The Potential of Tree Fruit Stone and Seed Wastes in Greece as Sources of Bioactive Ingredients
Abstract
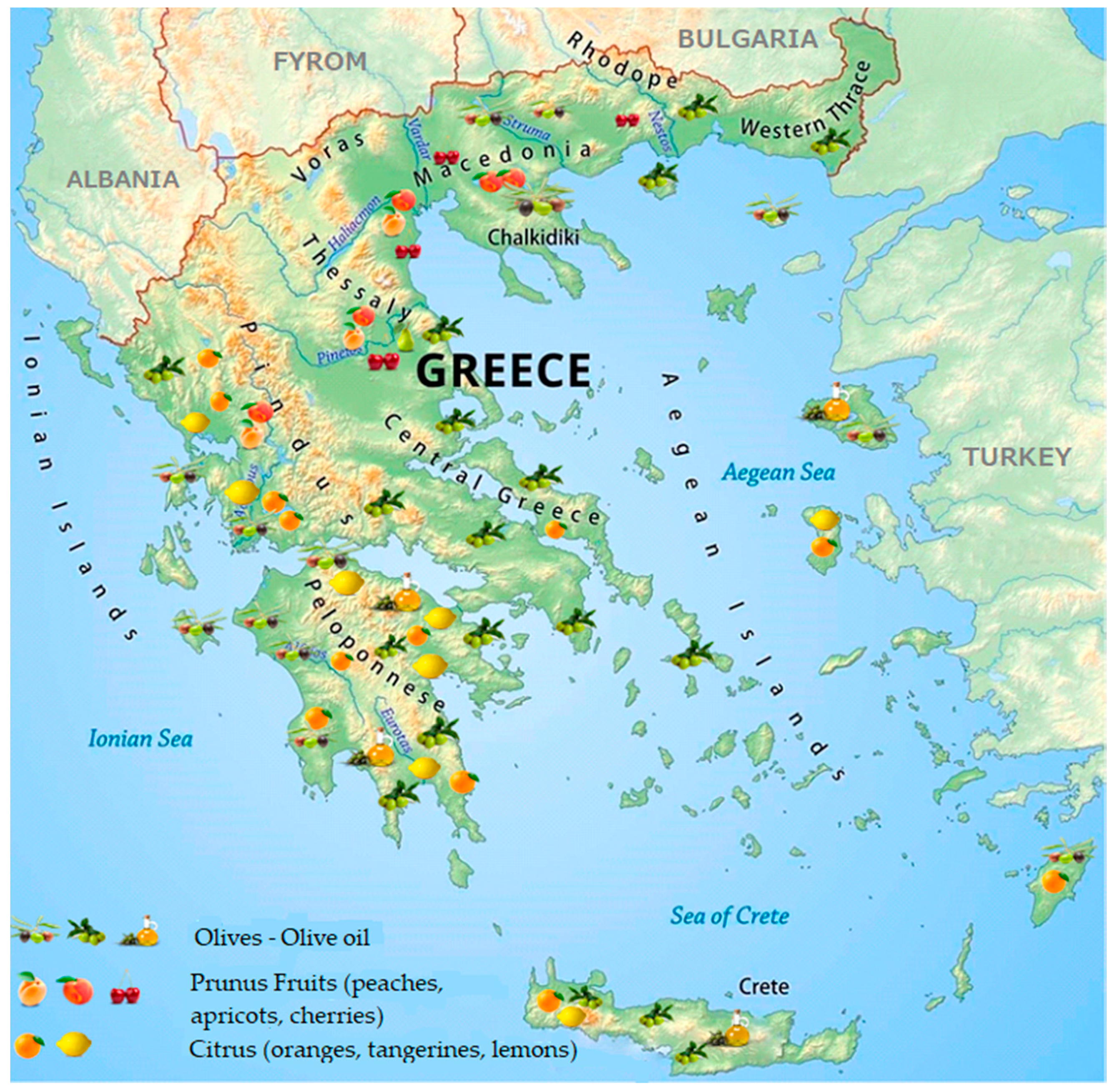
:1. The Fruit Sector in Greece
2. Bioactive Ingredients in the Tree Fruit Stones and Seeds
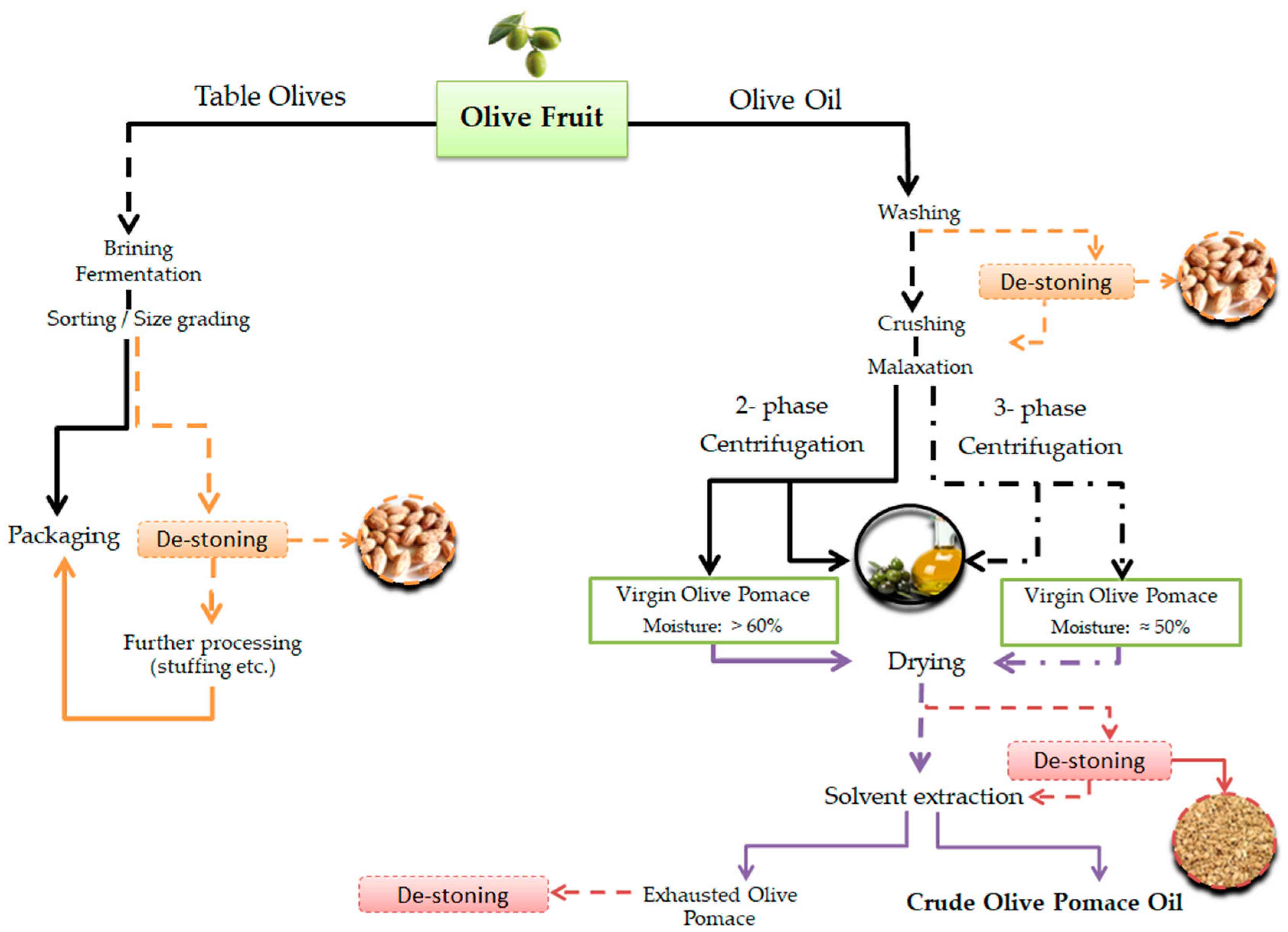
2.1. Olive Fruit Stones and Seeds
Table Olive and Olive Oil Sectors in Greece
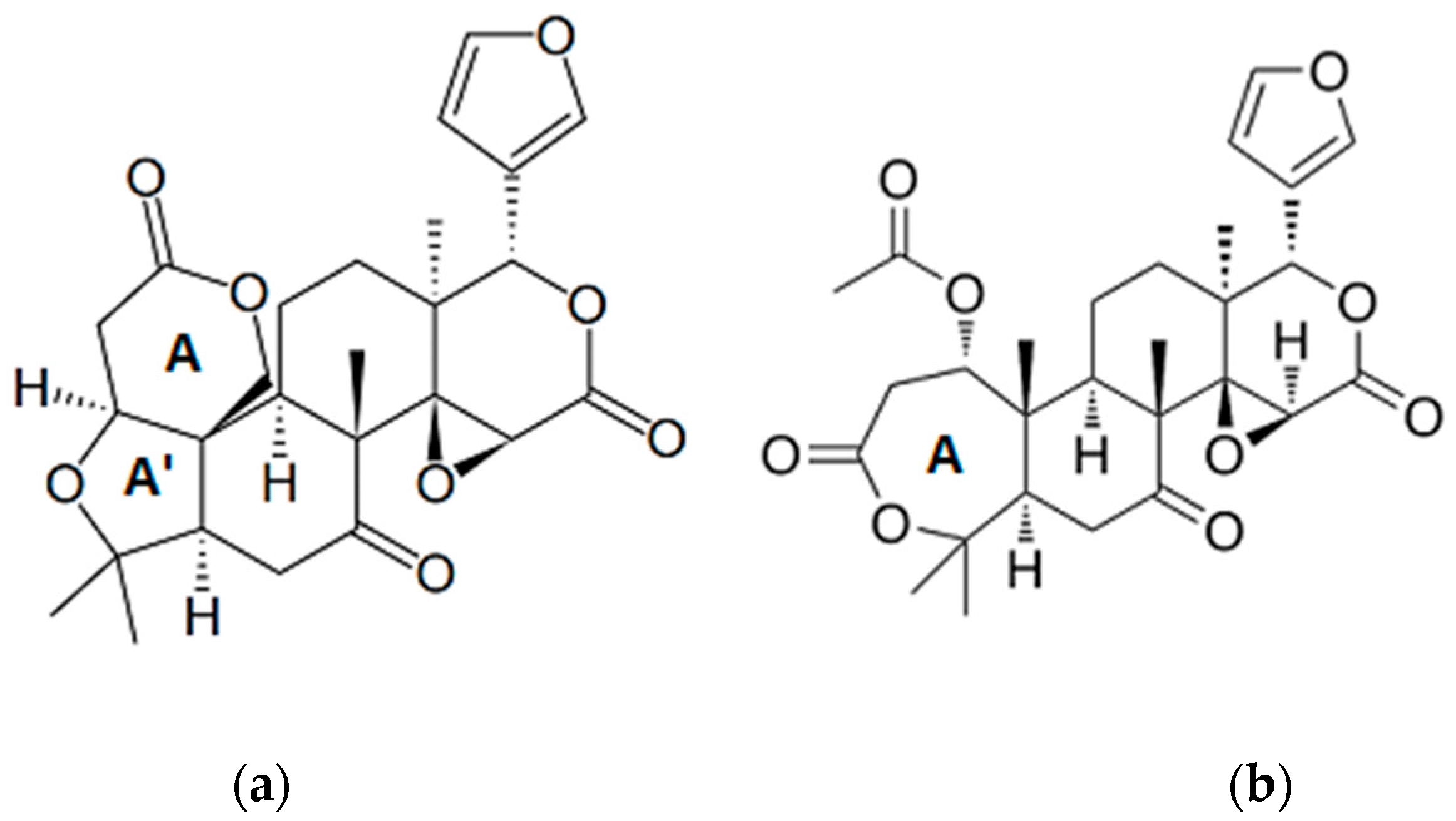
2.2. Prunus Fruit Stones and Seeds
Stone Fruit Canners and Juice Producers in Greece
2.3. Citrus Seeds
The Citrus Juice Sector in Greece
3. Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Petropoulos, D.P. Analysis of the “Balassa” Index of fruit and vegetables in Greece (1981-2013). IORE J. Agro Crop Sci. 2016, 2, 1–10. [Google Scholar] [CrossRef]
- European Comission Agriculture and Rural Development. Agriculture in the European Union: Statistical and Economic Information; Agriculture and Rural Development European Commission: Brussel, Belgium, 2013. [Google Scholar]
- Food and Agriculture Organisation of the United Nations Statistics Division. Available online: http://www.fao.org/faostat/en/#data/QI (accessed on 5 February 2018).
- Klonaris, S.; Agiangkatzoglou, A. Competitiveness of Greek virgin olive oil in the main destination markets. Br. Food J. 2018, 120, 80–95. [Google Scholar] [CrossRef]
- Eurostat. Agriculture, Forestry and Fishery Statistics; Eurostat: Luxembourg, 2014; ISBN 9789279330056.
