Thermogravimetric Investigation of the Lead Volatilization from Waste Cathode-Ray Tube Glass
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Thermogravimetric Analysis
2.3. Tube Reactor Experiments
2.4. Kinetic Investigation
3. Results and Discussion
3.1. Identification of Reaction Steps
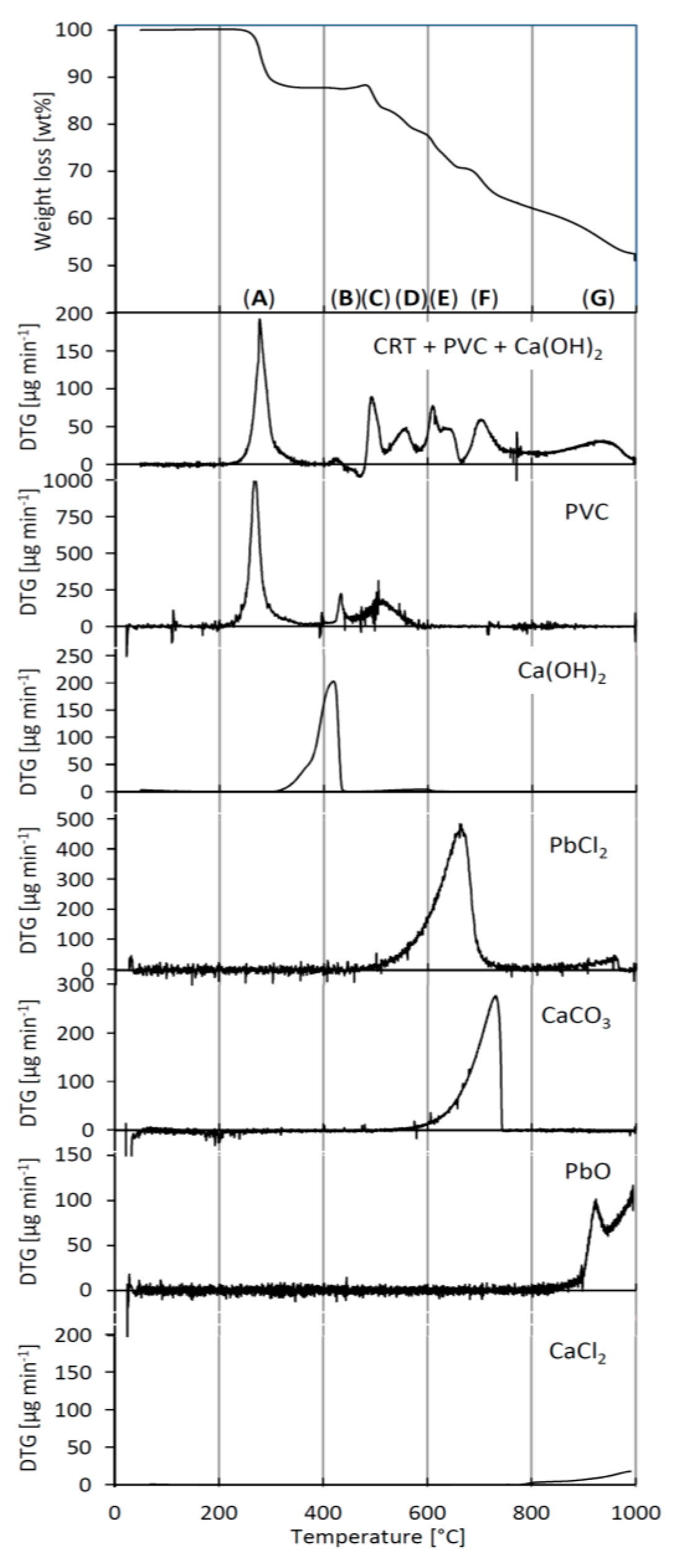
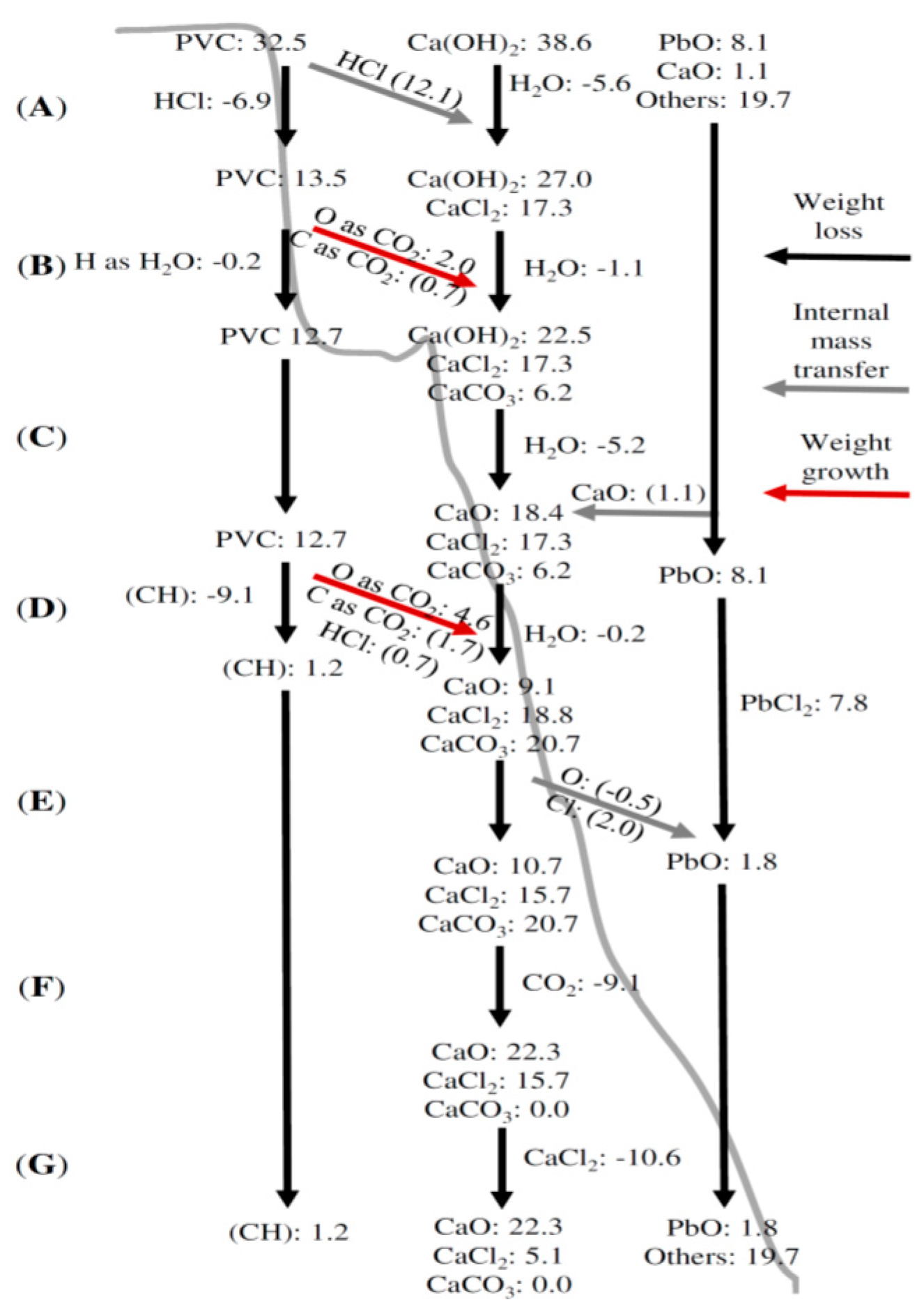
3.2. TGA Mass Balance
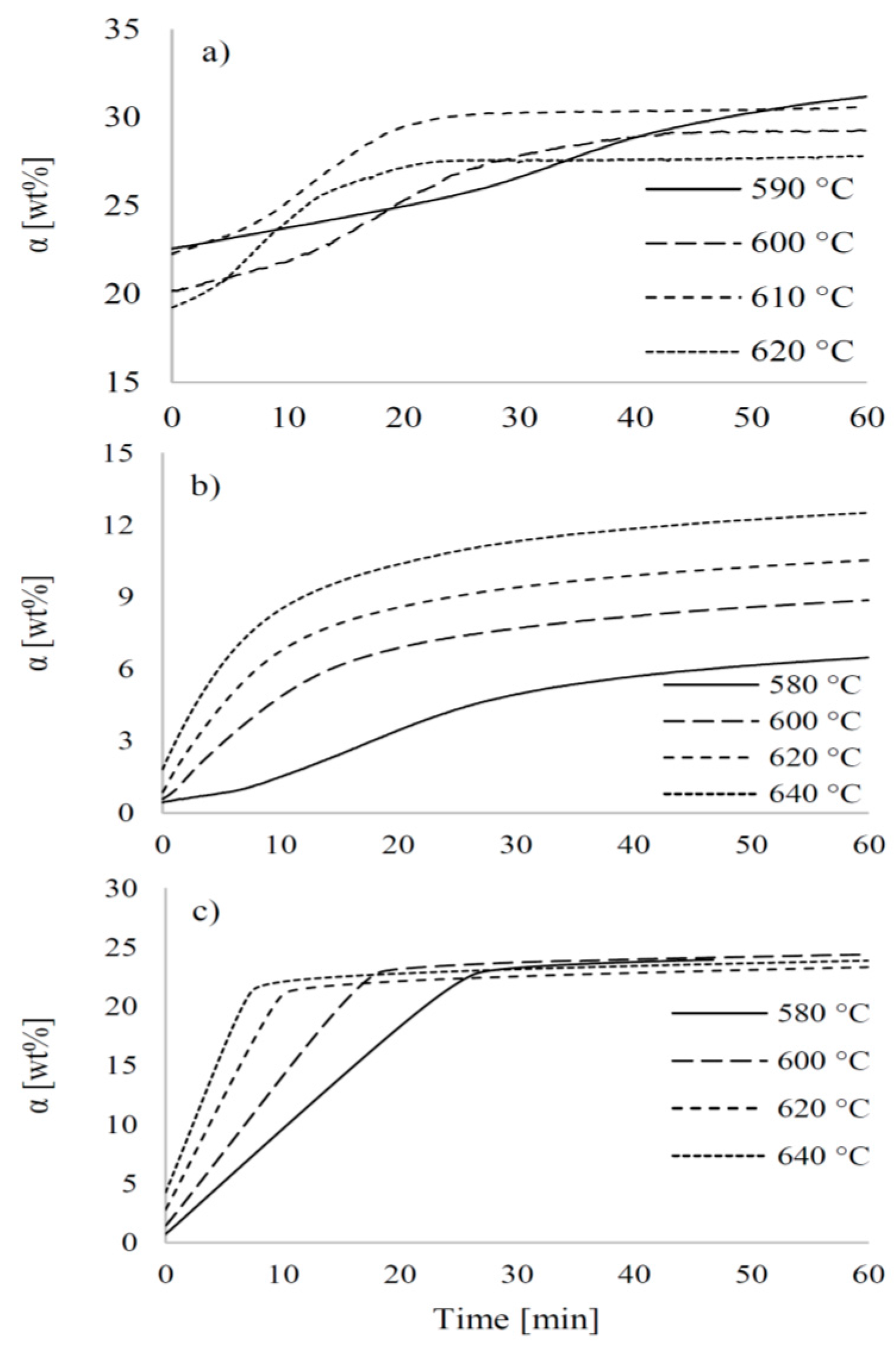
3.3. Kinetic Parameters
| Sample | Reaction Step | EA [kJ·mol−1] | A [min−1] | Model |
|---|---|---|---|---|
| CRT (TGA) | Step 1 | 204–220 | - | A3,A4,P2,P3,P4 |
| Step 2 | 180 | - | D2,D3,D4 | |
| CRT (tube reactor) | 101 | 4770 | F2 | |
| Amorphous PbSiO4 | Step 1 | - | - | A2,A3,P2,P3 |
| Step 2 | 190 | - | D1,D2,D3,D4 | |
| 173 | 2.1 × 107 | P2/3 | ||
| Crystalline PbSiO4 | 116 | 4.0 × 104 | R3 | |
| 117 | 1.5 × 105 | F1 |
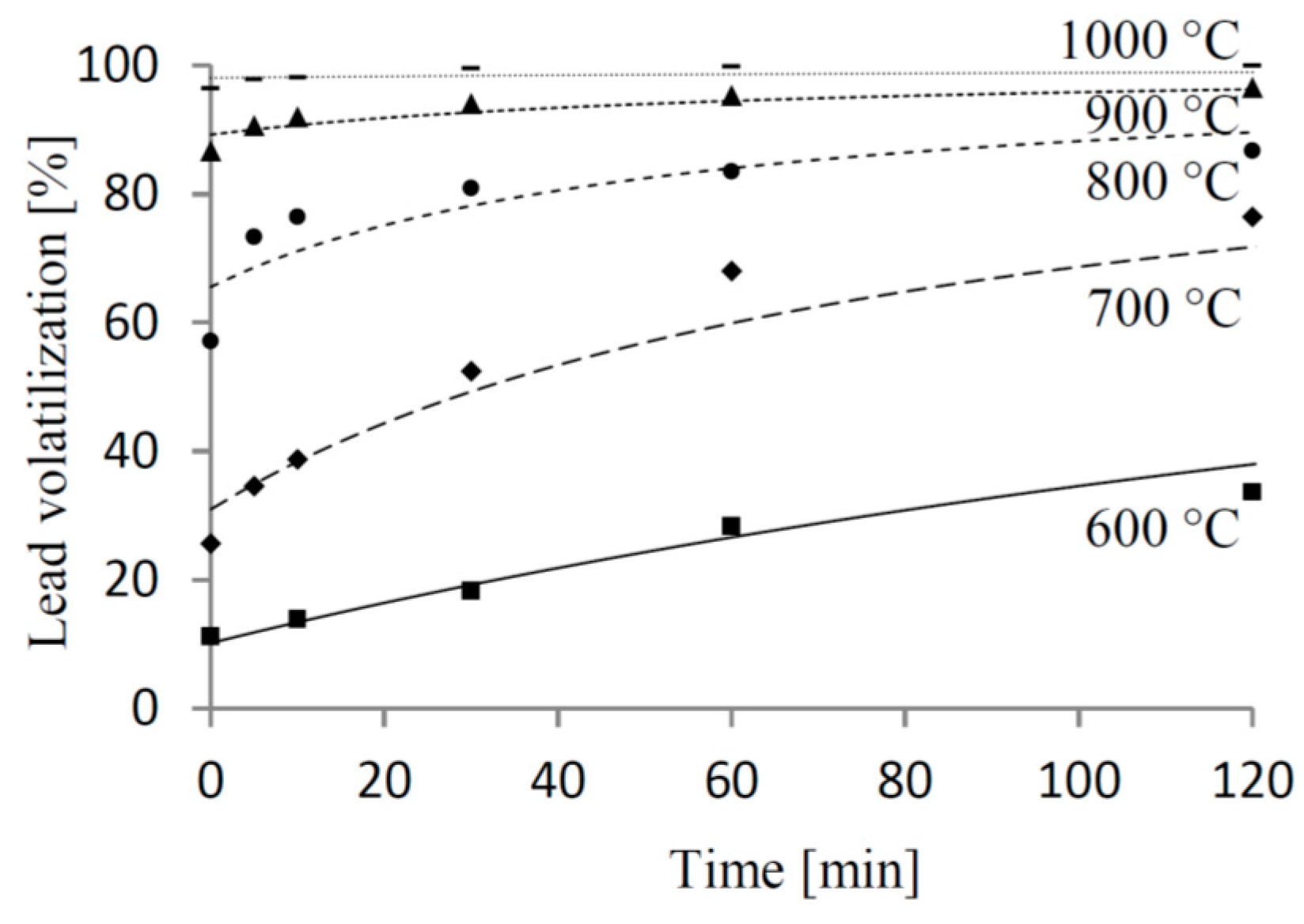
4. Conclusions
Supplementary Materials
Acknowledgments
Author contributions
Conflicts of Interest
References
- Pant, D.; Singh, P. Pollution due to hazardous glass waste. Environ. Sci. Pollut. Res. 2014, 21, 2414–2436. [Google Scholar] [CrossRef] [PubMed]
- Csavina, J.; Taylor, M.P.; Félix, O.; Rine, K.P.; Eduardo Sáez, A.; Betterton, E.A. Size-resolved dust and aerosol contaminants associated with copper and lead smelting emissions: Implications for emission management and human health. Sci. Total Environ. 