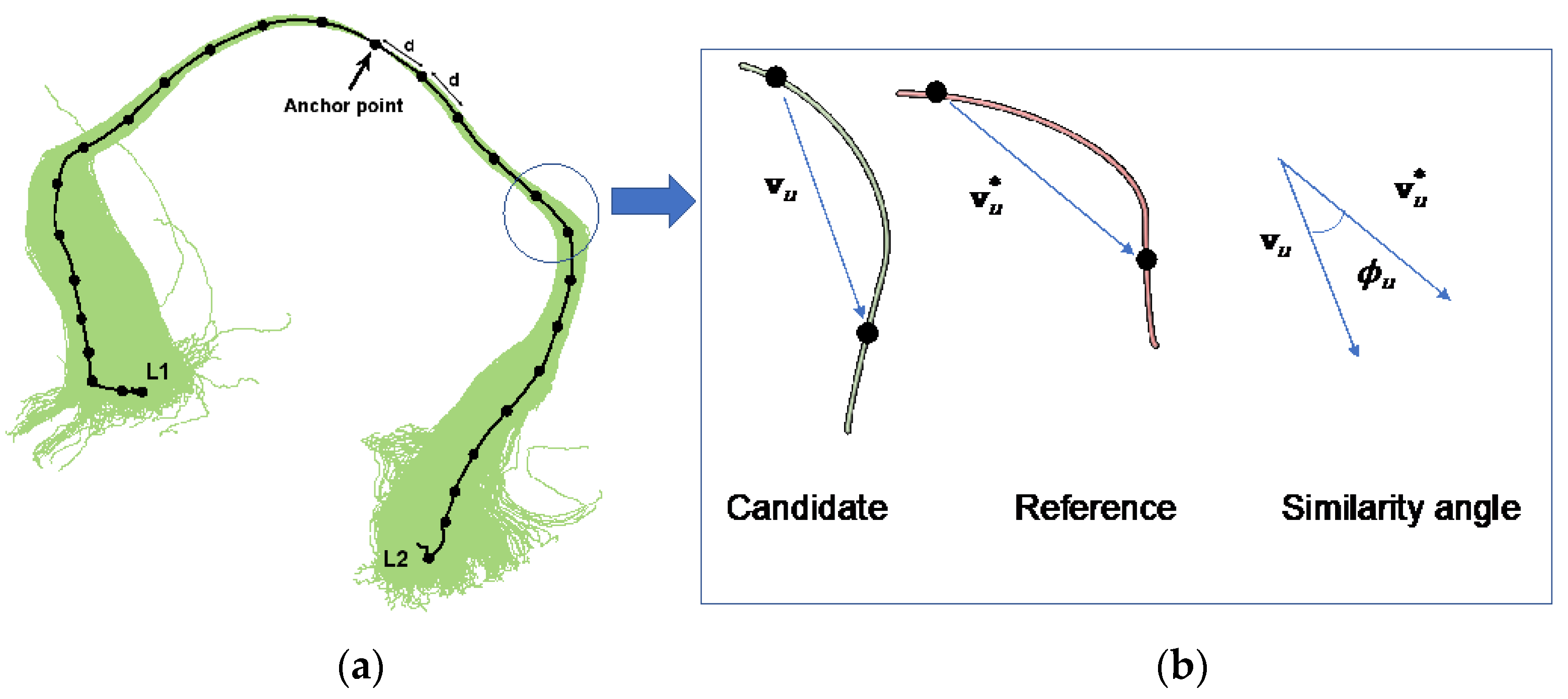
2.4. Reference Tracts
2.4.1. Atlas-Based Reference Tracts
2.4.2. Data-Based Reference Tracts
2.5. Creation of Matching Models
2.6. Testing of Reference Tracts and Matching Models
2.7. Sampling from PNT Models
- Identify the image voxel corresponding to the reference anchor point, and choose a specific starting location from a uniform distribution over that voxel. Note this as the first pseudo-knot point.
- Sample and from their respective distributions, thereby obtaining the length of the sample streamline either side of the anchor point.
- Beginning at the point obtained in step 1, sample sequentially for u {−1, ..., }. In each case, take a step of length d in the direction of from the current pseudo-knot point to arrive at the next pseudo-knot point.
- Return to the point obtained in step 1, and sample sequentially for u {1, ..., }, analogously to step 3.
