1. Introduction
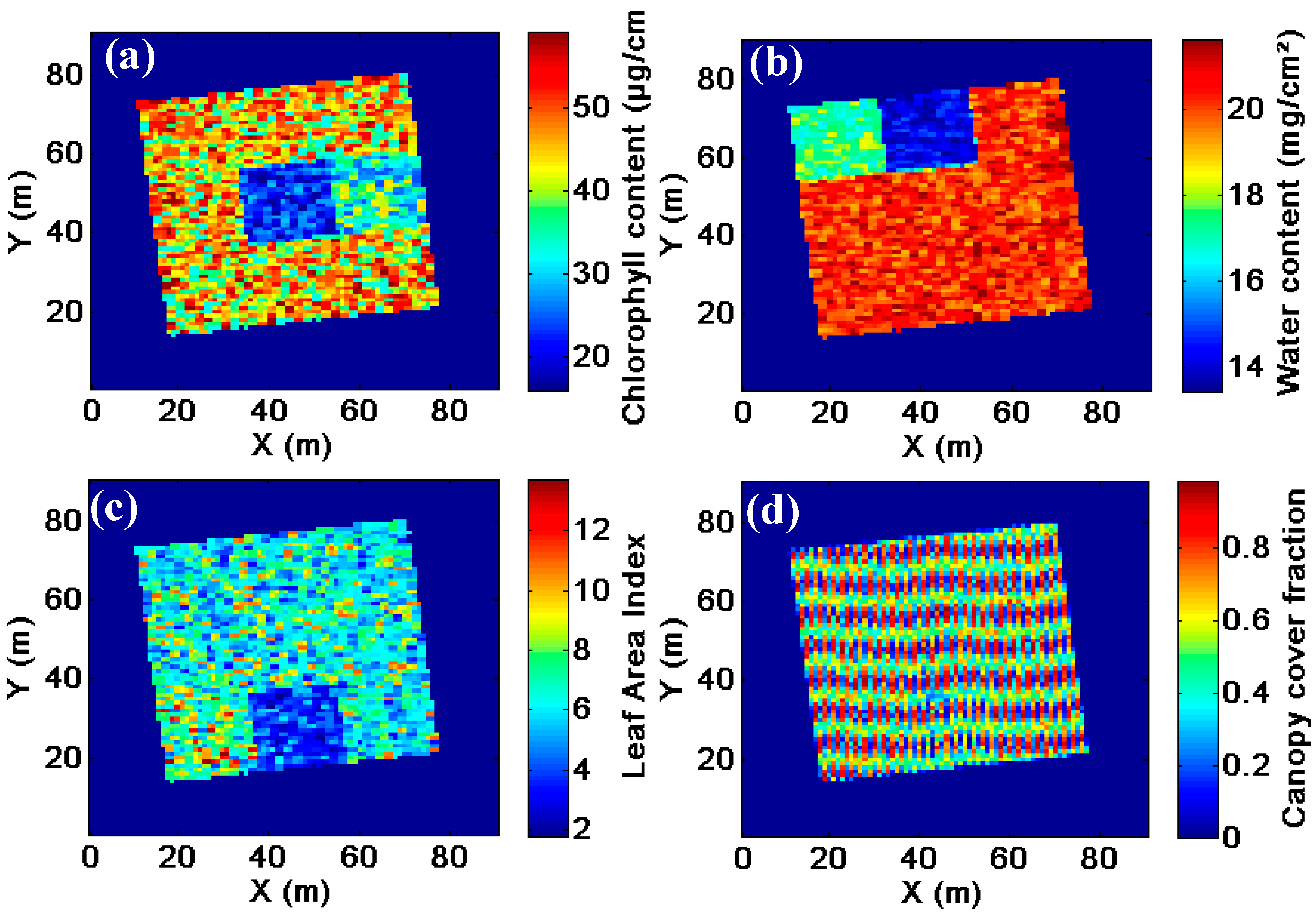
Monitoring and managing of capital intensive orchard crops through precision agriculture is centered around the estimation of stress-related biophysical variables such as chlorophyll content [
1,
2], water content [
2,
3,
4] and Leaf Area Index (LAI) [
5]. Time consuming, labor intensive and destructive
in situ measurements of biophysical variables could be circumvented through remotely sensed imagery and spectral vegetation indices [
6,
7]. Examples include the Photochemical Reflectance Index (PRI) [
8] for chlorophyll content [
1,
3], the Water Index (WI) [
9] for water content [
2] and the standardized LAI-Determining Index (sLAIDI) for LAI [
5]. On the one hand, practical use of remotely sensed spectral imagery in temperate climates requires near-to-daily revisit times because of the high cloud cover [
10]. On the other hand, heterogeneous orchards require high spatial resolution imagery to provide frequent and accurate information on field and plant conditions [
11]. Currently, this combination of both high spatial and temporal resolutions is feasible with high spatial resolution satellite sensors capable of off-nadir viewing, such as GeoEye-1, Quickbird, Pleaides, WorldView-1, WorldView-2 and WorldView-3.
