Pyrrolidinium FSI and TFSI-Based Polymerized Ionic Liquids as Electrolytes for High-Temperature Lithium-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Stability
2.2. Ionic Conductivities
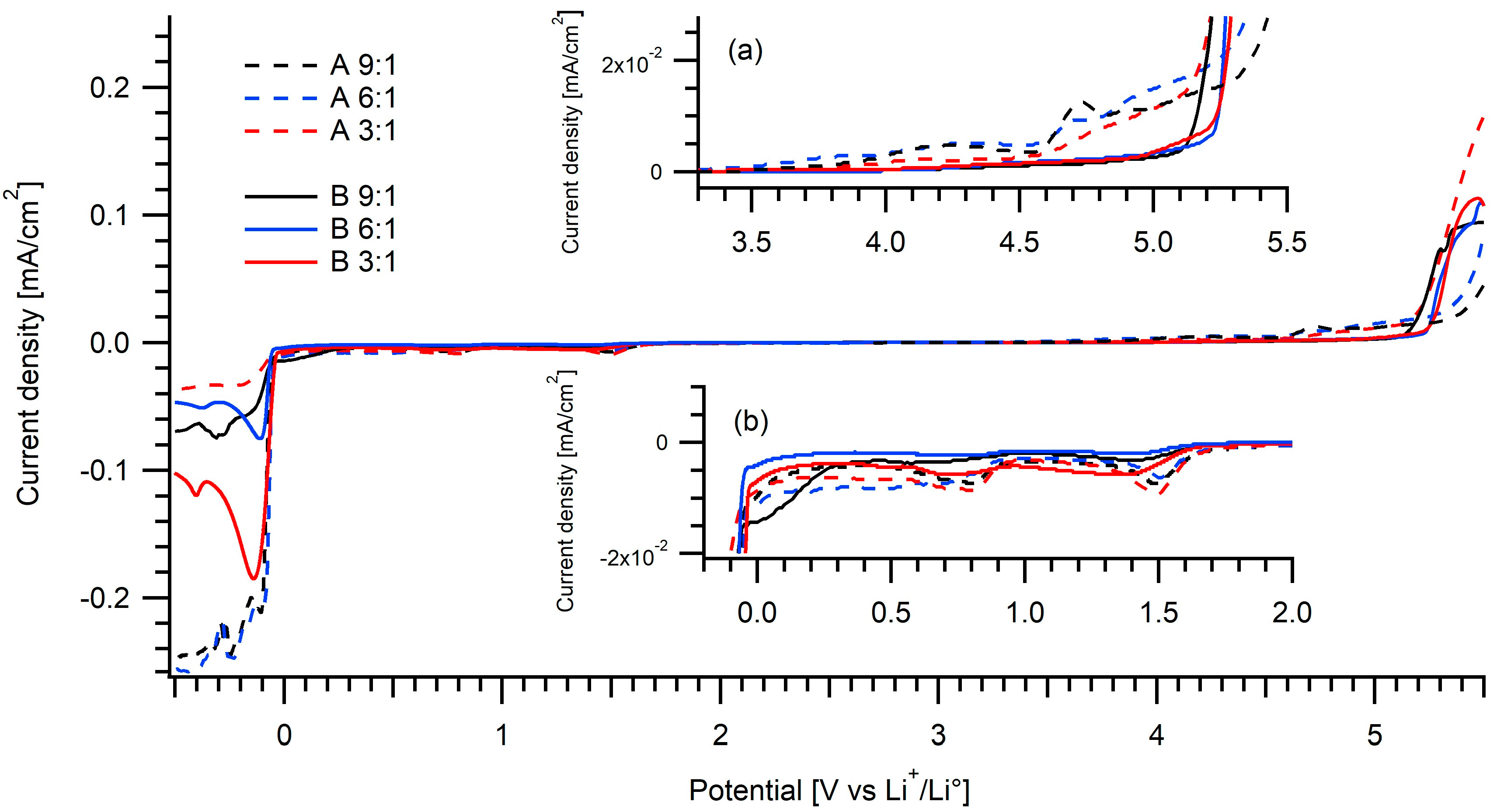
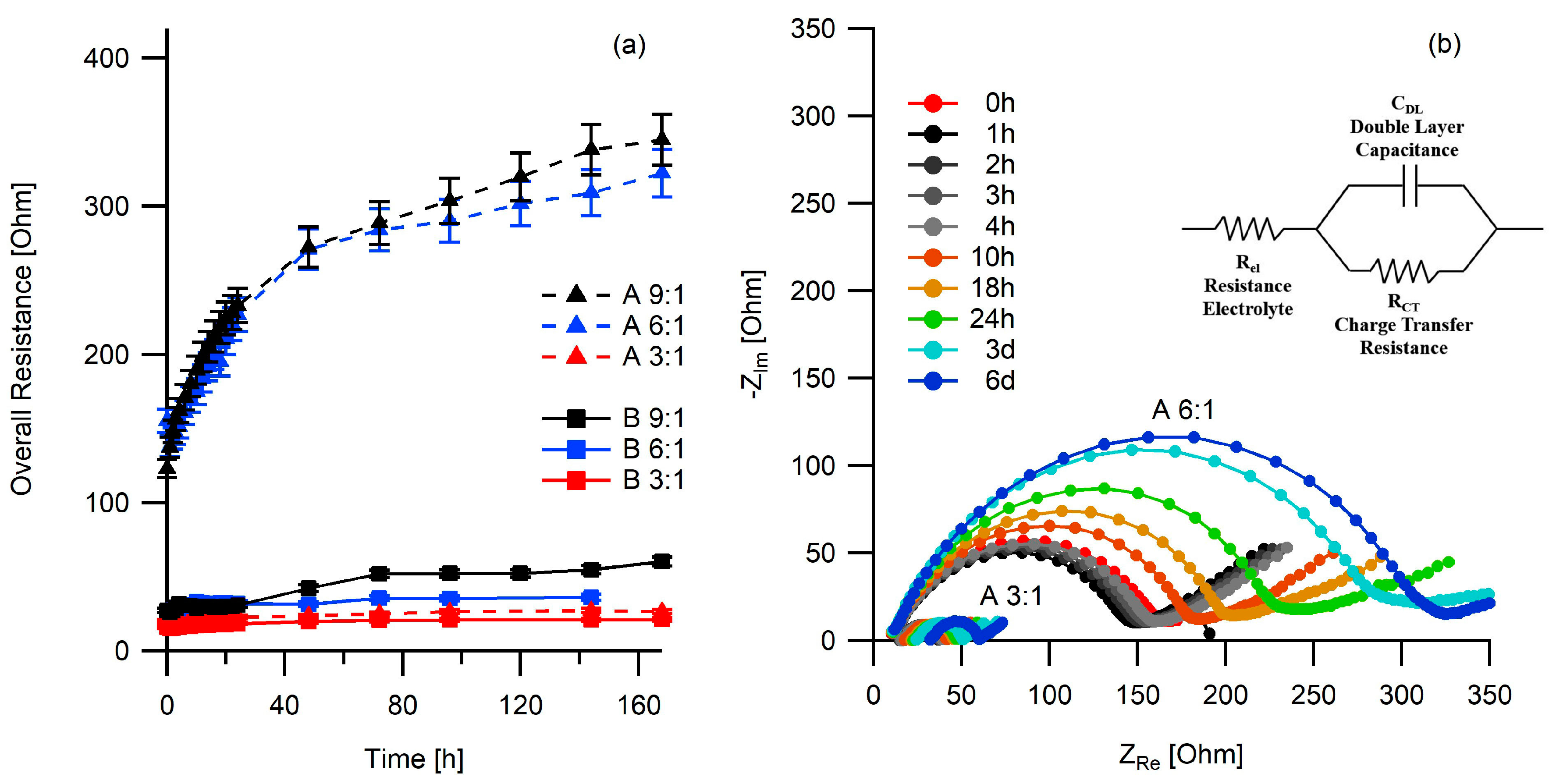
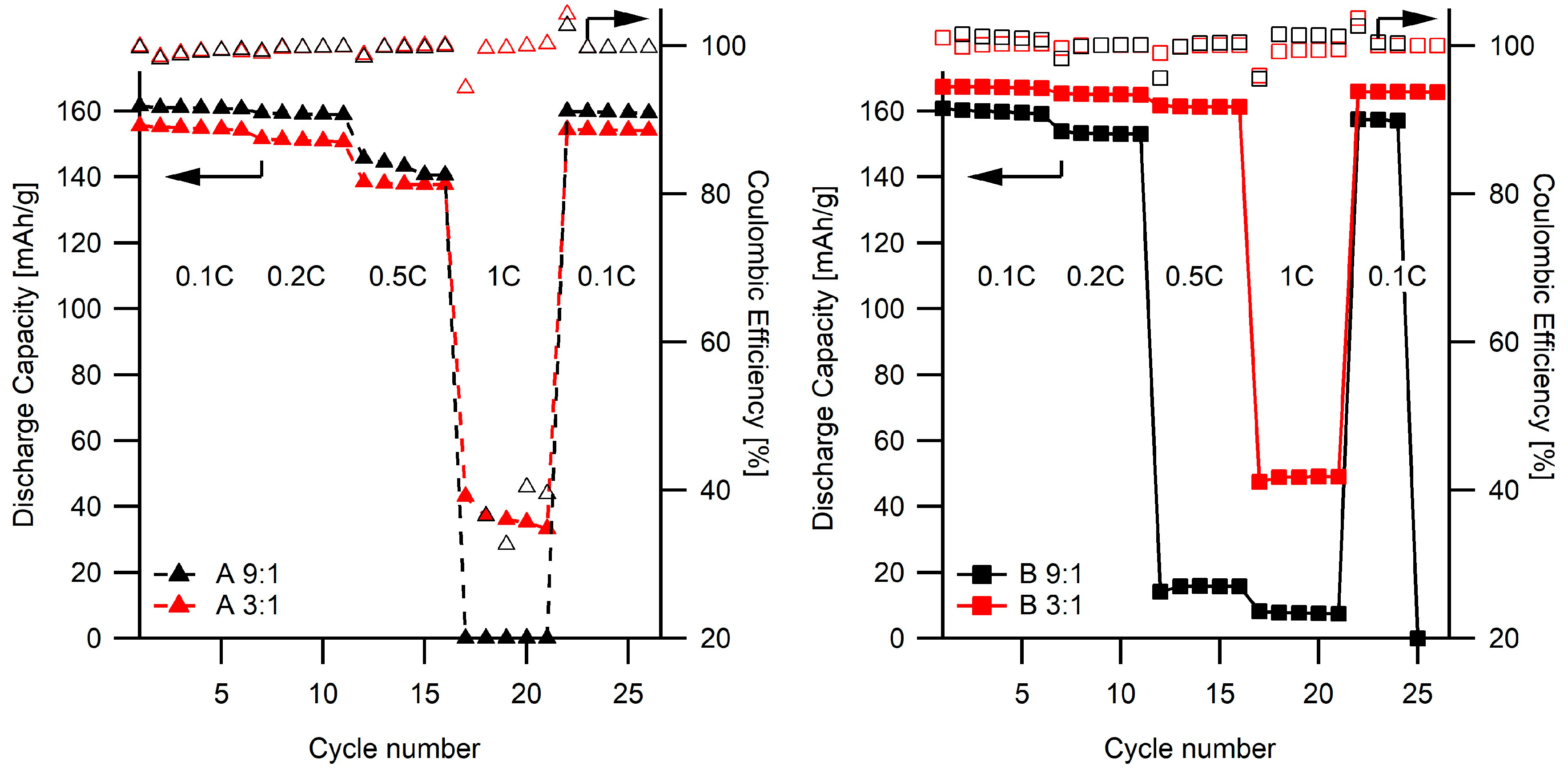
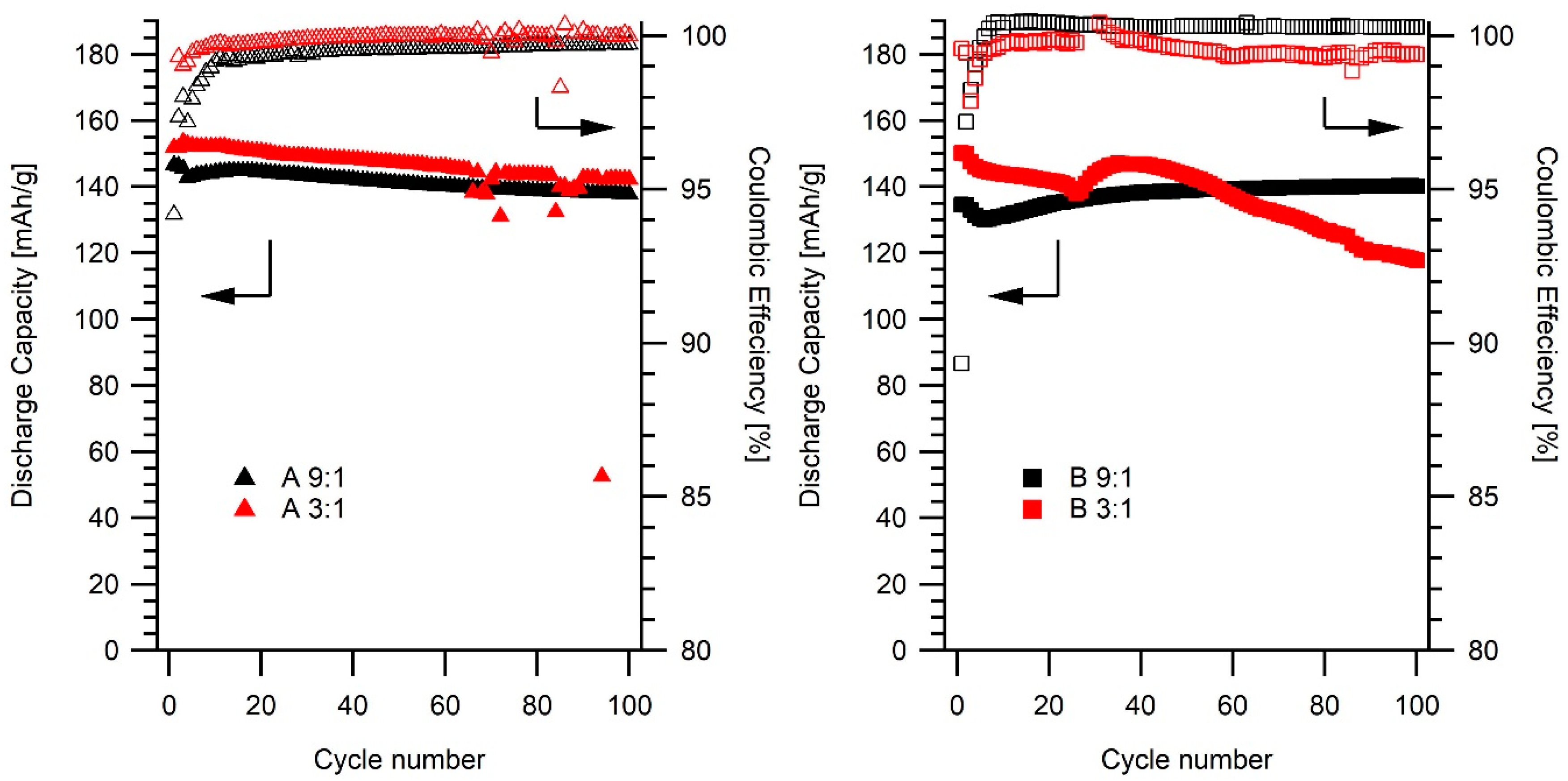
2.3. Electrochemical Studies
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Sloop, S.E.; Pugh, J.K.; Wang, S.; Kerr, J.B.; Kinoshita, K. Chemical Reactivity of PF5 and LiPF6 in Ethylene Carbonate/Dimethyl Carbonate Solutions. Electrochem. Solid-State Lett. 2001, 4, A42–A44. [Google Scholar] [CrossRef]
- Andersson, A.M.; Edstroöm, K. Chemical Composition and Morphology of the Elevated Temperature SEI on Graphite. J. Electrochem. Soc. 2001, 148, A1100–A1109. [Google Scholar] [CrossRef]
- Lang, G.; Kitanoski, F.; Kussmann, C. Principal Aspects and Simulation of a Hybrid Demonstrator Vehicle’s Cooling System. SAE Tech. Pap. 2007, 1–3483. [Google Scholar] [CrossRef]
- Santee, S.; Xiao, A.; Yang, L.; Gnanaraj, J.; Lucht, B.L. Effect of combinations of additives on the performance of lithium ion batteries. J. Power Sources 2009, 194, 1053–1060. [Google Scholar] [CrossRef]
