Untreated Natural Graphite as a Graphene Source for High-Performance Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene
2.2. Sample Characterisation
2.3. Electrochemical Measurements
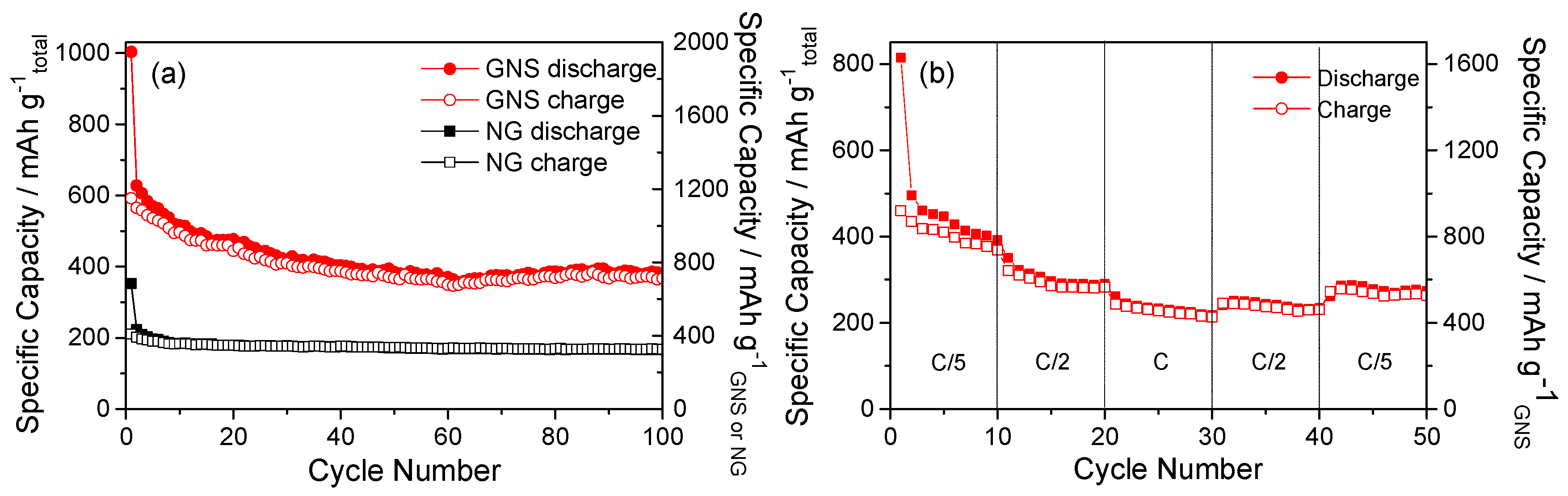
3. Results and Discussion
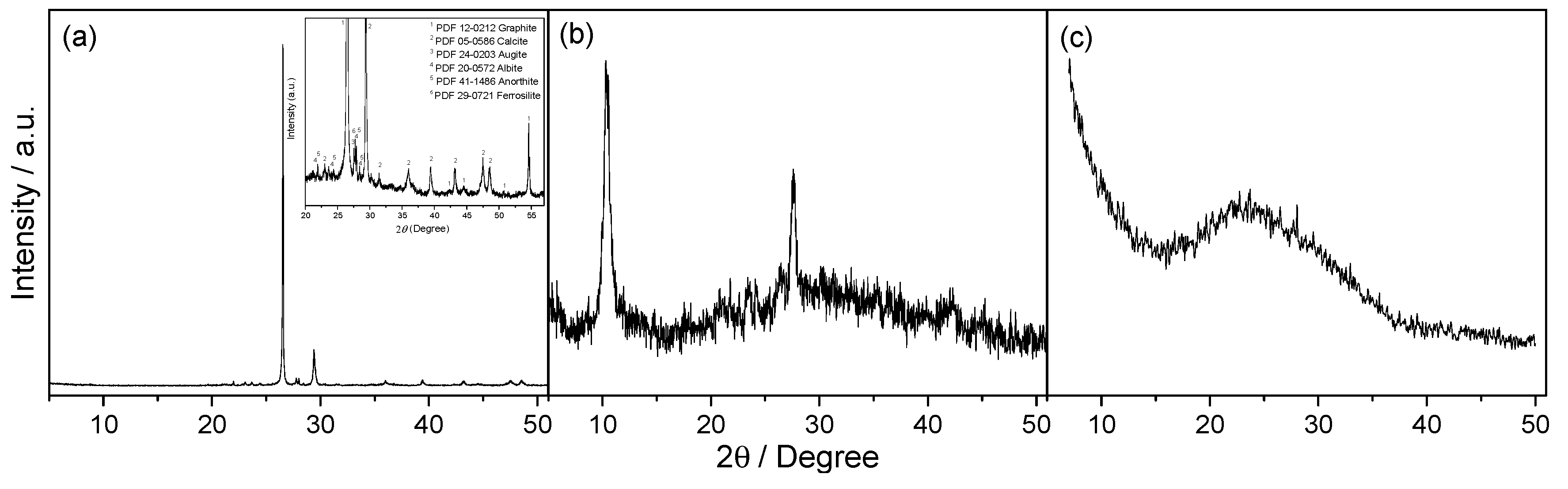
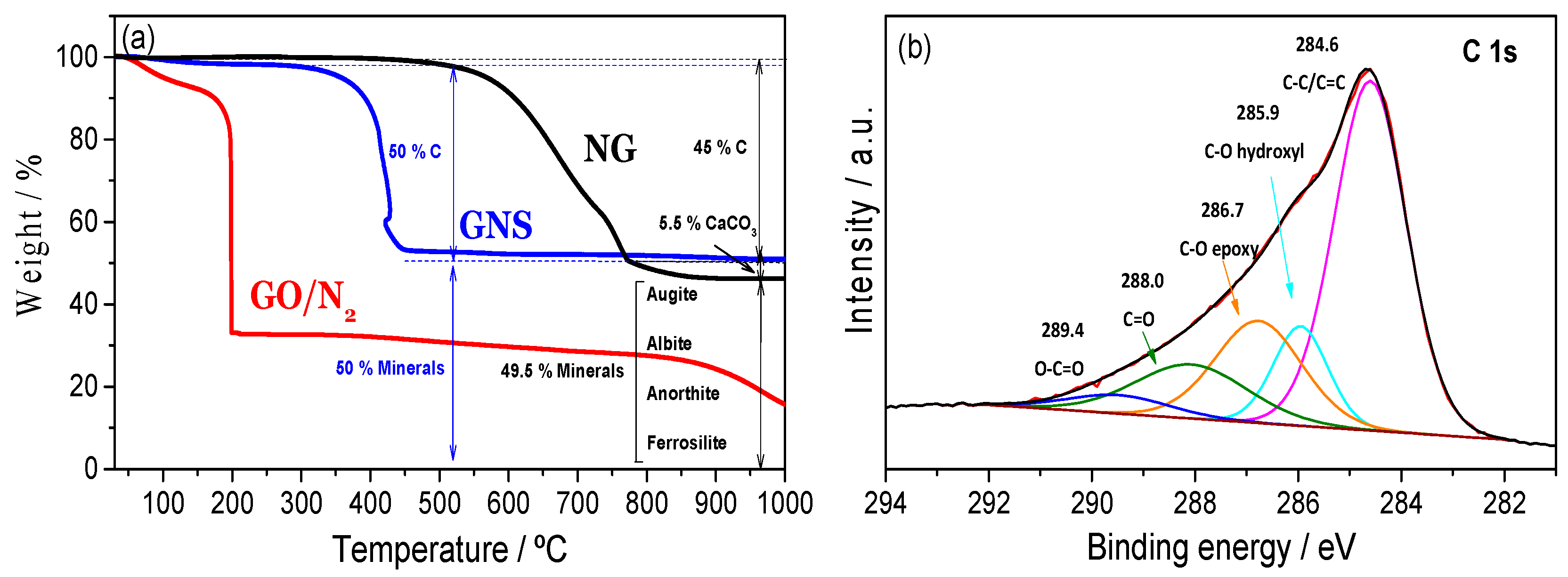
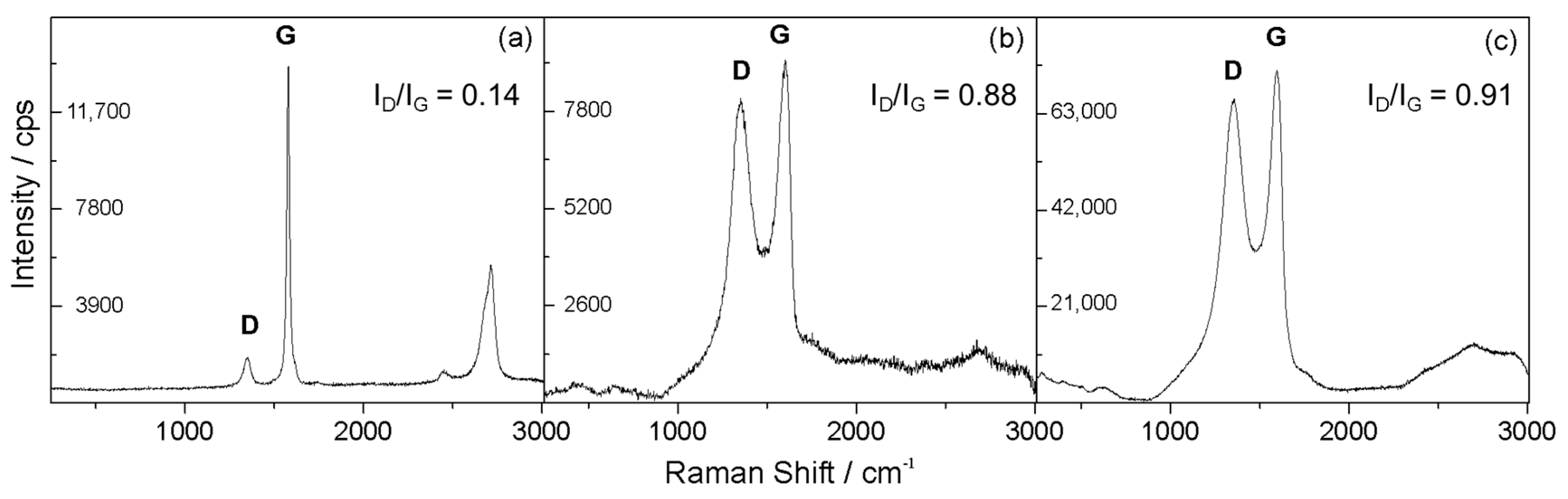
3.1. Structural, Compositional and Morphological Characterization
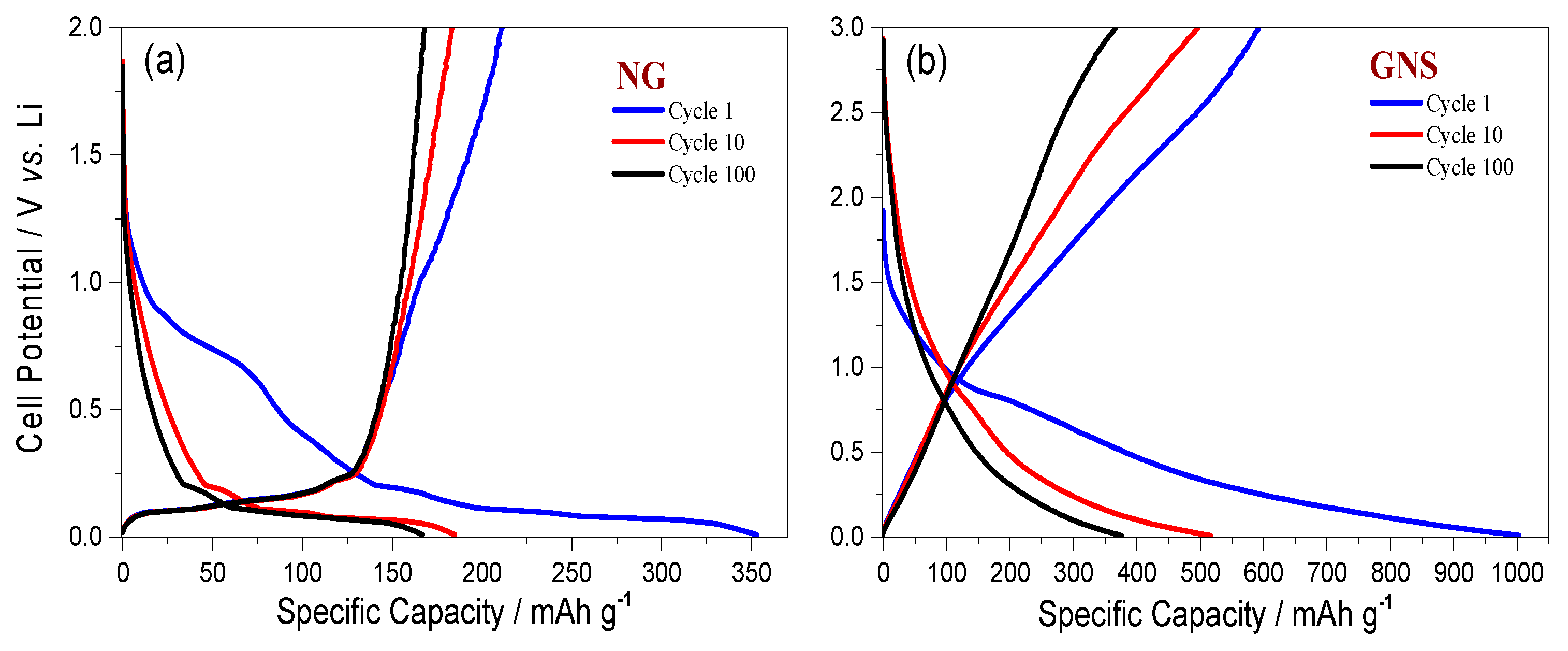
3.2. Electrochemical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, Y.Q.; Wu, Q.O.; Shi, G.Q. Graphene based new energy materials. Energy Environ. Sci. 2011, 4, 1113–1132. [Google Scholar] [CrossRef]
