Effect of Mixed Li+/Na+-ion Electrolyte on Electrochemical Performance of Na4Fe3(PO4)2P2O7 in Hybrid Batteries
Abstract
:1. Introduction
2. Result and Discussion
2.1. Crystal Structure
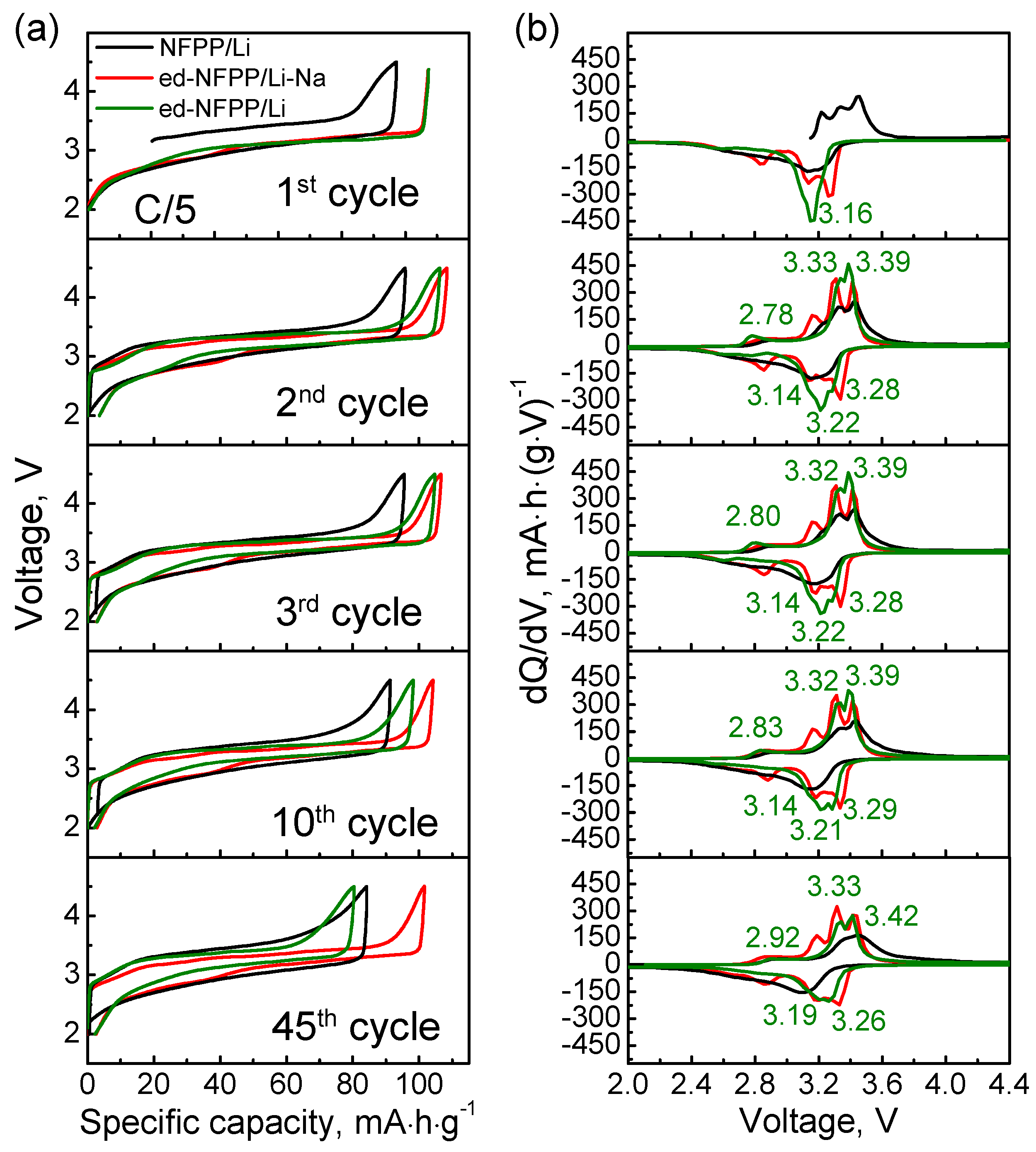
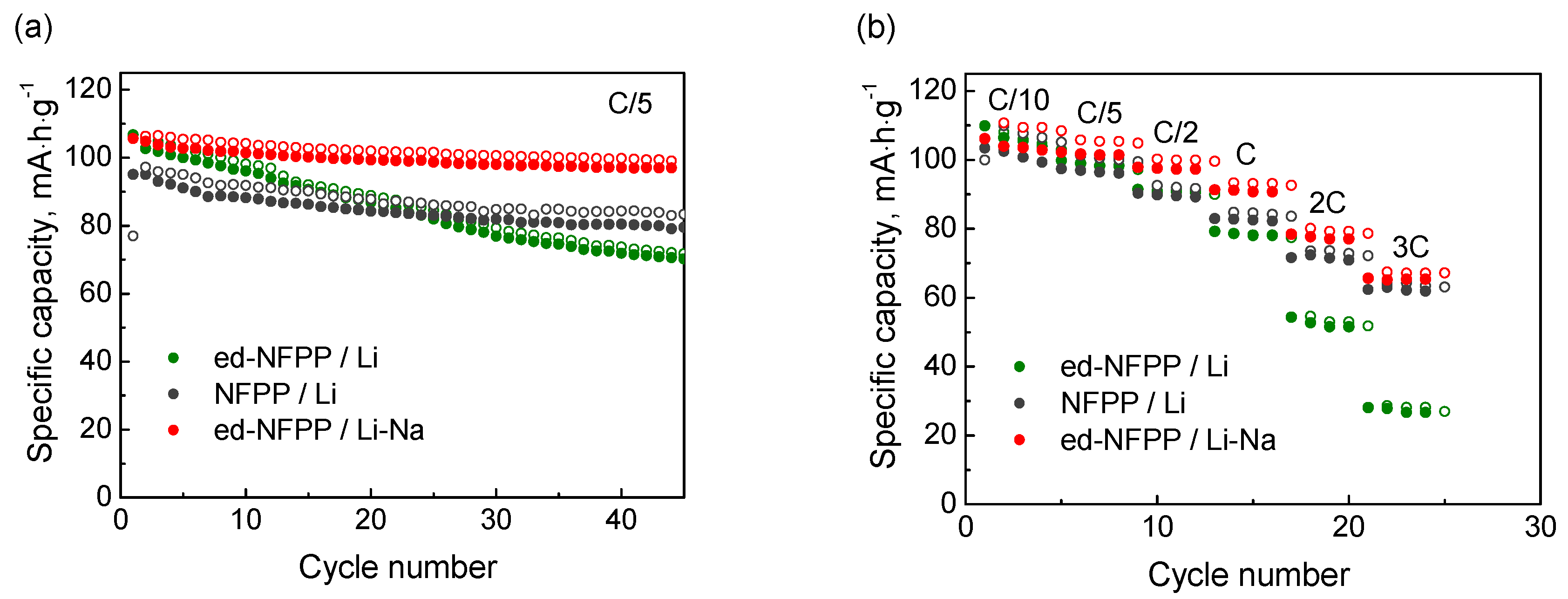
2.2. Electrochemical Cycling
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, B.; Murugesan, C.; Sharma, L.; Lochab, S.; Barpanda, P. An overview of mixed polyanionic cathode materials for sodium-ion batteries. Small Methods 2018, 1800253. [Google Scholar] [CrossRef]
- Sagane, F.; Abe, T.; Iriyama, Y.; Ogumi, Z. Li+ and Na+ transfer through interfaces between inorganic solid electrolytes and polymer or liquid electrolytes. J. Power Sources 2005, 146, 749–752. [Google Scholar] [CrossRef]
- Yao, H.-R.; You, Y.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Rechargeable dual-metal-ion batteries for advanced energy storage. Phys. Chem. Chem. Phys. 2016, 18, 9326–9333. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Gover, R.K.B.; Burns, P.; Bryan, A.J. Hybrid-ion a lithium-ion cell based on a sodium insertion material. Electrochem. Solid-State Lett. 2006, 9, A190–A192. [Google Scholar] [CrossRef]
- Barker, J.; Gover, R.K.B.; Burns, P.; Bryan, A.J. Li4/3Ti5/3O4 || Na3V2(PO4)2F3: An example of a hybrid-ion cell using a non-graphitic anode. J. Electrochem. Soc. 2007, 154, A882–A887. [Google Scholar] [CrossRef]
- Song, W.; Ji, X.; Pan, C.; Zhu, Y.; Chen, Q.; Banks, C.E. A Na3V2(PO4)3 cathode material for use in hybrid lithium ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 14357–14363. [Google Scholar] [CrossRef] [PubMed]
- Kosova, N.V.; Rezepova, D.O. Na1+yVPO4F1+y (0 ≤ y ≤ 0.5) as cathode materials for hybrid Na/Li batteries. Inorganics 2017, 5, 19. [Google Scholar] [CrossRef]
- Song, W.; Ji, X.; Wu, Z.; Zhu, Y.; Yao, Y.; Huangfu, K.; Chena, Q.; Banks, C.E. Na2FePO4F cathode utilized in hybrid-ion batteries: A mechanistic exploration of ion migration and diffusion capability. J. Mater. Chem. A 2014, 2, 2571–2577. [Google Scholar] [CrossRef]
