The Importance of Rare-Earth Additions in Zr-Based AB2 Metal Hydride Alloys
Abstract
:1. Introduction
2. Experimental Setup
3. Property Comparison of Elements and Intermetallic Compounds of Y, La, Ce, Pr, and Nd
4. Results and Discussion
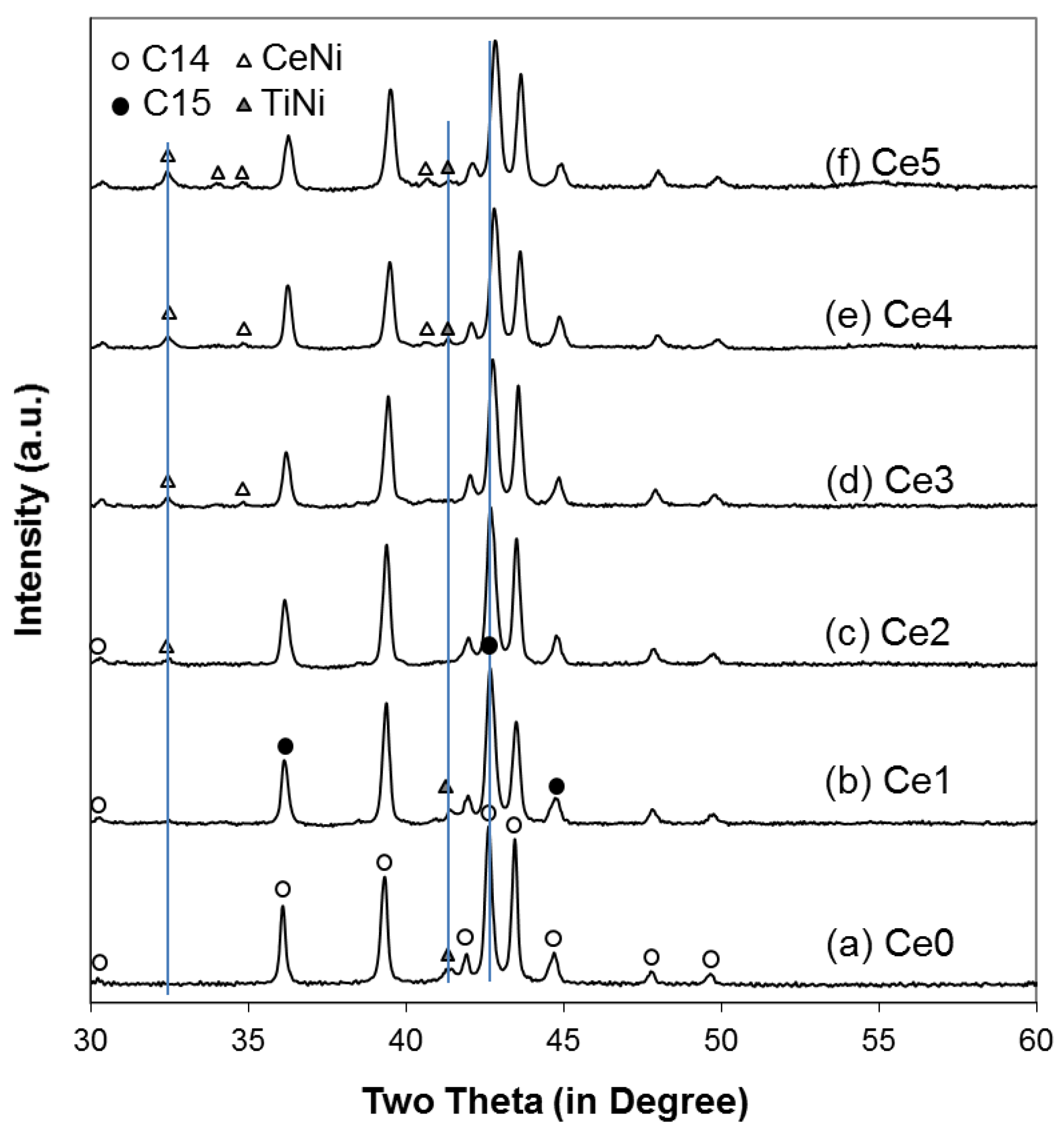
4.1. X-Ray Diffraction Analysis