- Use B-spline interpolation to recover a curve between the sequence of pseudo-knot points.
- Sample from the model.
- Establish a point, w, on the plane passing through the origin perpendicular to . The equation of this plane is , so any vector perpendicular to will do. We take , where = (0, 0, 1) unless this is collinear with , in which case we use = (1, 0, 0).
- Sample θ ~ (0, 2π), the angle around the locus circle.
- Rotate w by the angle θ around the unit vector , using Rodrigues’ rotation Formula (1):
- Scale w′ to the radius of the locus circle and translate it along the reference vector, to arrive at the final step vector, , as (2):
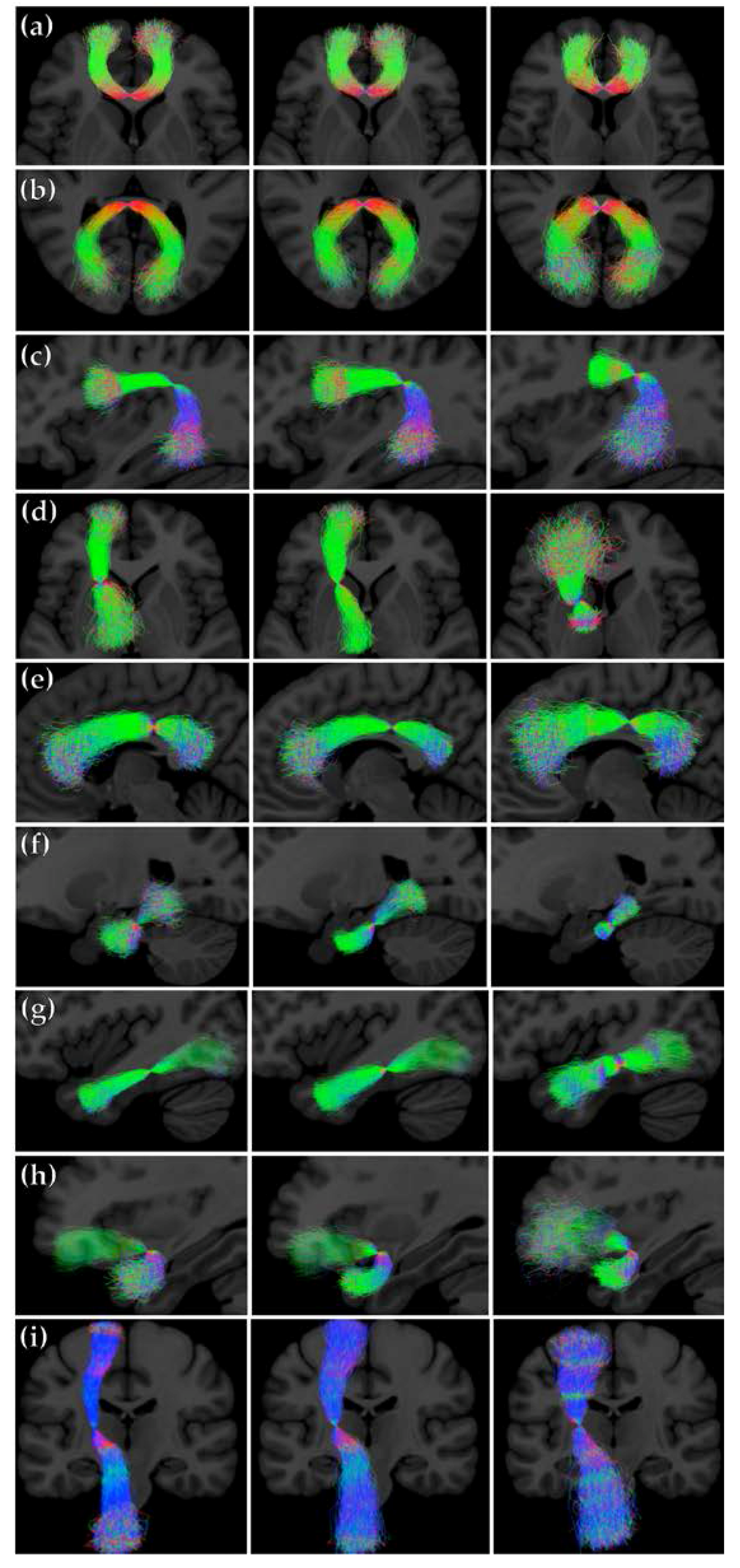
2.8. Creating Synthetic Tracts from PNT Models
3. Results
3.1. Testing of Reference Tracts and Matching Models
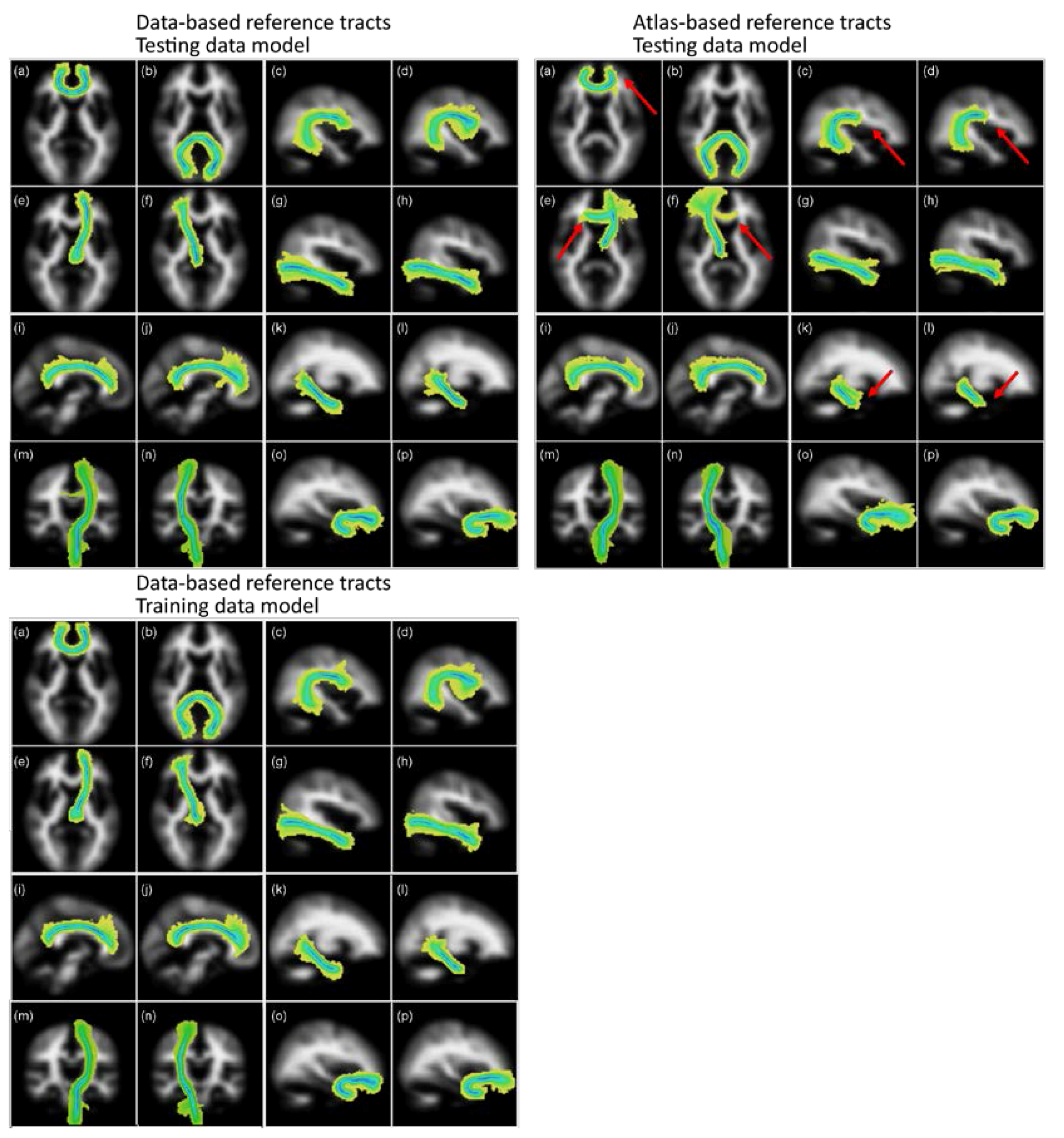
3.1.1. Visual Assessments
3.1.2. FA and MD Variability
3.1.3. Overlap Analysis
3.2. Assessment of Synthetic Tracts Sampled from PNT Models
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muñoz Maniega, S.; Bastin, M.E.; Deary, I.J.; Wardlaw, J.M.; Clayden, J.D. Improved reference tracts for unsupervised brain white matter tractography. In Medical Image Understanding and Analysis: 21st Annual Conference, MIUA 2017, Edinburgh, UK, July 11--13, 2017, Proceedings; Valdés Hernández, M., González-Castro, V., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 425–435. ISBN 978-3-319-60964-5. [Google Scholar]
- Tournier, J.D.; Mori, S.; Leemans, A. Diffusion tensor imaging and beyond. Magn. Reson. Med. 2011, 65, 1532–1556. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, O.; Catani, M.; Johansen-Berg, H.; Clark, C.; Thompson, A. Diffusion-based tractography in neurological disorders: Concepts, applications, and future developments. Lancet Neurol. 2008, 7, 715–727. [Google Scholar] [CrossRef]
- Johansen-Berg, H.; Behrens, T.E.J. Just pretty pictures? What diffusion tractography can add in clinical neuroscience. Curr. Opin. Neurol. 2006, 19, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Clayden, J.D.; Storkey, A.J.; Muñoz Maniega, S.; Bastin, M.E. Reproducibility of tract segmentation between sessions using an unsupervised modelling-based approach. Neuroimage 2009, 45, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Cllayden, J.D.D.; Storkey, A.J.J.; Bastin, M.E.E. A probabilistic