- United States Department of Agriculture. GAIN Report: Stone Fruit Annual EU-28; U.S. Mission to the European Union: Brussels, Belgium, 2017. [Google Scholar]
- Galanopoulos, K.; Mattas, K.; Baourakis, G. “Sixth Framework Program Priority 8.1 EU, Hellenic Ministry of Rural Development and Food. Market and Trade Policies for Mediterranean Agriculture: The Case of Fruit/Vegetable and Olive Oil”. Available online: http://www.minagric.gr/en/agro_pol/Works_en.htm (accessed on 17 December 2017).
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Naziri, E.; Nenadis, N.; Mantzouridou, F.T.; Tsimidou, M.Z. Valorization of the major agrifood industrial by-products and waste from Central Macedonia (Greece) for the recovery of compounds for food applications. Food Res. Int. 2014, 65, 350–358. [Google Scholar] [CrossRef]
- European Comission Environment DG. LIFE among the Olives: Good Practice in Improving Environmental Performance in the Olive oil Sector; Hervé, M., Ed.; European Comission Environment: Luxenburg, 2010; ISBN 978-92-79-14154-6. [Google Scholar]
- González-Hidalgo, I.; Bañón, S.; Ros, J.M. Evaluation of table olive by-product as a source of natural antioxidants. Int. J. Food Sci. Technol. 2012, 47, 674–681. [Google Scholar] [CrossRef]
- Tsimidou, M.Z. Virgin Olive Oil (VOO) and other olive tree products as sources of α-tocopherol. Updating and perspective. In Tocopherol: Sources, Uses and Health Benefits; Catala, A., Ed.; Nova Science Publisher: New York, NY, USA, 2012; pp. 1–21. [Google Scholar]