2014, 493, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.P.; Davies, P.J.; Kristensen, L.J.; Csavina, J.L. Licenced to pollute but not to poison: The ineffectiveness of regulatory authorities at protecting public health from atmospheric arsenic, lead and other contaminants resulting from mining and smelting operations. Aeolian Res. 2014, 14, 35–52. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, F.-S.; Zhu, J. Lead recovery and the feasibility of foam glass production from funnel glass of dismantled cathode ray tube through pyrovacuum process. J. Hazard. Mater. 2009, 161, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Inano, H.; Hiroyoshi, N. Recovery and immobilization of lead in cathode ray tube funnel glass by a combination of reductive and oxidative melting processes. J. Soc. Inf. Disp. 2012, 20, 508–516. [Google Scholar] [CrossRef]
- Yuan, W.; Li, J.; Zhang, Q.; Saito, F. Innovated application of mechanical activation to separate lead from scrap cathode ray tube funnel glass. Environ. Sci. Technol. 2012, 46, 4109–4114. [Google Scholar] [CrossRef] [PubMed]
- Erzat, A.; Zhang, F.-S. Evaluation of lead recovery efficiency from waste CRT funnel glass by chlorinating volatilization process. Environ. Technol. 2014, 35, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- Grause, G.; Yamamoto, N.; Kameda, T.; Yoshioka, T. Removal of lead from cathode ray tube funnel glass by chloride volatilization. Int. J. Environ. Sci. Technol. 2014, 11, 959–966. [Google Scholar] [CrossRef]
- Grause, G.; Takahashi, K.; Kameda, T.; Yoshioka, T. Lead removal from cathode ray tube glass by the action of calcium hydroxide and poly(vinyl chloride). Thermochim. Acta 2014, 596, 49–55. [Google Scholar] [CrossRef]