model-based approach to consistent white matter tract segmentation. IEEE Trans. Med. Imaging 2007, 26, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Yendiki, A.; Panneck, P.; Srinivasan, P.; Stevens, A.; Zöllei, L.; Augustinack, J.; Wang, R.; Salat, D.; Ehrlich, S.; Behrens, T.; Jbabdi, S.; et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinform. 2011, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.D.; Marshall, D.; Rosin, P.L.; Drage, N.; Richmond, S.; Jones, D.K. Fast and fully automated clustering of whole brain tractography results using shape-space analysis. In Proceedings of the ISMRM 21st Scientific Meeting & Exhibition, Salt Lake City, UT, USA, 20–26 April 2013; p. 778. [Google Scholar]
- O’Donnell, L.J.; Westin, C.-F.C. Automatic tractography segmentation using a high-dimensional white matter atlas. IEEE Trans. Med. Imaging 2007, 26, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.M.; Embleton, K.V.; Parker, G.J.M. Probabilistic fibre tracking: Differentiation of connections from chance events. Neuroimage 2008, 42, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.K. Determining and visualizing uncertainty in estimates of fiber orientation from diffusion tensor MRI. Magn. Reson. Med. 2003, 49, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hein, K.H.; Neher, P.F.; Houde, J.-C.; Côté, M.-A.; Garyfallidis, E.; Zhong, J.; Chamberland, M.; Yeh, F.-C.; Lin, Y.-C.; Ji, Q.; Reddick, W.E.; et al. The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun. 2017, 8, 1349. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.; Thiebaut De Schotten, M.A. Diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008, 44, 1105–1132. [Google Scholar] [CrossRef] [PubMed]
- Dickie, D.A.; Shenkin, S.D.; Anblagan, D.; Lee, J.; Blesa Cabez, M.; Rodriguez, D.; Boardman, J.P.; Waldman, A.; Job, D.E.; Wardlaw, J.M. Whole brain magnetic resonance image atlases: A systematic review of existing atlases and caveats for use in population imaging. Front. Neuroinform. 2017, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Anblagan, D.; Bastin, M.E.; Sparrow, S.; Piyasena, C.; Pataky, R.; Moore, E.J.; Serag, A.; Wilkinson, A.G.; Clayden, J.D.; Semple, S.I.; et al. Tract shape modeling detects changes associated with preterm birth and neuroprotective treatment effects. NeuroImage Clin. 2015, 8, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Maniega, S.; Bastin, M.; McIntosh, A.; Lawrie, S.; Clayden, J. Atlas-based reference tracts improve automatic white matter segmentation with neighbourhood tractography. In Proceedings of the ISMRM 16th Scientific Meeting & Exhibition, Toronto, ON, Canada, 3–9 May 2008; p. 3318. [Google Scholar]