- Kalogeropoulos, N.; Tsimidou, M. Antioxidants in Greek virgin olive oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.G. Olives and Olive Oil Bioactive Constituents, 1st ed.; Boskou, D.G., Ed.; AOCS: Urbana, IL, USA, 2015; ISBN 9781630670429. [Google Scholar]
- Blekas, G.; Vassilakis, C.; Harizanis, C.; Tsimidou, M.; Boskou, D.G. Biophenols in table olives. J. Agric. Food Chem. 2002, 50, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Pattara, C.; Cappelletti, G.M.; Cichelli, A. Recovery and use of olive stones: Commodity, environmental and economic assessment. Renew. Sustain. Energy Rev. 2010, 14, 1484–1489. [Google Scholar] [CrossRef]
- Amirante, P.; Clodoveo, M.L.; Tamborrino, A.; Leone, A.; Paice, A.G. Influence of the Crushing System: Phenol Content in Virgin Olive Oil Produced from Whole and De-Stoned Pastes; Elsevier Inc.: Amsterdam, The Netherlands, 2010; ISBN 9780123744203. [Google Scholar]
- Skoulou, V.; Zabaniotou, A. Investigation of agricultural and animal wastes in Greece and their allocation to potential application for energy production. Renew. Sustain. Energy Rev. 2007, 11, 1698–1719. [Google Scholar] [CrossRef]
- Regional Committee of Peloponesse. Available online: http://ppel.gov.gr/2016/12/ανακοίνωση-διαβούλευσης-στο-πλαίσιο/ (accessed on 5 Feburary 2018).