- Kosova, N.V.; Podugolnikov, V.R.; Devyatkina, E.T.; Slobodyuk, A.B. Structure and electrochemistry of NaFePO4 and Na2FePO4F cathode materials prepared via mechanochemical route. Mater. Res. Bull. 2014, 60, 849–857. [Google Scholar] [CrossRef]
- Kosova, N.V.; Rezepova, D.O.; Petrov, S.A.; Slobodyuk, A.B. Electrochemical and chemical Na+/Li+ ion exchange in Na-based cathode materials: Na1.56Fe1.22P2O7 and Na3V2(PO4)2F3. J. Electrochem. Soc. 2017, 164, A6192–A6200. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Y.; Shao, H. Enhanced cycleability and dendrite-free lithium deposition by addition of sodium ion in electrolyte for lithium metal batteries. Electrochim. Acta 2018, 271, 617–623. [Google Scholar] [CrossRef]
- Kim, H.; Park, I.; Seo, D.-H.; Lee, S.; Kim, S.-W.; Kwon, W.J.; Park, Y.-U.; Kim, C.S.; Jeon, S.; Kang, K. New iron-based mixed-polyanion cathodes for lithium and sodium rechargeable batteries: Combined first principles calculations and experimental study. J. Am. Chem. Soc. 2012, 134, 10369–10372. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, I.; Lee, S.; Kim, H.; Park, K.-Y.; Park, Y.-U.; Kim, H.; Kim, J.; Lim, H.-D.; Yoon, W.-S.; et al. Understanding the electrochemical mechanism of the new iron-based mixed-phosphate Na4Fe3(PO4)2(P2O7) in a Na rechargeable battery. Chem. Mater. 2013, 25, 3614–3622. [Google Scholar] [CrossRef]
- Kosova, N.V.; Belotserkovsky, V.A. Sodium and mixed sodium/lithium iron ortho-pyrophosphates: Synthesis, structure and electrochemical properties. Electrochim. Acta 2018, 278, 182–195. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, G.; Yang, Y. Sol-gel synthesis of Na4Fe3(PO4)2(P2O7)/C nanocomposite for sodium ion batteries and new insights into microstructural evolution during sodium extraction. J. Power Sources 2016, 327, 666–674. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, Y.; Shao, H.; Yang, Y. Understanding the electrochemical mechanism of high sodium selective material Na3V2(PO4)2F3 in Li+/Na+ dual-ion batteries. Electrochim. Acta 2018, 292, 234–246. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Guyomard, D. New electrolyte compositions stable over the 0 to 5 V voltage range and compatible with the Li1+xMn2O4/carbon Li-ion cells. Solid State Ionics 1994, 69, 293–305. [Google Scholar] [CrossRef]
- Bhide, A.; Hofmann, J.; Durr, A.K.; Janek, J.; Adelhelm, P. Electrochemical stability of non-aqueous electrolytes for sodium-ion batteries and their compatibility with Na0.7CoO2. Phys. Chem. Chem. Phys. 2014, 161987. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, V.A.; Fedotov, S.S.; Vassiliev, S.Y.; Samarin, A.S.; Khasanova, N.R.; Antipov, E.V. Transport and kinetic aspects of alkali metal ions intercalation into AVPO4F framework. J. Electrochem. Soc. 2017, 164, A6373–A6380. [Google Scholar] [CrossRef]



| Sample | a, Å | b, Å | c, Å | V, Å3 | Rwp/χ2, % |
|---|---|---|---|---|---|
| NFPP (this work) | 18.082 (1) | 6.5385 (4) | 10.6541 (6) | 1259.64 (13) | 7.7/2.17 |
| NFPP [13] | 18.07517 (7) | 6.53238 (2) | 10.64760 (4) | 1257.204 (1) | -/3.55 |
| ed-NFPP (this work) | 17.635 (2) | 6.3921 (6) | 10.691 (1) | 1205.1 (2) | 9.7/2.63 |
| NFPP after chemical oxidation with NO2BF4 [14] | 17.6613 (9) | 6.3966 (4) | 10.7012 (7) | 1208.948 (1) | 2.42/3.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosova, N.V.; Shindrov, A.A. Effect of Mixed Li+/Na+-ion Electrolyte on Electrochemical Performance of Na4Fe3(PO4)2P2O7 in Hybrid Batteries. Batteries 2019, 5, 39. https://doi.org/10.3390/batteries5020039
Kosova NV, Shindrov AA. Effect of Mixed Li+/Na+-ion Electrolyte on Electrochemical Performance of Na4Fe3(PO4)2P2O7 in Hybrid Batteries. Batteries. 2019; 5(2):39. https://doi.org/10.3390/batteries5020039
Chicago/Turabian StyleKosova, Nina V., and Alexander A. Shindrov. 2019. "Effect of Mixed Li+/Na+-ion Electrolyte on Electrochemical Performance of Na4Fe3(PO4)2P2O7 in Hybrid Batteries" Batteries 5, no. 2: 39. https://doi.org/10.3390/batteries5020039