- Hua, K.; Zhang, J.; Wakana, S.; Jiang, H.; Li, X.; Reich, D.S.; Calabresi, P.A.; Pekar, J.J.; van Zijl, P.C.M.; Mori, S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008, 39, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Penke, L.; Muñoz Maniega, S.; Houlihan, L.M.; Murray, C.; Gow, A.J.; Clayden, J.D.; Bastin, M.E.; Wardlaw, J.M.; Deary, I.J. White matter integrity in the splenium of the corpus callosum is related to successful cognitive aging and partly mediates the protective effect of an ancestral polymorphism in ADRB2. Behav. Genet. 2010, 40, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Bastin, M.E.; Muñoz Maniega, S.; Ferguson, K.J.; Brown, L.J.; Wardlaw, J.M.; MacLullich, A.M.J.; Clayden, J.D. Quantifying the effects of normal ageing on white matter structure using unsupervised tract shape modelling. Neuroimage 2010, 51, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickie, D.A.; Mikhael, S.; Job, D.E.; Wardlaw, J.M.; Laidlaw, D.H.; Bastin, M.E. Permutation and parametric tests for effect sizes in voxel-based morphometry of gray matter volume in brain structural MRI. Magn. Reson. Imaging 2015, 33, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Deary, I.J.; Gow, A.J.; Taylor, M.D.; Corley, J.; Brett, C.; Wilson, V.; Campbell, H.; Whalley, L.J.; Visscher, P.M.; Porteous, D.J.; et al. The Lothian Birth Cohort 1936: A study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr. 2007, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.K.; Williams, S.C.R.; Gasston, D.; Horsfield, M.A.; Simmons, A.; Howard, R. Isotropic resolution diffusion tensor imaging with whole brain acquisition in a clinically acceptable time. Hum. Brain Mapp. 2002, 15, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Smith, S.A. Global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Basser, P.J.; Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. Ser. B 1996, 111, 209–219. [Google Scholar] [CrossRef]