- Vlyssides, A.G.; Loizides, M.; Karlis, P.K. Integrated strategic approach for reusing olive oil extraction by-products. J. Clean. Prod. 2004, 12, 603–611. [Google Scholar] [CrossRef]
- Boskou, D.G. Phenolic Compounds in Olives and Olive Oil. In Olive oil Minor Constituents and Health; Boskou, D.G., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 11–44. ISBN 9781420059939. [Google Scholar]
- Angerosa, F.; Basti, C.; Vito, R.; Lanza, B. Effect of fruit stone removal on the production of virgin olive oil volatile compounds. Food Chem. 1999, 67, 295–299. [Google Scholar] [CrossRef]
- Luaces, P.; Romero, C.; Gutierrez, F.; Sanz, C.; Perez, A.G. Contribution of olive seed to the phenolic profile and related quality parameters of virgin olive oil. J. Sci. Food Agric. 2007, 87, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, E.; Mantzouridou, F.T. Current status and future challenges of table olive processing wastewater valorization. Biochem. Eng. J. 2016, 112, 103–113. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Barampouti, E.M.P.; Mai, S.T. Physical characteristics of olive stone wooden residues: Possible bulking material for composting process. Biodegradation 2008, 19, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Vlyssides, A.G.; Loizidou, M.; Zorpas, A.A. Characteristics of solid residues from olive oil processing as bulking material for co-composting with industrial wastewaters. J. Environ. Sci. Health Part A 1999, 34, 737–748. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; De Dios Alché, J.; Rodríguez-García, M.I. Characterization of olive seed storage proteins. Acta Physiol. Plant. 2007, 29, 439–444. [Google Scholar] [CrossRef]
- Esteve, C.; Marina, M.L.; García, M.C. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem. 2015, 167, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Prenzler, P.D.; Lavee, S.; Antolovich, M.; Robards, K. Quantitative changes in phenolic content during physiological development of the olive (Olea europaea) cultivar Hardy’s Mammoth. J. Agric. Food Chem. 2003, 51, 2532–2538. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Macchioni, A.; Montedoro, G.; Agrarie, I.; Costanzo, V.S.; Chimica, D.; Elce, V.; Sample, M.; et al. Phenolic compounds of olive fruit: One- and Two-Dimensional Nuclear Magnetic Resonance characterization of nuzhenide and its distribution in the constitutive parts of fruit. J. Agric. Food Chem. 1999, 12–18. [Google Scholar] [CrossRef]
- Maestro-Duran, R.; Leon-Cabello, R.; Ruiz-Gutierrez, V.; Fiestas, P.; Vazquez-Roncero, A. Glucosidos fenolicos amargos de las semillas del olivo (Olea europaea). Grasas Aceites 1994, 45, 332. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.V.; Boas, L.V. Phenolic compounds and antioxidant activity of Olea europaea L. Fruits and leaves. Food Sci. Technol. Int. 2006, 12, 385–396. [Google Scholar] [CrossRef]
- Moghaddam, G.; Heyden, Y.V.; Rabiei, Z.; Sadeghi, N.; Oveisi, M.R.; Jannat, B.; Araghi, V.; Hassani, S.; Behzad, M.; Hajimahmoodi, M. Characterization of different olive pulp and kernel oils. J. Food Compos. Anal. 2012, 28, 54–60. [Google Scholar] [CrossRef]
- Ranalli, A.; Pollastri, L.; Contento, S.; Di Loreto, G.; Iannucci, E.; Lucera, L.; Russi, F. Acylglycerol and fatty acid components of pulp, seed, and whole olive fruit oils. Their use to characterize fruit variety by chemometrics. J. Agric. Food Chem. 2002, 50, 3775–3779. [Google Scholar] [CrossRef] [PubMed]
- Ben Mansour, A.; Flamini, G.; Ben Selma, Z.; Le Dréau, Y.; Artaud, J.; Abdelhedi, R.; Bouaziz, M. Olive oil quality is strongly affected by cultivar, maturity index and fruit part: Chemometrical analysis of volatiles, fatty acids, squalene and quality parameters from whole fruit, pulp and seed oils of two Tunisian olive cultivars. Eur. J. Lipid Sci. Technol. 2015, 117, 976–987. [Google Scholar] [CrossRef]
- Ranalli, A.; Pollastri, L.; Contento, S.; Loreto, G.D.; Lannucci, E.; Lucera, L.; Russi, F. Sterol and alcohol components of seed, pulp and whole olive fruit oils. Their use to characterise olive fruit variety by multivariates. J. Sci. Food Agric. 2002, 82, 854–859. [Google Scholar] [CrossRef]
- da Silva, A.C.; Jorge, N. Bioactive compounds of the lipid fractions of agro-industrial waste. Food Res. Int. 2014, 66, 493–500. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M. Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind. Crops Prod. 2016, 83, 329–338. [Google Scholar] [CrossRef]
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar] [CrossRef]
- Tsimidou, M.Z. Squalene and tocopherols in olive oil. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 561–567. ISBN 9780123744203. [Google Scholar]
- Naziri, E.; Tsimidou, M.Z. Squalene, a desirable hydrocabon of virgin olive oil. In Olive Oil at the Core of the Mediterranean Diet; Greek Lipid Forum: Athens, Greece, 2015; pp. 44–55. [Google Scholar]
- E.C. BIC of Attica. Olive Oil and Olive Pomace Oil Sector Analysis & Brief analysis of Table Olives Sector; E.C. BIC of Attica: Athens, Greece, 2012. [Google Scholar]
- Table Olives. Available online: http://doepel.gr/en_US/ (accessed on 2 Feburary 2018).