- Kerner, M.; Plylahan, N.; Scheers, J.; Johansson, P. Ionic liquid based lithium battery electrolytes: Fundamental benefits of utilising both TFSI and FSI anions? Phys. Chem. Chem. Phys. 2015, 17, 19569–19581. [Google Scholar] [CrossRef] [PubMed]
- Plylahan, N.; Kerner, M.; Lim, D.-H.; Matic, A.; Johansson, P. Ionic liquid and hybrid ionic liquid/organic electrolytes for high temperature lithium-ion battery application. Electrochim. Acta 2016, 216, 24–34. [Google Scholar] [CrossRef]
- Bergman, M.; Bergfelt, A.; Sun, B.; Bowden, T.; Brandell, D.; Johansson, P. Graft copolymer electrolytes for high temperature Li-battery applications, using poly(methyl methacrylate) grafted poly(ethylene glycol)methyl ether methacrylate and lithium bis(trifluoromethanesulfonimide). Electrochim. Acta 2015, 175, 96–103. [Google Scholar] [CrossRef]
- Stephan, A.M.; Nahm, K.S. Review on composite polymer electrolytes for lithium batteries. Polymer (Guildf) 2006, 47, 5952–5964. [Google Scholar] [CrossRef]
- Armand, M. Polymers with Ionic Conductivity. Adv. Mater. 1990, 2, 278–286. [Google Scholar] [CrossRef]