- Liang, M.H.; Zhi, L.J. Graphene-based electrode materials for rechargeable lithium batteries. J. Mater. Chem. 2009, 19, 5871–5878. [Google Scholar] [CrossRef]
- Guo, F.; Silverberg, G.; Bowers, S.; Kim, S.P.; Datta, D.; Shenoy, V.; Hurt, R.H. Graphene-Based Environmental Barriers. Environ. Sci. Technol. 2012, 46, 7717–7724. [Google Scholar] [CrossRef] [PubMed]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Erdem, E. High-Capacitance Hybrid Supercapacitor Based on Multi-Colored Fluorescent Carbon-Dots. Sci. Rep. 2017, 7, 11222. [Google Scholar] [CrossRef] [PubMed]
- Repp, S.; Harputlu, E.; Gurgen, S.; Castellano, M.; Kremer, N.; Pompe, N.; Worner, J.; Hoffmann, A.; Thomann, R.; Emen, F.M.; et al. Synergetic effects of Fe3+ doped spinel Li4Ti5O12 nanoparticles on reduced graphene oxide for high surface electrode hybrid supercapacitors. Nanoscale 2018, 10, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Tang, H.; Kou, Z.; Pan, M.; Sun, X.; Zhang, J.; Mu, S. Engineered Graphene Materials: Synthesis and Applications for Polymer Electrolyte Membrane Fuel Cells. Adv. Mater. 2017, 29, 1601741. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Wei, Q.L.; Zhan, X.X.; Zhang, G.X.; Sun, S.H. The New Graphene Family Materials: Synthesis and Applications in Oxygen Reduction Reaction. Catalyst 2017, 7, 1. [Google Scholar] [CrossRef]
- Low, F.W.; Lai, C.W. Recent developments of graphene-TiO2 composite nanomaterials as efficient photoelectrodes in dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 82, 103–125. [Google Scholar] [CrossRef]
- Eck, M.; Pham, C.V.; Züfle, S.; Neukom, M.; Sessler, M.; Scheunemann, D.; Erdem, E.; Weber, S.; Borchert, H.; Ruhstaller, B.; et al. Improved efficiency of bulk heterojunction hybrid solar cells by utilizing CdSe quantum dot–graphene nanocomposites. Phys. Chem. Chem. Phys. 2014, 16, 12251–12260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials for energy storage. Energy Environ. Sci. 2011, 4, 668–674. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Carbon, Graphite, Diamond and Fullerenes: Properties, Processing and Applications; Noyes Publications: Park Ridge, NJ, USA, 1993. [Google Scholar]
- Olson, D.W. 2010 Minerals Yearbook: Graphite; United States Geological Survey (USGS): Reston, VA, USA, 2012; Volume 32.