- Mylonas, P. Olive Oil: Establishing the Greek Brand; National Bank of Greece: Athens, Greece, 2015. [Google Scholar]
- Siddiq, M. Stone fruits production, postharvest storage, processing and nutrition. In Agricultural and Food Biotechnology of Olea Europaea and Stone Fruits; Muzzalupo, I., Micali, S., Eds.; Bentham Science Publishers Ltd.: Sharjah, UAE, 2015; pp. 309–383. ISBN 978-1-68108-002-4. [Google Scholar]
- Valta, K.; Damala, P.; Panaretou, V.; Orli, E.; Moustakas, K.; Loizidou, M. Review and assessment of waste and wastewater treatment from fruits and vegetables processing industries in Greece. Waste Biomass Valoriz. 2016, 1–20. [Google Scholar] [CrossRef]
- Kamel, B.S.; Kakuda, Y. Characterization of the seed oil and meal from apricot, cherry, nectarine, peach and plum. J. Am. Oil Chem. Soc. 1992, 69, 492–494. [Google Scholar] [CrossRef]
- Arvelakis, S.; Gehrmann, H.; Beckmann, M.; Koukios, E.G. Preliminary results on the ash behavior of peach stones during fluidized bed gasification: Evaluation of fractionation and leaching as pre-treatments. Biomass Bioenergy 2005, 28, 331–338. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels. EFSA J. 2016, 14, 1–47. [Google Scholar] [CrossRef] [Green Version]
- García, M.C.; González-García, E.; Vásquez-Villanueva, R.; Marina, M.L. Apricot and other seed stones: Amygdalin content and the potential to obtain antioxidant, angiotensin I converting enzyme inhibitor and hypocholesterolemic peptides. Food Funct. 2016, 7, 4693–4701. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially- available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Transition of phenolics and cyanogenic glycosides from apricot and cherry fruit kernels into liqueur. Food Chem. 2016, 203, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, T.; Durmaz, G.; Ateş, B.; Erdoǧan, A. Protective effect of dietary apricot kernel oil supplementation on cholesterol levels and antioxidant status of liver in hypercholesteremic rats. J. Food Agric. Environ. 2009, 7, 61–65. [Google Scholar]
- Zhang, J.; Gu, H.-D.; Zhang, L.; Tian, Z.J.; Zhang, Z.Q.; Shi, X.C.; Ma, W.H. Protective effects of apricot kernel oil on myocardium against ischemia-reperfusion injury in rats. Food Chem. Toxicol. 2011, 49, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Lazos, E.S. Composition and oil characteristics of apricot, peach and cherry kernel. Grasas y Aceites 1991, 42, 127–131. [Google Scholar] [CrossRef]
- Matthaus, B.; Özcan, M.M. Fatty acids and tocopherol contents of some prunus spp. kernel oils. J. Food Lipids 2009, 16, 187–199. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Soliven, A.; Segliņa, D. Composition of bioactive compounds in kernel oils recovered from sour cherry (Prunus cerasus L.) by-products: Impact of the cultivar on potential applications. Ind. Crops Prod. 2016, 82, 44–50. [Google Scholar] [CrossRef]
- Chamli, D.; Bootello, M.A.; Bouali, I.; Jouhri, S.; Boukhchina, S.; Martínez-Force, E. Chemical characterization and thermal properties of kernel oils from Tunisian peach and nectarine varieties of Prunus persica. Grasas y Aceites 2017, 68, 1–9. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ünver, A.; Arslan, D. A research on evaluation of some fruit kernels and/or seeds as a raw material of vegetable oil industry. Qual. Assur. Saf. Crop. Foods 2014, 7, 187–191. [Google Scholar] [CrossRef]