- Liu, J.; Fu, J.; Ning, X.A.; Sun, S.; Wang, Y.; Xie, W.; Huang, S.; Zhong, S. An experimental and thermodynamic equilibrium investigation of the Pb, Zn, Cr, Cu, Mn and Ni partitioning during sewage sludge incineration. J. Environ.Sci. 2015, 35, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Osada, S.; Kuchar, D.; Matsuda, H. Effect of chlorine on volatilization of Na, K, Pb, and Zn compounds from municipal solid waste during gasification and melting in a shaft-type furnace. J. Mater. Cycles Waste Manag. 2009, 11, 367–375. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Wang, B.; Qiao, Y.; Xiang, J.; Hu, S.; Yao, H. Study on the behavior of heavy metals during thermal treatment of municipal solid waste (MSW) components. Environ. Sci. Pollut. Res. 2015, 23, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Yang, W.F. Metal volatility during plastic combustion. J. Environ. Sci. Health A 1998, 33, 783–799. [Google Scholar] [CrossRef]
- Rio, S.; Verwilghen, C.; Ramaroson, J.; Nzihou, A.; Sharrock, P. Heavy metal vaporization and abatement during thermal treatment of modified wastes. J. Hazard. Mater. 2007, 148, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Lee, H.T.; Shiao, C.C.; Hopper, J.R.; Bostick, W.D. Metal behavior during fluidized bed thermal treatment of soil. Waste Manag. 1995, 15, 325–334. [Google Scholar] [CrossRef]
- Nowak, B.; Aschenbrenner, P.; Winter, F. Heavy metal removal from sewage sludge ash and municipal solid waste fly ash—A comparison. Fuel Process. Technol. 2013, 105, 195–201. [Google Scholar] [CrossRef]