- Kelly, I.E.; Owen, J.R.; Steele, B.C.H. Poly(ethylene oxide) electrolytes for operation at near room temperature. J. Power Sources 1985, 14, 13–21. [Google Scholar] [CrossRef]
- Bolloré, Electric Vehicles, Solutions, 2017. Available online: http://www.bollore.com/en-us/activities/electricity-storage-and-solutions/electric-vehicles-solutions (accessed on 27 October 2017).
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Frömling, T.; Kunze, M.; Schönhoff, M.; Sundermeyer, J.; Roling, B. Enhanced lithium transference numbers in ionic liquid electrolytes. J. Phys. Chem. B 2008, 112, 12985–12990. [Google Scholar] [CrossRef] [PubMed]
- Guyomard-Lack, A.; Said, B.; Dupré, N.; Galarneau, A.; Le Bideau, J. Enhancement of lithium transport by controlling the mesoporosity of silica monoliths filled by ionic liquids. New J. Chem. 2016, 40, 4269–4276. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent Advances in Innovative Polymer Electrolytes based on Poly(ionic liquid)s. Electrochim. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Salamone, J.C.; Israel, S.C.; Taylor, P.; Snidert, B. Synthesis and homopolymerization studies of vinylimidazolium salts. Polymer (Guildf) 1973, 14, 639–644. [Google Scholar] [CrossRef]