- Femenia, A.; Rosselló, C.; Mulet, A.; Cañellas, J. Chemical composition of bitter and sweet apricot kernels. J. Agric. Food Chem. 1995, 43, 356–361. [Google Scholar] [CrossRef]
- Górnaś, P.; Ramos, M.J.; Montano, M.C.; Rudzińska, M.; Radziejewska-Kubzdela, E.; Grygier, A. Fruit pits recovered from 14 genotypes of apricot (Prunus armeniaca L.) as potential biodiesel feedstock. Eur. J. Lipid Sci. Technol. 2017, 1700147, 1–10. [Google Scholar] [CrossRef]
- Yilmaz, C.; Gökmen, V. Compositional characteristics of sour cherry kernel and its oil as influenced by different extraction and roasting conditions. Ind. Crops Prod. 2013, 49, 130–135. [Google Scholar] [CrossRef]
- Rudzińska, M.; Górnaś, P.; Raczyk, M.; Soliven, A. Sterols and squalene in apricot (Prunus armeniaca L.) kernel oils: The variety as a key factor. Nat. Prod. Res. 2017, 31, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Belitz, H.D., Grosch, W., Schieberle, P., Eds.; Springer: Berlin, Germany, 2009; Volume 107, ISBN 9783540699330. [Google Scholar]
- Radenkovs, V.; Feldmane, D. Profile of lipophilic antioxidants in the by-products recovered from six cultivars of sour cherry (Prunus cerasus L.). Nat. Prod. Res. 2017, 31, 2549–2553. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Radziejewska-Kubzdela, E.; Mišina, I.; Biegańska-Marecik, R.; Grygier, A.; Rudzińska, M. Tocopherols, tocotrienols and carotenoids in kernel oils recovered from 15 apricot (Prunus armeniaca L.) genotypes. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 693–699. [Google Scholar] [CrossRef]
- Bak, I.; Lekli, I.; Juhasz, B.; Varga, E.; Varga, B.; Gesztelyi, R.; Szendrei, L.; Tosaki, A. Isolation and analysis of bioactive constituents of sour cherry (Prunus cerasus) seed kernel: An emerging functional food. J. Med. Food 2010, 13, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.E.; Gallyas, E.; Bak, I.; Rakotovao, A.; Boucher, F.; De Leiris, J.; Nagy, N.; Varga, E.; Tosaki, A. Heme oxygenase-1-related carbon monoxide and flavonoids in ischemic/reperfused rat retina. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Lekli, I.; Juhasz, B.; Nagy, N.; Varga, E.; Varadi, J.; Gesztelyi, R.; Szabo, G.; Szendrei, L.; Bacskay, I.; et al. Cardioprotective mechanisms of Prunus cerasus (sour cherry) seed extract against ischemia-reperfusion-induced damage in isolated rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1329–H1336. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Priksz, D.; Lampé, N.; Bombicz, M.; Kurucz, A.; Szabó, A.; Pósa, A.; Szabó, R.; Kemény-Beke, Á.; Remenyik, J.; et al. Protective effect of Prunus cerasus (sour cherry) seed extract on the recovery of ischemia/reperfusion-induced retinal damage in Zucker diabetic fatty rat. Molecules 2017, 22, 1782. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Greece: Stone Fruit Annual; United States Department of Agriculture: Washington, DC, USA, 2014.