- Behrens, T.E.J.; Johansen-Berg, H.; Jbabdi, S.; Rushworth, M.F.S.; Woolrich, M.W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 2007, 34, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Clayden, J.D.; Muñoz Maniega, S.; Storkey, A.J.; King, M.D.; Bastin, M.E.; Clark, C.A. TractoR: Magnetic resonance imaging and tractography with R. J. Stat. Softw. 2011, 44, 1–18. [Google Scholar] [CrossRef]
- Clayden, J.D.; King, M.D.; Clark, C.A. Shape modelling for tract selection. In Lecture Notes in Computer Science, Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, London, UK, 20–24 September 2009; Yang, G.Z., Hawkes, D., Rueckert, D., Noble, A., Taylor, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5762, pp. 150–157. [Google Scholar]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Hill, C.S.; Clayden, J.D.; Kitchen, N.; Bull, J.; Harkness, W.; Clark, C.A. A feasibility study of neighbourhood tractography in the presence of paediatric brain tumours. In Proceedings of the Autumn Meeting of the Society of British Neurological Surgeons, Dublin, Ireland, 28–30 October 2009; p. 1191. [Google Scholar]
- Mori, S.; Crain, B.J.; Chacko, V.P.; van Zijl, P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999, 45, 265–269. [Google Scholar] [CrossRef]
- Seunarine, K.K.; Clayden, J.D.; Clark, C.A. Whole-brain neighbourhood tractography. In Proceedings of the ISMRM 21th Annual Meeting & Exhibition, Salt Lake City, UT, USA, 20–26 April 2013; p. 2132. [Google Scholar]
- Armitage, P.A.; Muñoz Maniega, S.; Bridson, J.; Poon, M.; Bastin, M.E. Improved white matter tract segmentation reproducibility using global diffusion tensor neighborhood tractography. In Proceedings of the ISMRM 20th Annual Meeting & Exhibition, Melbourne, Australia, 5–11 May 2012; p. 1905. [Google Scholar]


| Reference Tracts | Data-Based | Atlas-Based | |
|---|---|---|---|
| Model Trained on | Training Data | LBC1936 Data | LBC1936 Data |
| Genu | 100.0% | 100.0% | 96.0% |
| Splenium | 98.0% | 96.0% | 98.0% |
| L Arc | 100.0% | 100.0% | 98.0% |
| R Arc | 96.0% | 96.0% | 94.0% |
| L ATR | 100.0% | 100.0% | 32.0% |
| R ATR | 96.0% | 100.0% | 76.0% |
| L ILF | 100.0% | 100.0% | 100.0% |
| R ILF | 100.0% | 100.0% | 100.0% |
| L Cing | 98.0% | 98.0% | 100.0% |
| R Cing | 98.0% | 92.0% | 98.0% |
| L Cing, ventral | 98.0% | 100.0% | 98.0% |
| R Cing, ventral | 94.0% | 98.0% | 100.0% |
| L CST | 100.0% | 98.0% | 100.0% |