- Watanabe, M.; Yamada, S.-I.; Sanui, K.; Ogata, N. High ionic conductivity of new polymer electrolytes consisting of polypyridinium, pyridinium and aluminium chloride. J. Chem. Soc. Chem. Commun. 1993, 929. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Vlasov, P.S.; Lozinskaya, E.I.; Ponkratov, D.O.; Malyshkina, I.A.; Vidal, F.; Okatova, O.V.; Pavlov, G.M.; Wandrey, C.; Bhide, A.; et al. Polymeric ionic liquids: Comparison of polycations and polyanions. Macromolecules 2011, 44, 9792–9803. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Pont, A.-L.; Marcilla, R.; de Meatza, I.; Grande, H.; Mecerreyes, D. Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes. J. Power Sources 2009, 188, 558–563. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Kim, G.T.; Montanino, M.; Carewska, M.; Marcilla, R.; Mecerreyes, D.; De Meatza, I. Ternary polymer electrolytes containing pyrrolidinium-based polymeric ionic liquids for lithium batteries. J. Power Sources 2010, 195, 3668–3675. [Google Scholar] [CrossRef]
- Safa, M.; Chamaani, A.; Chawla, N.; El-Zahab, B. Polymeric Ionic Liquid Gel Electrolyte for Room Temperature Lithium Battery Applications. Electrochim. Acta 2016, 213, 587–593. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Girard, G.M.A.; Yunis, R.; MacFarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howletta, P.C.; Forsyth, M. Preparation and characterization of gel polymer electrolytes using poly(ionic liquids) and high lithium salt concentration ionic liquids. J. Mater. Chem. A 2017, 5, 23844–23852. [Google Scholar] [CrossRef]
- Sako, T.; Miyagawa, A.; Yamaguchi, M. Modulus enhancement of polycarbonate by addition of lithium perchlorate. J. Appl. Polym. Sci. 2017, 134, 1–5. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Hayamizu, K.; Seki, S. Origin of the Low-Viscosity of [emim][(FSO2)2N] Ionic Liquid and Its Lithium Salt Mixture: Experimental and Theoretical Study of Self-Diffusion Coefficients, Conductivities, and Intermolecular Interactions. J. Phys. Chem. B 2010, 114, 16329–16336. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Henderson, W.A.; Appetecchi, G.B.; Alessandrini, F.; Passerini, S. Recent developments in the ENEA lithium metal battery project. Electrochim. Acta 2005, 50, 3859–3865. [Google Scholar] [CrossRef]