- Chan, C.C.Y.; Kirk, D.W. Behaviour of metals under the conditions of roasting MSW incinerator fly ash with chlorinating agents. J. Hazard. Mater. 1999, 64, 75–89. [Google Scholar] [CrossRef]
- Jakob, A.; Stucki, S.; Struis, R.P.W.J. Complete heavy metal removal from fly ash by heat treatment: Influence of chlorides on evaporation rates. Environ. Sci. Technol. 1996, 30, 3275–3283. [Google Scholar] [CrossRef]
- Matsuno, M.; Tomoda, K.; Nakamura, T. Volatilization mechanism of Pb from fly ash in municipal waste incinerator. Mater. Trans. 2003, 44, 2481–2488. [Google Scholar] [CrossRef]
- Okada, T.; Tomikawa, H. Effects of chemical composition of fly ash on efficiency of metal separation in ash-melting of municipal solid waste. Waste Manag. 2013, 33, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, Y.; Sun, J.; Cao, Z.; Zhou, J.; Pan, Y.; Qian, G. Inhibiting evaporation of heavy metal by controlling its chemical speciation in MSWI fly ash. Fuel 2015, 158, 764–769. [Google Scholar] [CrossRef]
- Luengos, M.A.; Ambrosio, E.; Bohé, A.E.; Pasquevich, D.M. Thermal behavior of galena ore in chlorine atmospheres. J. Therm. Anal. Calorim. 2000, 59, 775–789. [Google Scholar] [CrossRef]
- Lee, G.-S.; Song, Y.J. Recycling eaf dust by heat treatment with PVC. Miner. Eng. 2007, 20, 739–746. [Google Scholar] [CrossRef]
- Yoo, J.-M.; Kim, B.-S.; Lee, J.; Kim, M.-S.; Nam, C.-W. Kinetics of the volatilization removal of lead in electric arc furnace dust. Mater. Trans. 2005, 46, 323–328. [Google Scholar] [CrossRef]
- Cong, J.; Yan, D.; Li, L.; Cui, J.; Jiang, X.; Yu, H.; Wang, Q. Volatilization of heavy metals (As, Pb, Cd) during co-processing in cement kilns. Environ. Eng. Sci. 2015, 32, 425–435. [Google Scholar] [CrossRef]