The main downside of using these agile satellites is the influence of variable viewing angles on the relationship between biophysical variables and spectral measurements or vegetation indices [
4,
12,
13]. Although information from multiple view angles is considerably greater than from a single angle and could provide information regarding structural variables (
i.e., plant cover, canopy height and biomass) [
14,
15], varying viewing angles within and between time series could obstruct change detection due to the confusion between genuine changes and viewing geometry influences. In the past, several methods were constructed to remove the influence of variable viewing and illumination angles. The most common is the use of Bidirectional Reflectance Distribution Function (BRDF) models to standardize and produce nadir equivalent reflectance values [
16,
17,
18,
19,
20,
21]. However, this could remove useful information regarding vegetation structure [
19,
21,
22] or plant stress [
4] and requires a wide range of viewing angles over a short period of time. Therefore, existing research focused mostly on imagery of low spatial resolution images (
i.e., > 20 m spatial resolution e.g., Moderate Resolution Imaging Spectroradiometer (MODIS)) [
17,
19,
21,
23] and/or at fixed view angles (e.g., +/− 55°, +/− 36° and 0° for the pushbroom CHRIS (Compact High Resolution Imaging Spectrometer) sensor mounted on the PROBA (Project for On-Board Autonomy) platform) [
24].
In addition, the research on the effects of variable viewing conditions focused mostly on vegetative systems with a continuous canopy cover, such as forests [
18,
24,
25], grasslands [
24] and soybeans [
26]. However, high spatial resolution satellite imagery over orchard cropping systems will always contain mixtures of canopies and background (
i.e., soil, grass and shadow) [
27,
28]. This mixture effects can be further aggravated by the variable viewing conditions. Recent studies have shown the necessity and usefulness of the removal or reduction of background effects through either unmixing algorithms [
29] or vegetation index corrections [
28]. However, both methods were developed for nadir viewing imagery and assumed the presence of the full range of canopy fractions.
The goal of this study was to investigate the view-angle sensitivity of common spectral vegetation indices on the estimation of biophysical variables—i.e., chlorophyll content, water content and LAI—in orchards. In high spatial resolution imagery, changing view angles causes both BRDF- and mixture-related differences. As the latter influence can be minimized through a correction method, the view-angle sensitivity of common vegetation indices for high spatial resolution imagery of hedgerow cropping systems can be assessed and minimize the effects of varying viewing angles on change detection within and between time series. Synthetic imagery of a virtual orchard was used to include variable orchard conditions, as well as different background scenarios—i.e., non-vegetated, vegetated and partially vegetated. Finally, imagery from a satellite with off-nadir viewing capacities over an experimental orchard was investigated to highlight the importance of changing viewing geometry towards the estimation of biophysical variables.