- Kühnel, R.-S.; Böckenfeld, N.; Passerini, S.; Winter, M.; Balducci, A. Mixtures of ionic liquid and organic carbonate as electrolyte with improved safety and performance for rechargeable lithium batteries. Electrochim. Acta 2011, 56, 4092–4099. [Google Scholar] [CrossRef]
- Agostini, M.; Ulissi, U.; di Lecce, D.; Ahiara, Y.; Ito, S.; Hassoun, J. A Lithium-Ion Battery based on an Ionic Liquid Electrolyte, Tin-Carbon Nanostructured Anode, and Li2O-ZrO2-Coated Li[Ni0.8Co0.15Al0.05]O2 Cathode. Energy Technol. 2015, 3, 632–637. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.C.; Hinokuma, K. Optimized LiFePO4 for Lithium Battery Cathodes. J. Electrochem. Soc. 2001, 148, A224. [Google Scholar] [CrossRef]
- Xu, C.; Sun, B.; Gustafsson, T.; Edström, K.; Brandell, D.; Hahlin, M. Interface layer formation in solid polymer electrolyte lithium batteries: An XPS study. J. Mater. Chem. A 2014, 2, 7256–7264. [Google Scholar] [CrossRef]
- Li, M.; Yang, B.; Wang, L.; Zhang, Y.; Zhang, Z.; Fang, S.; Zhang, Z. New polymerized ionic liquid (PIL) gel electrolyte membranes based on tetraalkylammonium cations for lithium ion batteries. J. Memb. Sci. 2013, 447, 222–227. [Google Scholar] [CrossRef]