- Greek Ministry of Rural Development and Food Apricots. Available online: http://www.minagric.gr/index.php/el/for-farmer-2/crop-production/oporokipeytika/1773-berikoka (accessed on 7 Febuary 2018).
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Manners, G.D. Citrus Limonoids: Analysis, bioactivity, and biomedical prospects. J. Agric. Food Chem. 2007, 55, 8285–8294. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Brodbelt, J.S.; Bhat, N.G.; Patil, B.S. Methods for the separation of limonoids from citrus. ACS Symp. Ser. 2006, 936, 34–51. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.; Torre, G.; Saro, M.; Dugo, P.; Mondello, L. Underestimated sources of flavonoids, limonoids and dietary fiber: Availability in lemon’s by-products. J. Funct. Foods 2014, 9, 18–26. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.; Inferrera, V.; Dugo, P.; Mondello, L. Underestimated sources of flavonoids, limonoids and dietary fiber: Availability in orange’s by-products. J. Funct. Foods 2015, 12, 150–157. [Google Scholar] [CrossRef]
- Minamisawa, M.; Yoshida, S.; Uzawa, A. The functional evaluation of waste yuzu (Citrus junos) seeds. Food Funct. 2014, 5, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Miyake, M.; Inaba, N.; Ayano, S.; Ifuku, Y.; Hasegawa, S. Limonoid glucosides of Satsuma mandarin (Citrus unshiu Marcov.) and its processing products. In Citrus Limonoids: Functional Chemicals in Agriculture and Foods; Berhow, M., Hasegawa, S., Manners, G., Eds.; American Chemical Society: Washington, DC, USA, 2000; pp. 107–119. [Google Scholar]
- Miller, E.G.; Taylor, S.E.; Berry, C.W.; Zimmerman, J.A. Citrus limonoids: Increasing importance as anticancer agents. In Citrus Limonoids: Functional Chemicals in Agriculture and Foods; Berhow, M., Hasegawa, S., Manners, G., Eds.; American Chemical Society: Washington, DC, USA, 2000; pp. 132–144. [Google Scholar]
- Wang, H.; Chen, G.; Guo, X.; Abbasi, A.M.; Liu, R.H. Influence of the stage of ripeness on the phytochemical profiles, antioxidant and antiproliferative activities in different parts of Citrus reticulata Blanco cv. Chachiensis. LWT Food Sci. Technol. 2016, 69, 67–75. [Google Scholar] [CrossRef]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crops Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Bocco, A.; Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidant activity and phenolic composition of Citrus peel and seed extracts. J. Agric. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- Habib, M.A.; Hammam, M.A.; Sakr, A.A.; Ashoush, Y.A. Chemical evaluation of egyptian citrus seeds as potential sources of vegetable oils. J. Am. Oil Chem. Soc. 1986, 63, 1192–1196. [Google Scholar] [CrossRef]
- Cassia, R.M.; Mieko, K.; Neuza, J. Phytochemicals and antioxidant activity of Citrus seed oils. Food Sci. Technol. Res. 2012, 18, 399–404. [Google Scholar] [CrossRef]
- Jorge, N.; da Silva, A.C.; Aranha, C.P.M. Antioxidant activity of oils extracted from orange (Citrus sinensis) seeds. An. Acad. Bras. Cienc. 2016, 88, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Matthaus, B.; Özcan, M.M. Chemical evaluation of citrus seeds, an agro-industrial waste, as a new potential source of vegetable oils. Grasas y Aceites 2012, 63, 313–320. [Google Scholar] [CrossRef]