5. Discussion
The use of remote sensing within capital intensive orchard crops provides alternatives to time consuming, labor intensive and destructive
in situ measurements. Practical use in hedgerow orchards under temperate climate conditions requires the use of high spatial resolution satellite sensors with off-nadir viewing capabilities [
10,
11]. However, this presence of variable viewing angles could affect the relationship between biophysical variables and spectral measurements [
4,
12,
13]. Moreover, variable viewing angles within and between time series could obstruct the distinction between genuine changes and viewing geometry influences. In this study, the distribution of R
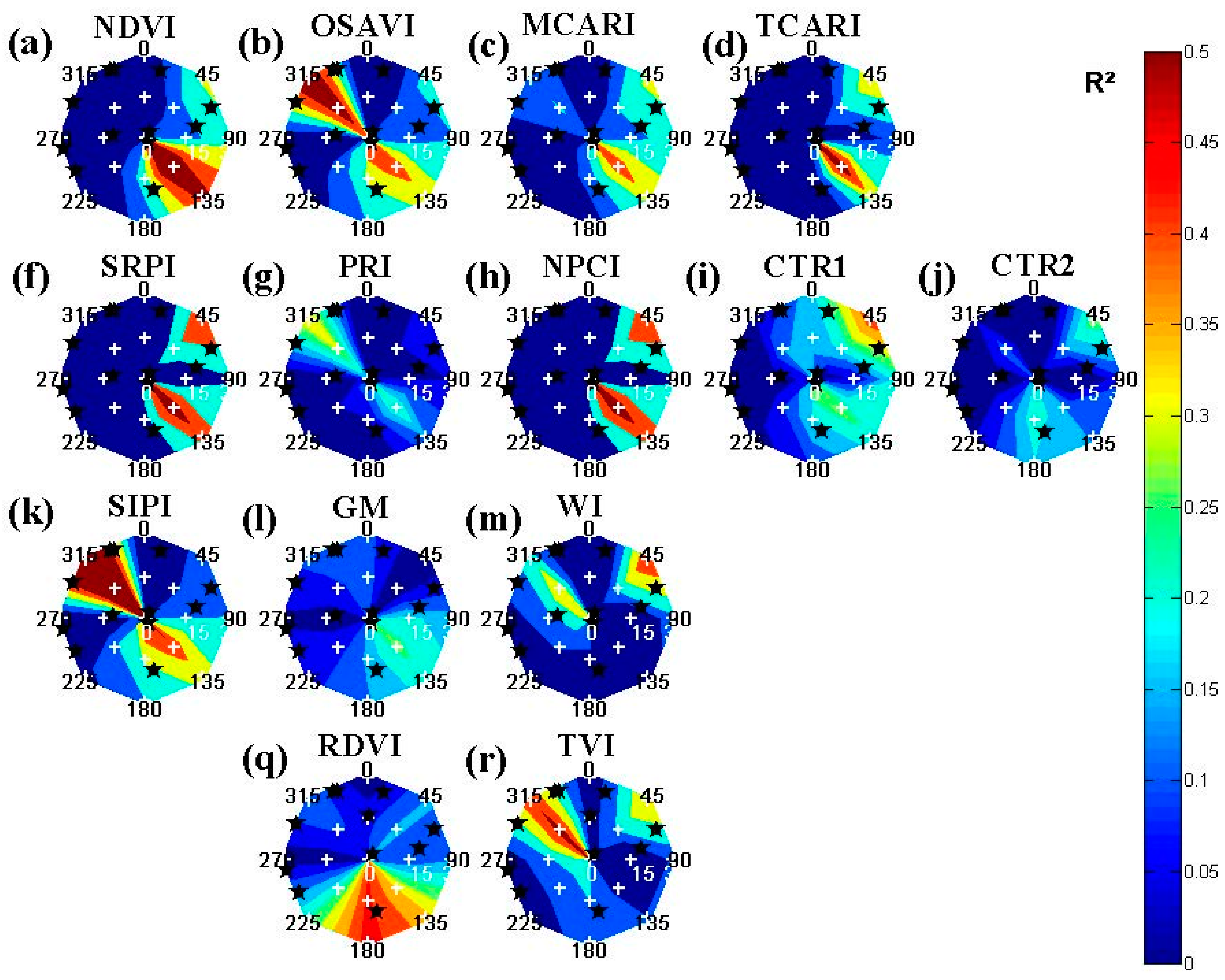
2 values for different viewing compositions (
Figure 4,
Figure 5,
Figure 6 and
Figure 7) and the ranges for different background scenarios (
Table 4) showed the necessity of careful selection of vegetation indices for applications using spectral imagery acquired under or subject to multiple viewing geometries.