- Boukamp, B.A. A package for impedance/admittance data analysis. Solid State Ion. 1986, 18–19, 136–140. [Google Scholar] [CrossRef]
- Boukamp, B.A. A nonlinear least squares fit procedure for analysis of immittancs data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, J.W.; Cheruvally, G.; Kim, J.U.; Ahn, J.H.; Cho, G.B.; Kim, K.-W.; Ahn, H.-J. A modified mechanical activation synthesis for carbon-coated LiFePO4 cathode in lithium batteries. Mater. Lett. 2007, 61, 3822–3825. [Google Scholar] [CrossRef]
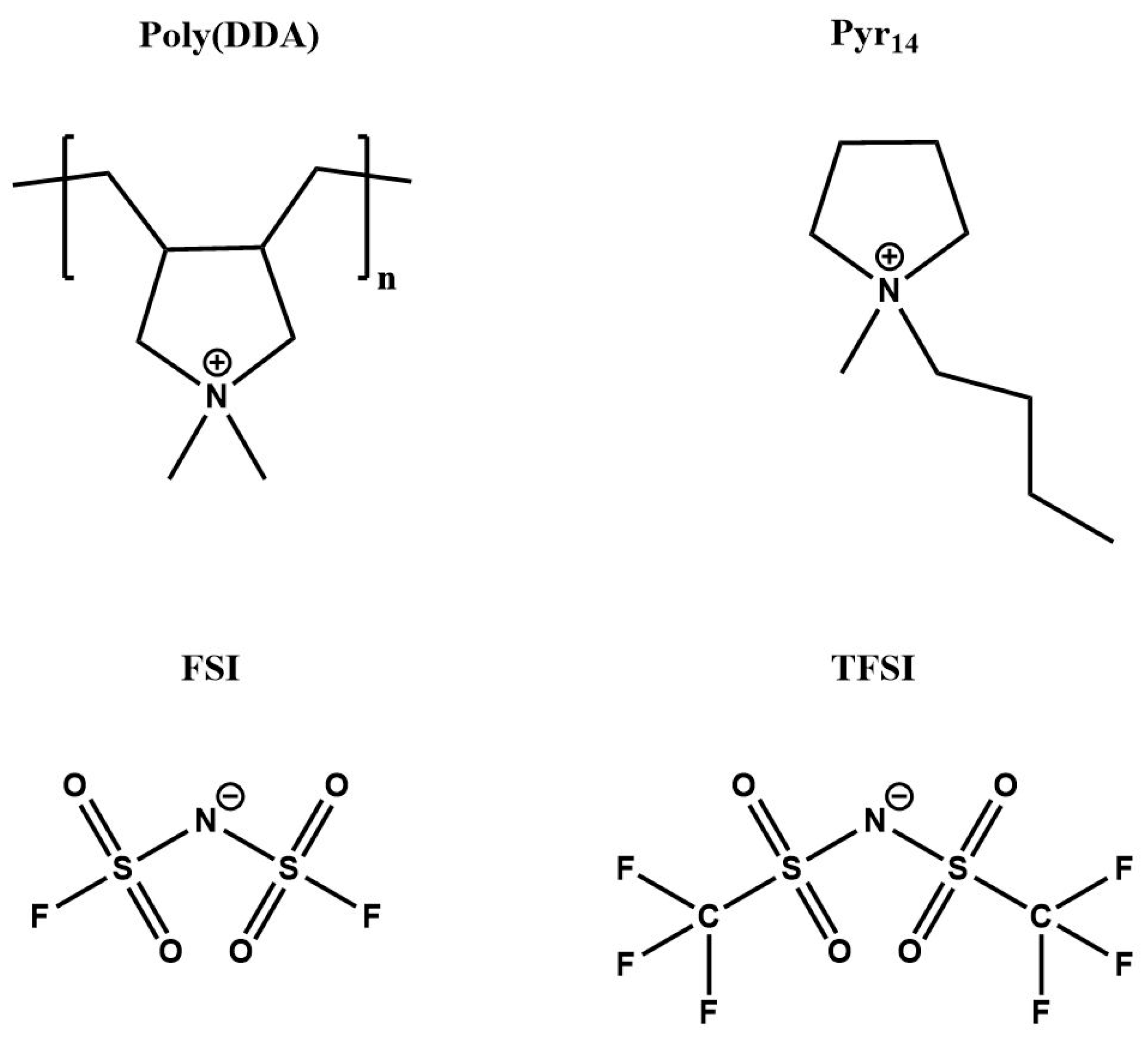
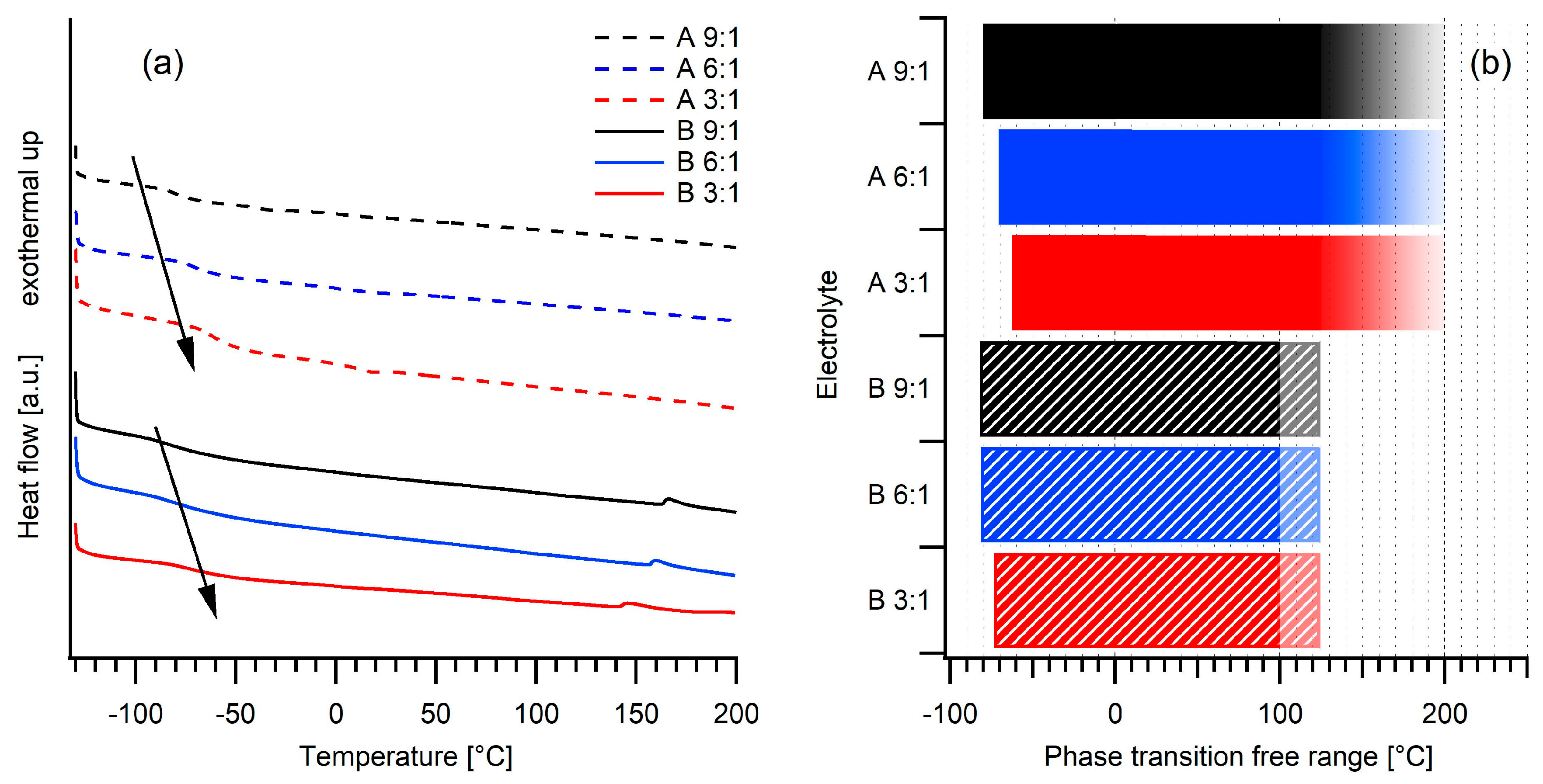
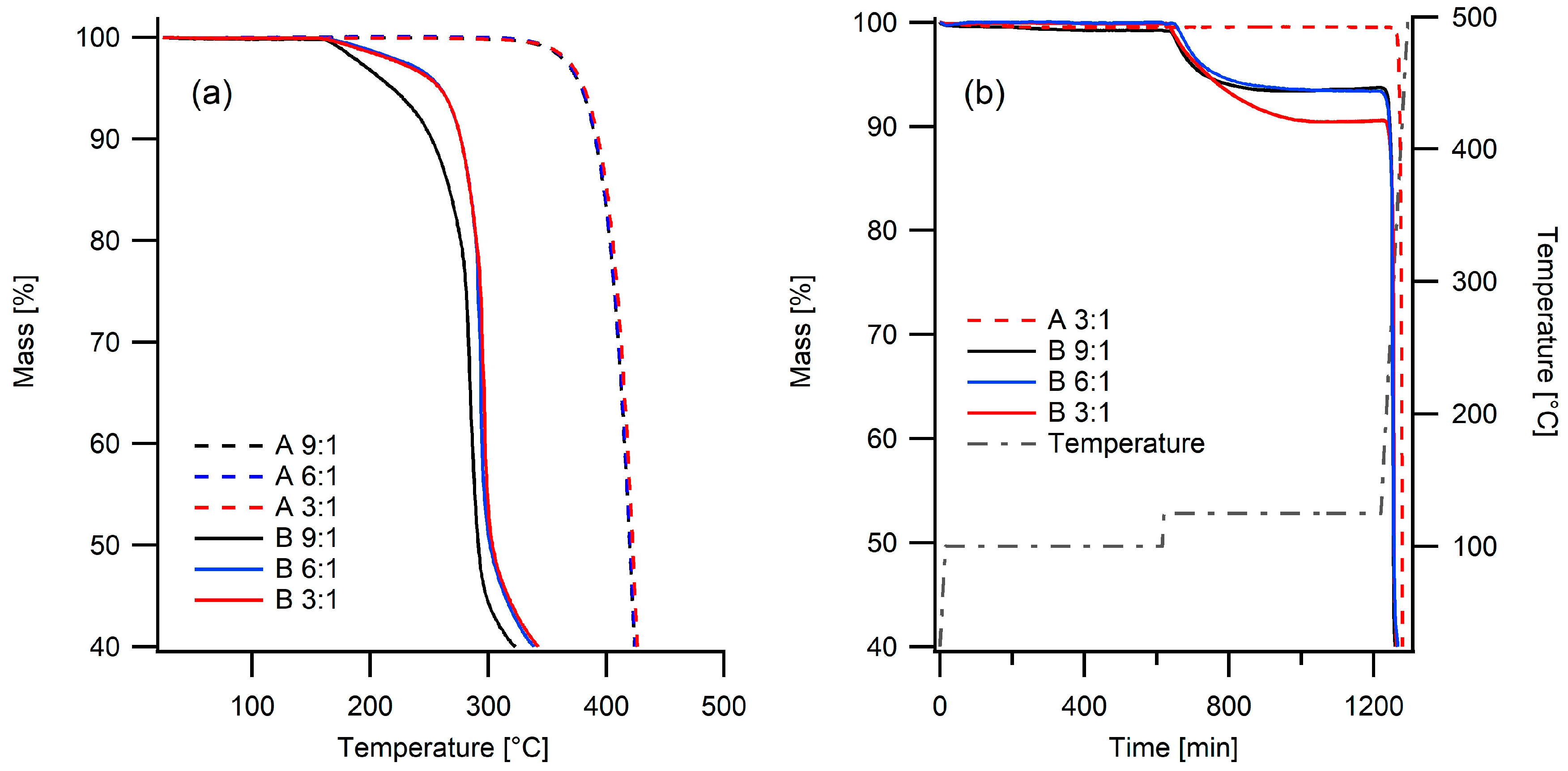
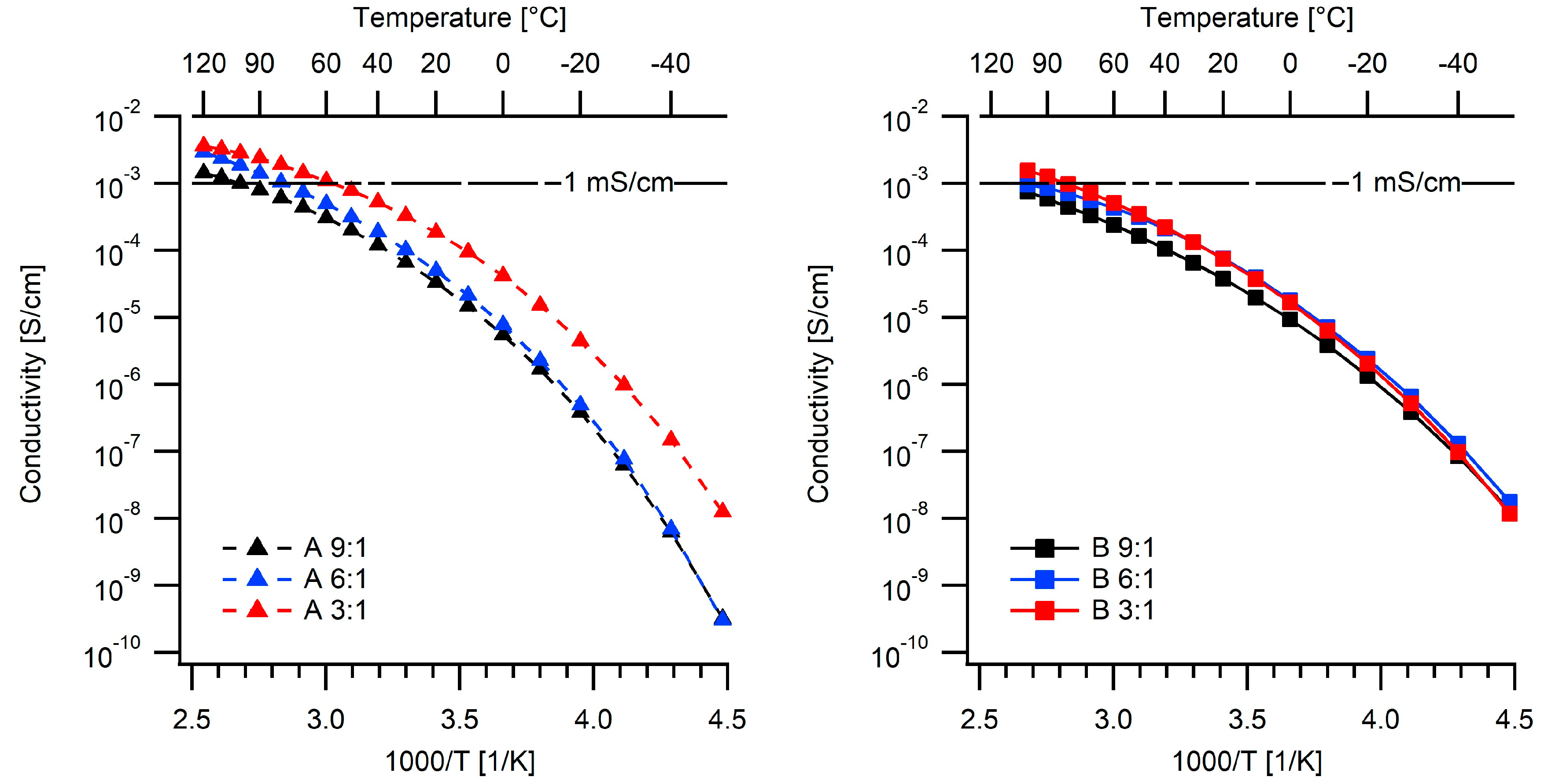

| Electrolyte | Tg (°C) | Td (°C) | σ20°C (S/cm) | σ80°C (S/cm) |
|---|---|---|---|---|
| A 9:1 | −81 | 349 | 3.3 × 10−5 | 6.0 × 10−4 |
| A 6:1 | −72 | 352 | 5.0 × 10−5 | 1.0 × 10−3 |
| A 3:1 | −63 | 352 | 1.9 × 10−4 | 1.9 × 10−3 |
| B 9:1 | −82 | 174 | 3.8 × 10−5 | 4.5 × 10−4 |
| B 6:1 | −82 | 193 | 7.7 × 10−5 | 7.1 × 10−4 |
| B 3:1 | −74 | 188 | 7.4 × 10−5 | 1.0 × 10−3 |