| R CST | 100.0% | 100.0% | 100.0% |
| L Unc | 96.0% | 92.0% | 88.0% |
| R Unc | 100.0% | 100.0% | 100.0% |
| Mean | 98.3% | 98.1% | 92.4% |
| FA | MD (10−6 mm2/s) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Atlas-Based | Data-Based | Atlas-Based | Data-Based | ||||||||||||||
| Model Training | LBC1936 Data | LBC1936 Data | Training Data | LBC1936 Data | LBC1936 Data | Training Data | ||||||||||||
| Mean (sd) | CV | Mean (sd) | CV | Mean (sd) | CV | Mean (sd) | CV | Mean (sd) | CV | Mean (sd) | CV | |||||||
| Genu | 0.41 | (0.05) | 0.11 | 0.39 | (0.05) | 0.12 | 0.39 | (0.05) | 0.12 | 776.91 | (65.59) | 0.08 | 799.20 | (75.46) | 0.09 | 799.85 | (74.59) | 0.09 |
| Splenium | 0.45 * | (0.09) | 0.20 | 0.52 * | (0.06) | 0.12 | 0.51 * | (0.08) | 0.15 | 1117.26 * | (220.22) | 0.20 | 807.61 * | (108.59) | 0.13 | 837.77 * | (162.71) | 0.19 |
| L Arc | 0.46 | (0.05) | 0.10 | 0.45 | (0.04) | 0.09 | 0.45 | (0.04) | 0.10 | 663.30 | (49.21) | 0.07 | 661.30 | (49.26) | 0.07 | 659.82 | (49.73) | 0.08 |
| R Arc | 0.43 | (0.05) | 0.12 | 0.42 | (0.04) | 0.10 | 0.43 | (0.04) | 0.09 | 646.56 | (55.00) | 0.09 | 645.36 | (48.93) | 0.08 | 644.13 | (45.30) | 0.07 |
| L ATR | 0.34 | (0.05) | 0.14 | 0.34 | (0.03) | 0.10 | 0.34 | (0.03) | 0.10 | 757.89 | (81.23) | 0.11 | 755.39 | (60.94) | 0.08 | 746.41 | (60.30) | 0.08 |
| R ATR | 0.35 * | (0.04) | 0.10 | 0.36 * | (0.03) | 0.08 | 0.33 * | (0.04) | 0.12 | 747.07 * | (54.08) | 0.07 | 704.05 * | (50.40) | 0.07 | 766.81 * | (74.85) | 0.10 |
| L ILF | 0.42 | (0.05) | 0.12 | 0.41 | (0.05) | 0.12 | 0.40 | (0.05) | 0.12 | 740.50 | (75.45) | 0.10 | 752.41 | (67.06) | 0.09 | 745.86 | (61.13) | 0.08 |
| R ILF | 0.39 | (0.05) | 0.14 | 0.40 | (0.04) | 0.11 | 0.38 | (0.05) | 0.12 | 788.00 | (142.54) | 0.18 | 750.31 | (83.70) | 0.11 | 755.39 | (87.47) | 0.12 |
| L Cing | 0.45 | (0.05) | 0.12 | 0.46 | (0.06) | 0.12 | 0.46 | (0.06) | 0.12 | 647.29 | (51.00) | 0.08 | 638.39 | (45.15) | 0.07 | 640.95 | (47.46) | 0.07 |
| R Cing | 0.42 | (0.06) | 0.13 | 0.43 | (0.04) | 0.10 | 0.42 | (0.05) | 0.11 | 619.92 | (36.16) | 0.06 | 626.56 | (36.03) | 0.06 | 630.97 | (33.82) | 0.05 |
| L Cing, ventral | 0.32 | (0.06) | 0.19 | 0.29 | (0.04) | 0.12 | 0.29 | (0.04) | 0.12 | 752.54 | (155.54) | 0.21 | 728.86 | (62.50) | 0.09 | 733.07 | (69.52) | 0.09 |