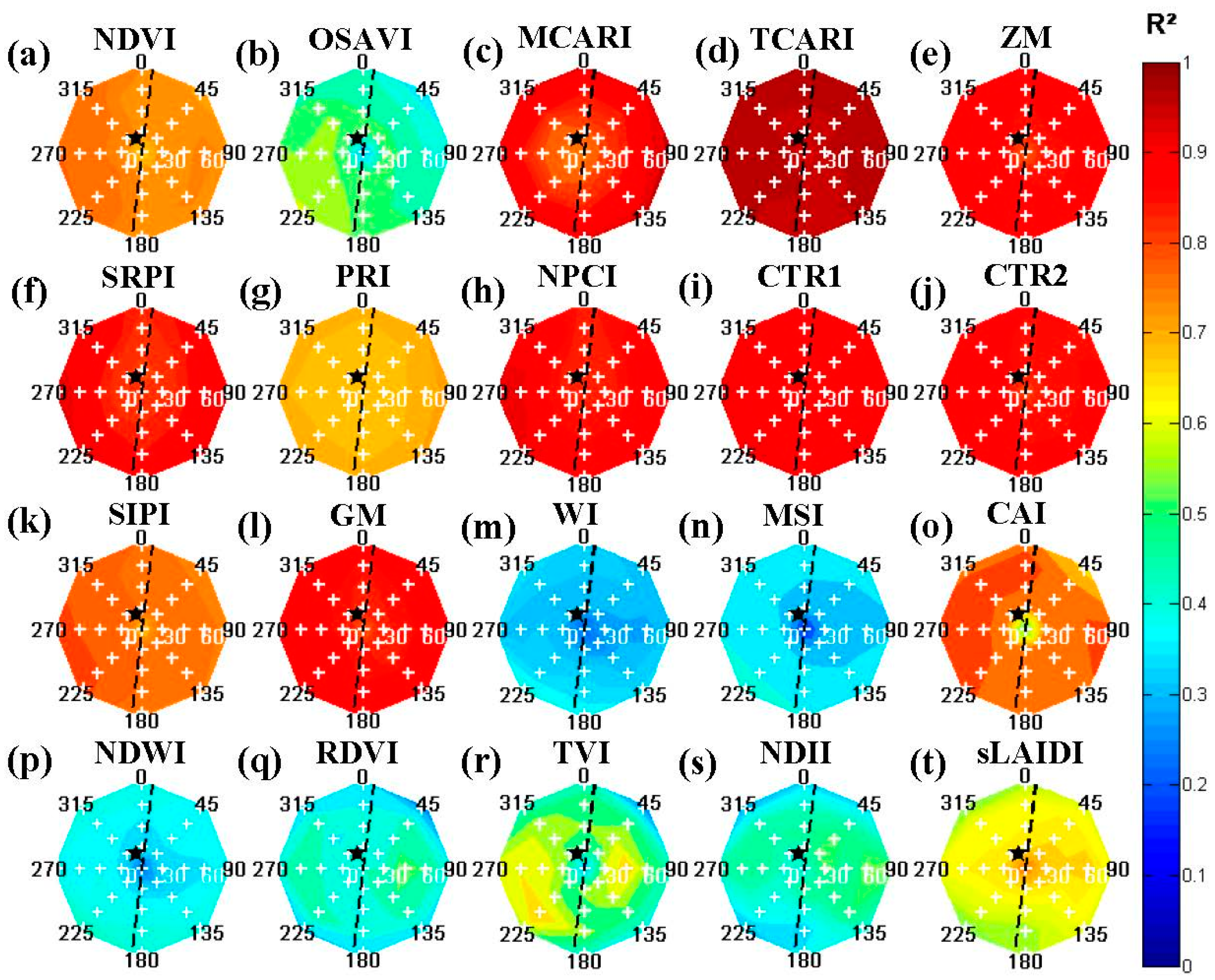
For high spatial resolution imagery, changing viewing geometries would cause both differences related to Bidirectional Reflectance Distribution Function (BRDF) influences [
21] and to variable canopy fraction mixtures. The former was visible for the tree endmembers, rendered without background and extracted from pure canopy pixels (
Figure 4). For these reference images, several indices showed a variable distribution favoring more nadir viewing—
i.e., sLAIDI (
Figure 4t)—or off-nadir viewing scenes—
i.e., OSAVI (
Figure 4b), WI (
Figure 4m), CAI (
Figure 4o) and TVI (
Figure 4r). These effect could be attributed to the inherent BRDF effects and should be avoided or corrected for [
16,
17,
18,
19,
20,
21]. However, other indices showed a less significant change in R
2 values and were less affected for these ideal circumstances.