| A | B | |||||
|---|---|---|---|---|---|---|
| XIL/XLiTFSI | ||||||
| 9:1 | 6:1 | 3:1 | 9:1 | 6:1 | 3:1 | |
| wt% | ||||||
| LiTFSI | 2.9 | 4.4 | 8.4 | 3.8 | 5.6 | 10.5 |
| Pyr14TFSI | 39.1 | 38.5 | 36.9 | - | - | - |
| Pyr14FSI | - | - | - | 38.2 | 37.5 | 35.5 |
| Poly(DDA)TFSI | 58.0 | 57.2 | 54.8 | - | - | - |
| Poly(DDA)FSI | - | - | - | 58.0 | 56.9 | 53.9 |
| Name | A 9:1 | A 6:1 | A 3:1 | B 9:1 | B 6:1 | B 3:1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerner, M.; Johansson, P. Pyrrolidinium FSI and TFSI-Based Polymerized Ionic Liquids as Electrolytes for High-Temperature Lithium-Ion Batteries. Batteries 2018, 4, 10. https://doi.org/10.3390/batteries4010010
Kerner M, Johansson P. Pyrrolidinium FSI and TFSI-Based Polymerized Ionic Liquids as Electrolytes for High-Temperature Lithium-Ion Batteries. Batteries. 2018; 4(1):10. https://doi.org/10.3390/batteries4010010
Chicago/Turabian StyleKerner, Manfred, and Patrik Johansson. 2018. "Pyrrolidinium FSI and TFSI-Based Polymerized Ionic Liquids as Electrolytes for High-Temperature Lithium-Ion Batteries" Batteries 4, no. 1: 10. https://doi.org/10.3390/batteries4010010