- Ueno, H.; Tanaka, M.; Machmudah, S.; Sasaki, M.; Goto, M. Supercritical carbon dioxide extraction of valuable compounds from Citrus junos seed. Food Bioprocess Technol. 2008, 1, 357–363. [Google Scholar] [CrossRef]
- Guneser, B.A.; Yilmaz, E. Bioactives, aromatics and sensory properties of cold-pressed and hexane-extracted lemon (Citrus limon L.) seed oils. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 723–731. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Varzakas, T.H. Fruit/fruit juice waste management: Treatment methods and potential uses of treated waste. In Waste Management for the Food Industries; Arvanitoyannis, I.S., Ed.; Elsevier Academic Press: Amsterdam, the Netherland, 2008; pp. 569–628. [Google Scholar]
- Karaouzas, I. Agro-industrial wastewater pollution in Greek river ecosystems. In The Rivers of Greece: Evolution, Current Status and Perspectives; Skoulikidis, N., Dimitriou, E., Karaouzas, I., Eds.; Springer-Verlag: Berlin, Germany, 2018; pp. 169–204. [Google Scholar]
- U.S. Department of Agriculture. GAIN Report: Greece Citrus Annual 2014; U.S. Department of Agriculture: Rome, Italy, 2014.
- Greek Ministry of Rural Development and Food Citrus. Available online: http://www.minagric.gr/index.php/el/for-farmer-2/crop-production/oporokipeytika/505-esperidoeidi/1713-stoixeia-esper-greece (accessed on 9 Febuary 2018).
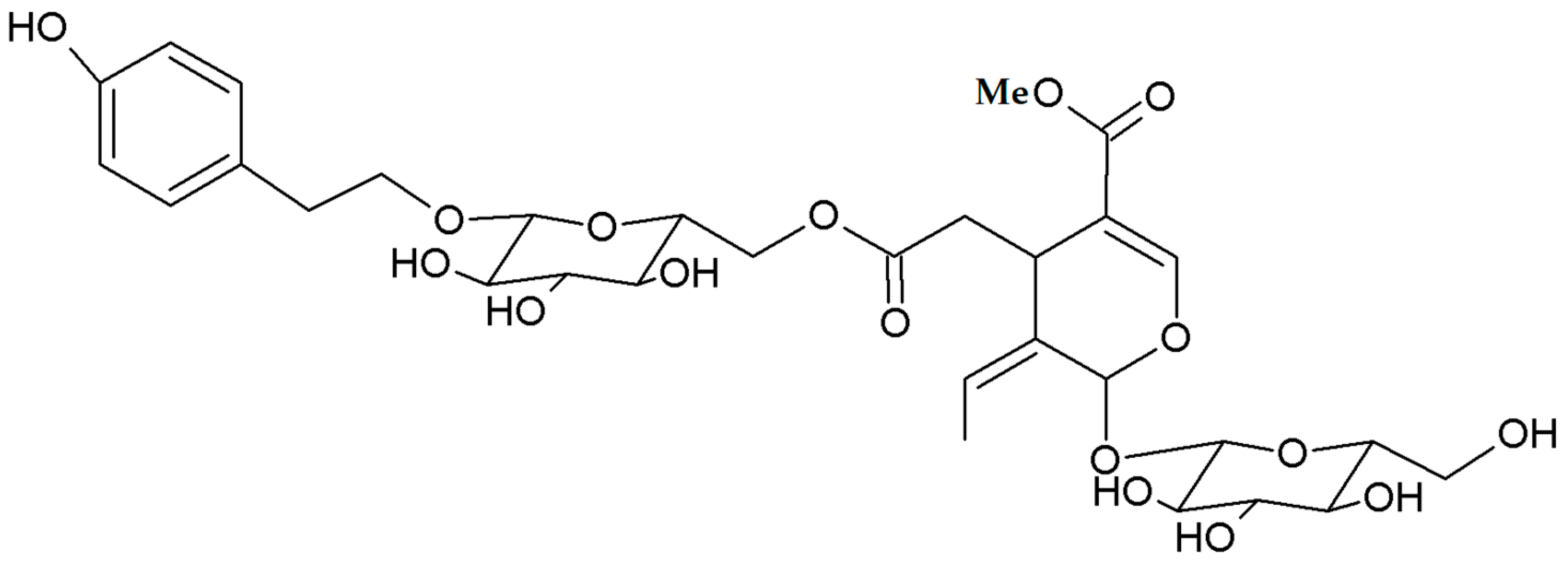

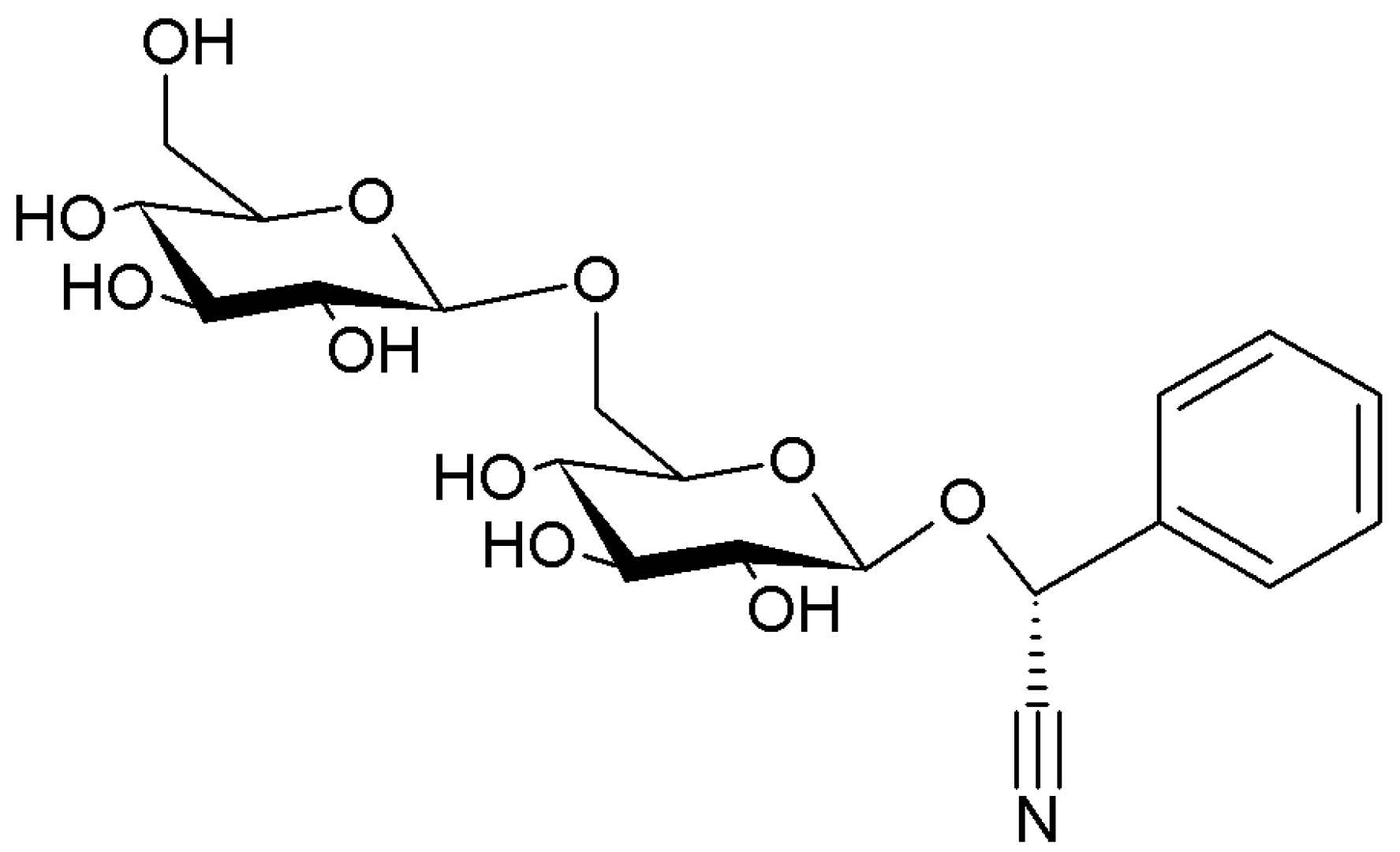

| Olive | Prunus | Citrus |
|---|---|---|
| Seed extracts with polar solvents | ||
| Phenols | Cyanogenic glycosides | Phenols |
| Secoiridoids | Amygdalin, prunasin | Flavanones |
| nüzhenide | hesperidin | |
| oleuropein | naringin | |
| eriocitrin | ||
| glycosylated forms | ||
| Phenolic alcohols | Phenolic acids | |
| tyrosol | Limonoids | |
| hydroxytyrosol | aglycones | |
| limonin | ||
| nomalin | ||
| glycosides | ||
| Seed oil | ||
| Sterols | Sterols | Sterols |
| β-sitosterol | β-sitosterol | β-sitosterol |
| campesterol | campesterol | campesterol |
| Squalene | Squalene | |
| Tocopherols | Tocopherols | Tocopherols |
| α-tocopherol | γ-tocopherol | α-tocopherol |
| α-tocopherol | ||
| Tocotrienols | ||
| α-tocotrienol | ||
| γ-tocotrienol | ||
| Carotenoids | Carotenoids | Carotenoids |
| lutein | ||
| β-carotene | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordoudi, S.A.; Bakirtzi, C.; Tsimidou, M.Z. The Potential of Tree Fruit Stone and Seed Wastes in Greece as Sources of Bioactive Ingredients. Recycling 2018, 3, 9. https://doi.org/10.3390/recycling3010009
Ordoudi SA, Bakirtzi C, Tsimidou MZ. The Potential of Tree Fruit Stone and Seed Wastes in Greece as Sources of Bioactive Ingredients. Recycling. 2018; 3(1):9. https://doi.org/10.3390/recycling3010009
Chicago/Turabian StyleOrdoudi, Stella A., Christina Bakirtzi, and Maria Z. Tsimidou. 2018. "The Potential of Tree Fruit Stone and Seed Wastes in Greece as Sources of Bioactive Ingredients" Recycling 3, no. 1: 9. https://doi.org/10.3390/recycling3010009