- Wu, Z.S.; Ren, W.C.; Gao, L.B.; Liu, B.L.; Jiang, C.B.; Cheng, H.M. Synthesis of high-quality graphene with a pre-determined number of layers. Carbon 2009, 47, 493–499. [Google Scholar] [CrossRef]
- Badenhorst, H. Microstructure of natural graphite flakes revealed by oxidation: Limitations of XRD and Raman techniques for crystallinity estimates. Carbon 2014, 66, 674–690. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhu, S.F.; Wang, L. Purification of natural graphite by microwave assisted acid leaching. Carbon 2013, 55, 377–378. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Chen, B.; Gao, X.-M. Depuration of scaly graphites by high temperature graphitization. Carbon Tech. 2001, 2, 39–40. [Google Scholar]
- Kuang, J.; Xu, H.; Xie, W.; Tang, W.; Deng, Y.; Wang, T.; Peng, S. Investigation of Purification Technology for Aphanitic Graphite by Ammonium Fluoride and Hydrochloric Acid. Mater. Rev. 2013, 27, 9–12. [Google Scholar]
- Rao, R.B.; Patnaik, N. Preparation of high pure graphite by alkali digestion method. Scand. J. Metall. 2004, 33, 257–260. [Google Scholar] [CrossRef]
- Cohen-Solal, M.E.; Baudoin, C.; Omouri, M.; Kuntz, D.; De Vernejoul, M.C. Bone mass in middle-aged osteoporotic men and their relatives: Familial effect. J. Bone Miner. Res. 1998, 13, 1909. [Google Scholar] [CrossRef] [PubMed]
- Rodas, M.; Barrenechea, J.F.; Luque, F.J. Fluid-deposited graphite: The mineralization from Huelma (Jaén). Geogaceta 1996, 20, 1573. [Google Scholar]
- Vargas, O.A.; Caballero, A.; Morales, J. Can the performance of graphene nanosheets for lithium storage in Li-ion batteries be predicted? Nanoscale 2012, 4, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.M.; Crain, J.N.; Guisinger, N.P.; Li, T.; First, P.N.; Stroscio, J.A. Scattering and interference in epitaxial graphene. Science 2007, 317, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.I.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Ren, W.C.; Xu, L.; Li, F.; Cheng, H.M. Doped Graphene Sheets As Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Abouimrane, A.; Compton, O.C.; Amine, K.; Nguyen, S.T. Non-Annealed Graphene Paper as a Binder-Free Anode for Lithium-Ion Batteries. J. Phys. Chem. C 2010, 114, 12800–12804. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A Green Approach to the Synthesis of Graphene Nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.H.; Wang, Y.Y.; Yu, T.; Shen, Z.X. Raman Spectroscopy and Imaging of Graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Holtstiege, F.; Bärmann, P.; Nölle, R.; Winter, M.; Placke, T. Pre-Lithiation Strategies for Rechargeable Energy Storage Technologies: Concepts, Promises and Challenges. Batteries 2018, 4, 4. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.Y.; Zhang, Y.F.; Sun, C.C.; Yan, Q.Y.; Dong, X.C. Cobalt silicate hierarchical hollow spheres for lithium-ion batteries. Nanotechnology 2016, 27, 7. [Google Scholar] [CrossRef] [PubMed]
- Arrebola, J.C.; Caballero, A.; Hernan, L.; Morales, J. Graphitized Carbons of Variable Morphology and Crystallinity: A Comparative Study of Their Performance in Lithium Cells. J. Electrochem. Soc. 2009, 156, A986–A992. [Google Scholar] [CrossRef]
- Hassoun, J.; Bonaccorso, F.; Agostini, M.; Angelucci, M.; Betti, M.G.; Cingolani, R.; Gemmi, M.; Mariani, C.; Panero, S.; Pellegrini, V.; et al. An Advanced Lithium-Ion Battery Based on a Graphene Anode and a Lithium Iron Phosphate Cathode. Nano Lett. 2014, 14, 4901–4906. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Xu, Y.; Wang, X.; Shi, X.; Mulder, F.M. The electrochemical performance of super P carbon black in reversible Li/Na ion uptake. Sci. China Phys. Mech. Astron. 2017, 60, 064611. [Google Scholar] [CrossRef]
- Chen, S.; Bao, P.; Xiao, L.; Wang, G. Large-scale and low cost synthesis of graphene as high capacity anode materials for lithium-ion batteries. Carbon 2013, 64, 158–169. [Google Scholar] [CrossRef]

| Element | NG | GO | GNS |
|---|---|---|---|
| Carbon | 45.73 | 34.78 | 41.84 |
| Hydrogen | 0.36 | 1.79 | 0.97 |
| Nitrogen | 0.40 | 0.87 | 1.28 |
| Sulfur | 0.01 | 1.63 | 0.98 |
| Oxygen | 13.58 | 37.46 | 19.22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simón, M.; Benítez, A.; Caballero, A.; Morales, J.; Vargas, O. Untreated Natural Graphite as a Graphene Source for High-Performance Li-Ion Batteries. Batteries 2018, 4, 13. https://doi.org/10.3390/batteries4010013
Simón M, Benítez A, Caballero A, Morales J, Vargas O. Untreated Natural Graphite as a Graphene Source for High-Performance Li-Ion Batteries. Batteries. 2018; 4(1):13. https://doi.org/10.3390/batteries4010013
Chicago/Turabian StyleSimón, María, Almudena Benítez, Alvaro Caballero, Julián Morales, and Oscar Vargas. 2018. "Untreated Natural Graphite as a Graphene Source for High-Performance Li-Ion Batteries" Batteries 4, no. 1: 13. https://doi.org/10.3390/batteries4010013