| R Cing, ventral | 0.30 | (0.06) | 0.20 | 0.30 | (0.05) | 0.15 | 0.29 | (0.04) | 0.14 | 760.68 | (95.07) | 0.12 | 748.37 | (79.00) | 0.11 | 748.73 | (88.67) | 0.12 |
| L CST | 0.48 | (0.03) | 0.07 | 0.46 | (0.04) | 0.08 | 0.46 | (0.04) | 0.08 | 655.47 | (36.72) | 0.06 | 672.26 | (37.18) | 0.06 | 675.52 | (38.65) | 0.06 |
| R CST | 0.49 | (0.03) | 0.07 | 0.49 | (0.03) | 0.07 | 0.50 | (0.04) | 0.07 | 653.82 * | (32.72) | 0.05 | 676.03 * | (32.36) | 0.05 | 676.37 * | (31.99) | 0.05 |
| L Unc | 0.34 | (0.03) | 0.10 | 0.33 | (0.03) | 0.10 | 0.34 | (0.04) | 0.11 | 767.04 | (53.54) | 0.07 | 767.63 | (60.41) | 0.08 | 764.88 | (60.65) | 0.08 |
| R Unc | 0.33 | (0.03) | 0.10 | 0.33 | (0.03) | 0.10 | 0.33 | (0.04) | 0.11 | 756.22 | (41.27) | 0.05 | 758.75 | (41.27) | 0.05 | 754.75 | (41.77) | 0.06 |
| Mean | 0.40 | (0.06) | 0.13 | 0.40 | (0.07) | 0.10 | 0.40 | (0.07) | 0.11 | 740.65 | (115.51) | 0.10 | 718.28 | (58.64) | 0.08 | 723.83 | (61.36) | 0.09 |
| Reference Tracts | Data-Based | Atlas-Based | |
|---|---|---|---|
| Model Trained on | Training Data | LBC1936 Data | LBC1936 Data |
| Genu | 0.46 | 0.50 | 0.43 |
| Splenium | 0.63 | 0.62 | 0.48 |
| L Arc | 0.34 | 0.34 | 0.21 |
| R Arc | 0.36 | 0.34 | 0.22 |
| L ATR | 0.31 | 0.31 | 0.28 |
| R ATR | 0.37 | 0.34 | 0.35 |
| L ILF | 0.44 | 0.43 | 0.4 |
| R ILF | 0.50 | 0.49 | 0.48 |
| L Cing | 0.52 | 0.49 | 0.57 |
| R Cing | 0.52 | 0.51 | 0.58 |
| L Cing, ventral | 0.47 | 0.48 | 0.45 |
| R Cing, ventral | 0.49 | 0.49 | 0.47 |
| L CST | 0.52 | 0.52 | 0.52 |
| R CST | 0.65 | 0.63 | 0.57 |
| L Unc | 0.27 | 0.26 | 0.22 |
| R Unc | 0.29 | 0.28 | 0.29 |
| Range | 0.27–0.65 | 0.26–0.63 | 0.21–0.58 |
| Mean | 0.45 | 0.44 | 0.41 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz Maniega, S.; Bastin, M.E.; Deary, I.J.; Wardlaw, J.M.; Clayden, J.D. Reference Tracts and Generative Models for Brain White Matter Tractography. J. Imaging 2018, 4, 8. https://doi.org/10.3390/jimaging4010008
Muñoz Maniega S, Bastin ME, Deary IJ, Wardlaw JM, Clayden JD. Reference Tracts and Generative Models for Brain White Matter Tractography. Journal of Imaging. 2018; 4(1):8. https://doi.org/10.3390/jimaging4010008
Chicago/Turabian StyleMuñoz Maniega, Susana, Mark E. Bastin, Ian J. Deary, Joanna M. Wardlaw, and Jonathan D. Clayden. 2018. "Reference Tracts and Generative Models for Brain White Matter Tractography" Journal of Imaging 4, no. 1: 8. https://doi.org/10.3390/jimaging4010008