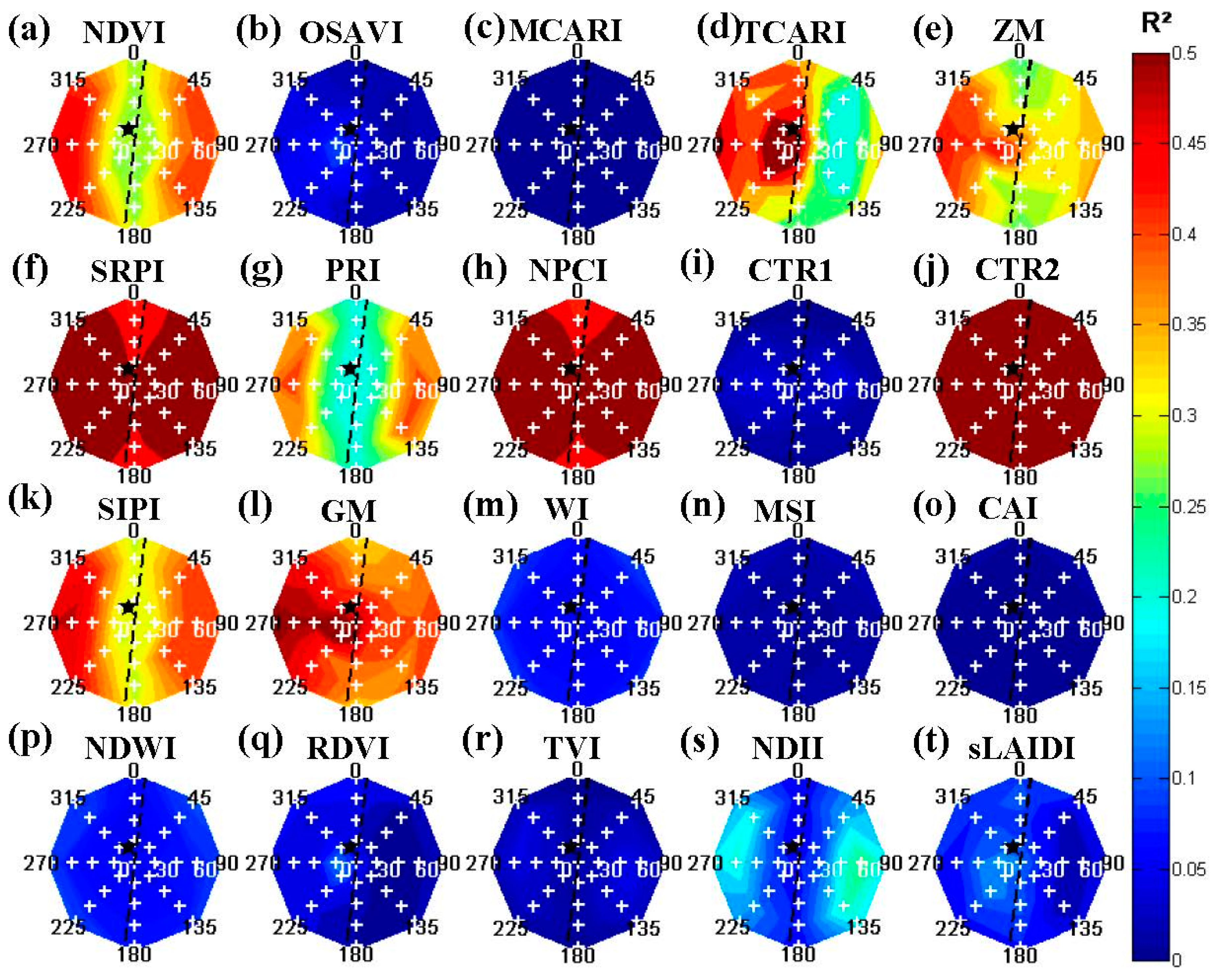
The synthetic imagery also demonstrated a high dependence of several indices towards canopy fraction distribution (
Figure 5), which was removed through a vegetation index correction (
Figure 6). After the correction, the lowest R
2 values from the synthetic imagery were found for the most variable background scenario—
i.e., the soil and weed background or S3 (
Table 4). Generally, the differences between different background scenarios were relatively small. This was most likely the result of the vegetation index correction [
28], which removed most of the influence of background mixtures and variable canopy cover fractions. Exceptions hereof were indices that were related to vegetative cover fraction, e.g., NDVI [
71], which showed significantly higher R
2 values for vegetated backgrounds (S2 and S4,
Table 4) compared to non-vegetated backgrounds (S1 and S3,
Table 4). This could be the result of the assumption of Lambertian behavior for the soil backgrounds. Although the range of R
2 values will be similar, the R
2 values could be lower for non-Lambertian backgrounds. Overall, the R
2 values were significantly higher between vegetation indices and chlorophyll content compared to LAI and water content, because of larger differences between treatments and the inherent relationship between leaf area and the amount of canopy water content [
5].
For the synthetic imagery, several commonly used vegetation indices presented an influence towards variable viewing geometries. PRI, NDII and SRPI values showed higher R
2 values perpendicular to the row orientation compared to parallel to rows for higher off-nadir view angles (
Figure 6). This might be attributed to the inclusion of background with parallel viewing to the rows, as imagery perpendicular to the rows would not present any background. Overall, differences between off-nadir viewing parallel or perpendicular to the row orientation might be greater without the use of the vegetation index correction to normalize for different canopy cover fractions. On the other hand, this view angle sensitivity of PRI was similar to [