- Wu, Z.F.; Su, Y.Y.; Zhao, Y.Q. Transfer characteristics of heavy metal Pb in municipal solid waste incineration. Adv. Mater. Res. 2014, 864–867, 2001–2006. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Xiang, J.; Hu, S.; Su, S. Kinetic vaporization of heavy metals during fluidized bed thermal treatment of municipal solid waste. Waste Manag. 2013, 33, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Xu, B.; Zhao, D.-N.; Jin, B.-S. Experimental analysis of heavy metals behavior during melting process of fly ashes from MSWI under different atmospheres. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE), Chengdu, China, 18–20 June 2010.
- Fraissler, G.; Jöller, M.; Brunner, T.; Obernberger, I. Influence of dry and humid gaseous atmosphere on the thermal decomposition of calcium chloride and its impact on the remove of heavy metals by chlorination. Chem. Eng. Process. Process Intensif. 2009, 48, 380–388. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Xiang, J.; Hu, S.; Su, S.; Qiu, J. Vaporization of heavy metals during thermal treatment of model solid waste in a fluidized bed incinerator. Chemosphere 2012, 86, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Haiying, Z.; Youcai, Z.; Jingyu, Q. Thermal characterization of fly ash from one municipal solid waste incinerator (MSWI) in Shanghai. Process Saf. Environ. Protect. 2010, 88, 269–275. [Google Scholar] [CrossRef]