24,
25,
72], finding a large difference between PRI values from forward (shaded) and backward (sunlit) scattered scenes. Other indices, such as NDVI, GM, ZM and SIPI, showed a decrease in R
2 values from nadir to off-nadir viewing scenes (
Figure 6). These results were similar to studies indicating a high dependence of NDVI towards variable viewing angles [
24,
73,
74] and a degraded correlation with NDVI-derived products [
18].
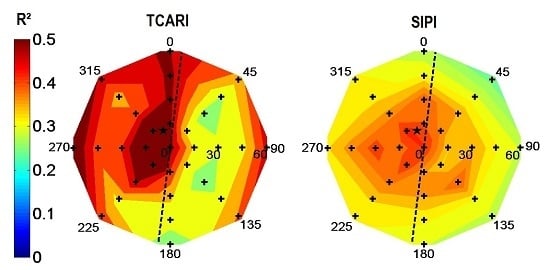
The R
2 values of satellite derived vegetation indices (
Table 5) were significantly lower compared to the synthetic images (
Table 4). This was most likely by the restricted amount of point measurements for the real imagery and the use of different row orientations within and between the orchards. On the other hand, also a suboptimal vegetation index correction could cause a decrease in R
2 values between indices and variables. For the rainfed orchard, R
2 increased significantly because of the similar row azimuth for all ground control plots. For the irrigated orchard, a significant decrease in R
2 values was the result of both sunlit and shaded canopies within one scene, as both 41 and 131° row orientations were monitored. The increased influence of shadow compared to the synthetic imagery was caused by a decrease in illumination elevation and the presence of a hedgerow cropping system causing one predominantly shaded and sunlit side [
41,
75]. Several studies have shown the negative effect of shaded canopy parts on the correlation between vegetation indices and biophysical variables [
3,
4,
76]. As the virtual orchard trees consisted of spherical canopies, shaded canopy sections and the effects of shadow on the vegetation indices were of less importance [
3].
The vegetation index correction algorithm could not mitigate the inclusion of shadow on the variation of index values, as it is not directly related to canopy cover fraction. Conversely, problems could be avoided by segmenting or classifying the canopies into sunlit and shaded areas—e.g., Stagakis
et al. [
2,
3]. However, for hedgerow systems problems with canopy anisotropy would be enlarged and cause significant differences between both faces of the canopy [
13], obstructing derivation of structural variables—
i.e., plant cover, canopy height and biomass—from multiple viewing angles. Another solution would be the minimization of shaded pixels in the analysis [
3,
76,
77]. To illustrate the usefulness of this approach, a selection was made for all satellite images (
Section 2.2) based on row orientation, viewing and illumination angles. Similarly to [
4], scenes with high off-nadir viewing angles—
i.e., off-nadir viewing angles over 20°—and viewing geometries opposite to the sun were removed as they consisted mostly of shaded canopies. Off-nadir viewing angles under 20° would result in partially sunlit scenes and were shown less affected [
4]. The average R
2 values for all indices after the selection of sunlit image pixels are shown in
Table 6.
Table 6 illustrated the usefulness of pixel selection based on scene illumination for the irrigated orchard, as a significant increase in R
2 values was found between biophysical variables and index values compared to
Table 5. The indices showing a significant increase also showed higher R
2 values for sunlit scene compositions in
Figure 7. On the other hand, indices which presented higher R
2 values for shaded scene compositions—
i.e., NDVI, CTR2, MCARI, OSAVI and TCARI—showed similar or decreased R
2 values.
For the rainfed orchard, a significant decrease of R
2 values was present for almost all indices after the selection of sunlit pixels compared to
Table 5. This was the effect of the different growing systems within both orchards. On the one hand, the Spindle bush system (rainfed orchard) resulted in open tree canopies and more sunlit areas [
41]. On the other hand, the hedgerow V-system (irrigated orchard) resulted in variable canopy faces—
i.e., one predominantly sunlit and shaded side [
41].
With regards to the optimal vegetation indices for high spatial resolution imagery, PRI, CTR1, SIPI and GM provided more stable correlations with chlorophyll content for variable viewing geometries (
Figure 6,
Table 5 and
Table 6). However, these indices were highly affected by the canopy fraction distribution and should be corrected or normalized prior to analysis (
Figure 5 and
Figure 6). With regards to water content and LAI, WI and sLAIDI provided good correlations for nadir viewing scenes, with a significant decrease towards off-nadir viewing angles. Other vegetation indices could be used in certain circumstances, but should be avoided in time series with variable viewing angles, e.g., NDVI, OSAVI, MCARI, TCARI, RDVI and TVI (
Figure 6,
Table 5 and
Table 6). Although the vegetation indices (
Table 2) were selected based on previous studies in fruit orchards, the list was not complete [
1,
2,
64]. Further research is required for vegetation indices not represented here prior to their use for applications with multiple viewing angles.
Overall, the results illustrated the necessity of a vegetation index selection or correction based on each specific circumstance and data set. However, through the careful selection of vegetation indices, an optimal derivation of biophysical variable should be plausible for applications with multiple viewing angles. Moreover, depending on the size of the available time series for a fixed target location, index values could be normalized with regards to view angle—e.g., Seaquist and Olsson [
73]. However, the most important limitation to this approach is the high amount of images required within a relatively small time window to minimize influences of a changing solar angle.