- Wang, S.-J.; Zhang, H.; Shao, L.-M.; Liu, S.-M.; He, P.-J. Thermochemical reaction mechanism of lead oxide with poly(vinyl chloride) in waste thermal treatment. Chemosphere 2014, 117, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Falcoz, Q.; Gauthier, D.; Abanades, S.; Flamant, G.; Patisson, F. Kinetic rate laws of Cd, Pb, and Zn vaporization during municipal solid waste incineration. Environ. Sci. Technol. 2009, 43, 2184–2189. [Google Scholar] [CrossRef] [PubMed]
- Bessada, C.; Massiot, D.; Coutures, J.; Douy, A.; Coutures, J.P.; Taulelle, F. 29Si MAS-NMR in lead silicates. J. Non-Cryst. Solids 1994, 168, 76–85. [Google Scholar] [CrossRef]
- Grause, G.; Ishibashi, J.; Kameda, T.; Bhaskar, T.; Yoshioka, T. Kinetic studies of the decomposition of flame retardant containing high-impact polystyrene. Polym. Degrad. Stab. 2010, 95, 1129–1137. [Google Scholar] [CrossRef]
- Aracil, I.; Font, R.; Conesa, J.A. Thermo-oxidative decomposition of polyvinyl chloride. J. Anal. Appl. Pyrolysis 2005, 74, 215–223. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grause, G.; Takahashi, K.; Yoshioka, T. Thermogravimetric Investigation of the Lead Volatilization from Waste Cathode-Ray Tube Glass. Recycling 2016, 1, 111-121. https://doi.org/10.3390/recycling1010111
Grause G, Takahashi K, Yoshioka T. Thermogravimetric Investigation of the Lead Volatilization from Waste Cathode-Ray Tube Glass. Recycling. 2016; 1(1):111-121. https://doi.org/10.3390/recycling1010111
Chicago/Turabian StyleGrause, Guido, Kenshi Takahashi, and Toshiaki Yoshioka. 2016. "Thermogravimetric Investigation of the Lead Volatilization from Waste Cathode-Ray Tube Glass" Recycling 1, no. 1: 111-121. https://doi.org/10.3390/recycling1010111