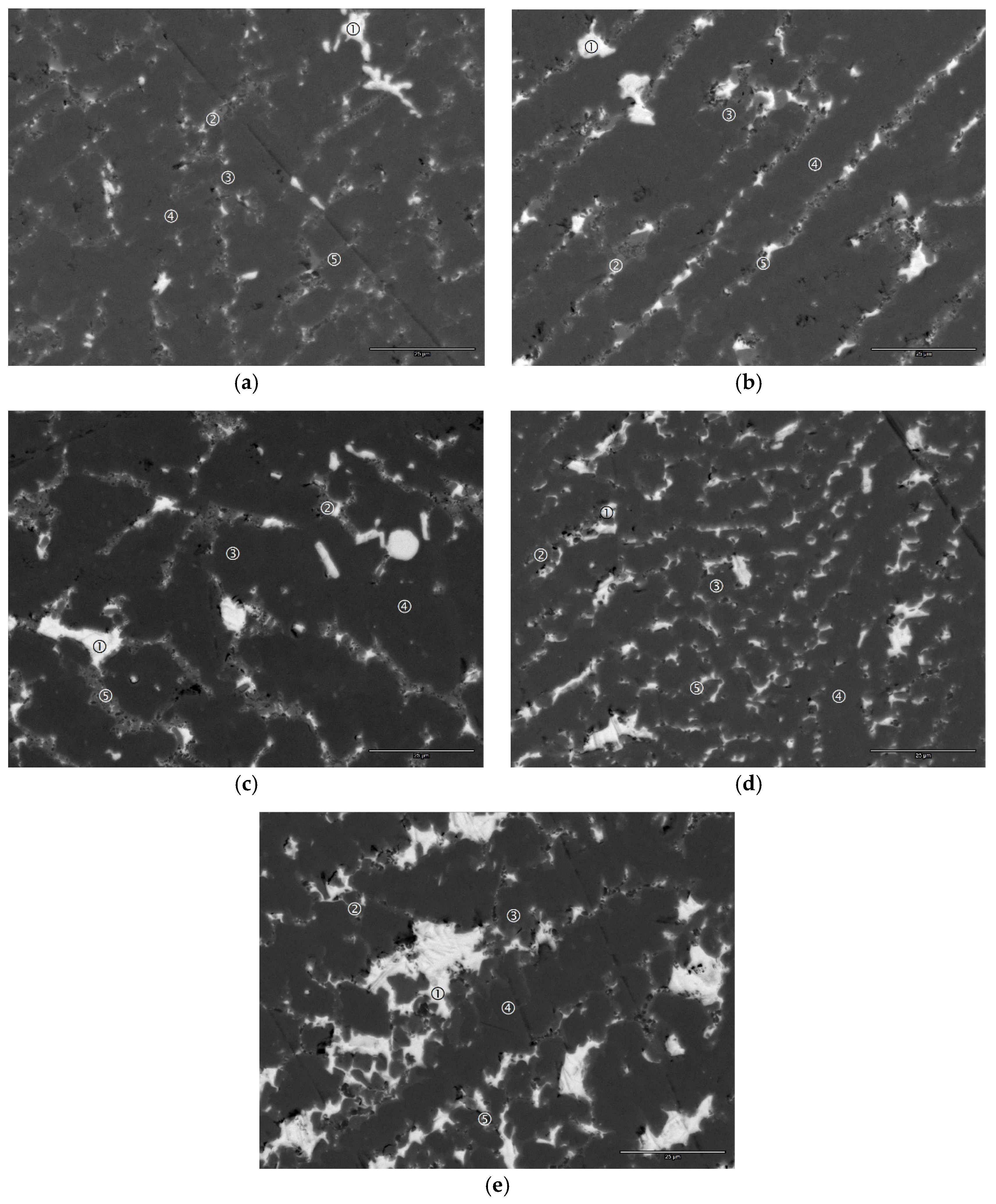
4.2. Scanning Electron Microscope/Energy Dispersive Spectroscopy Analysis
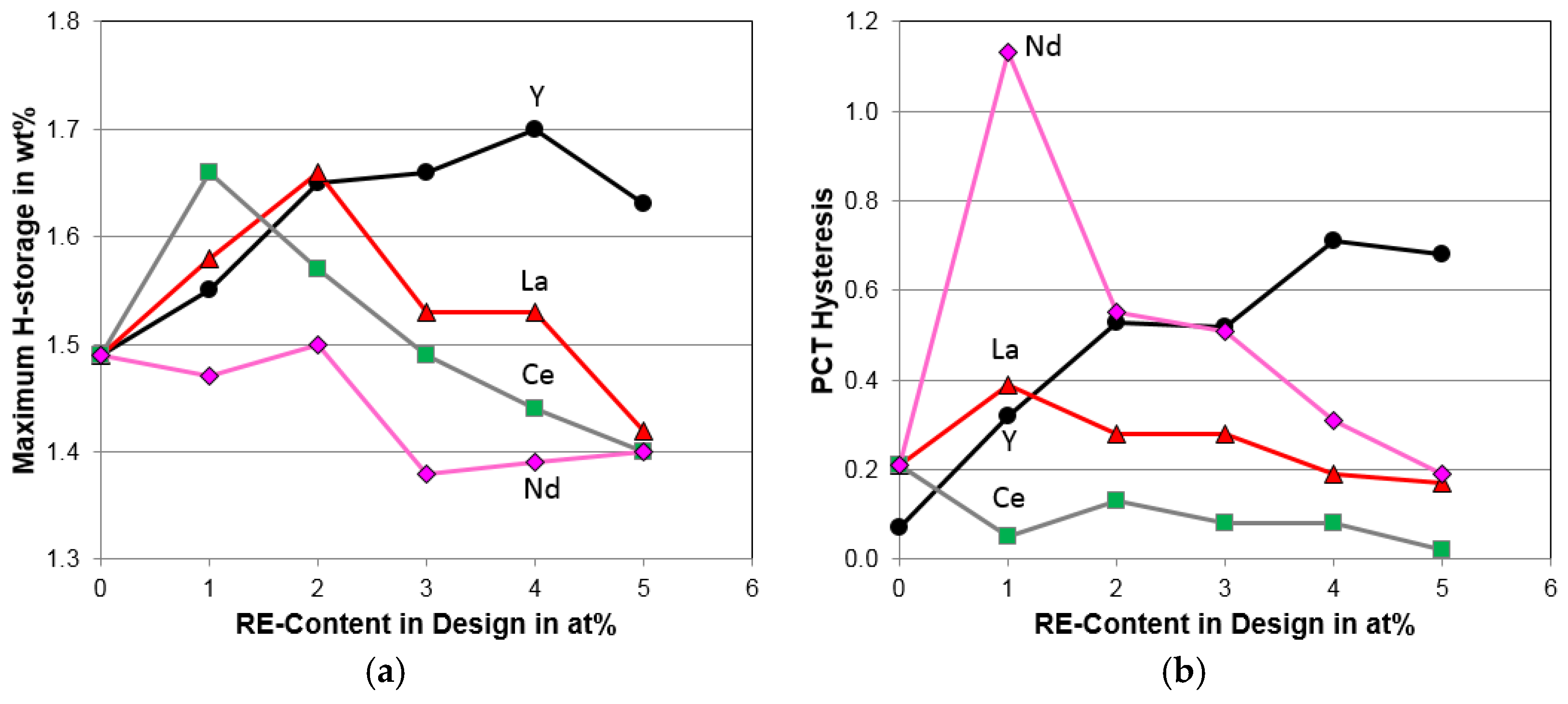
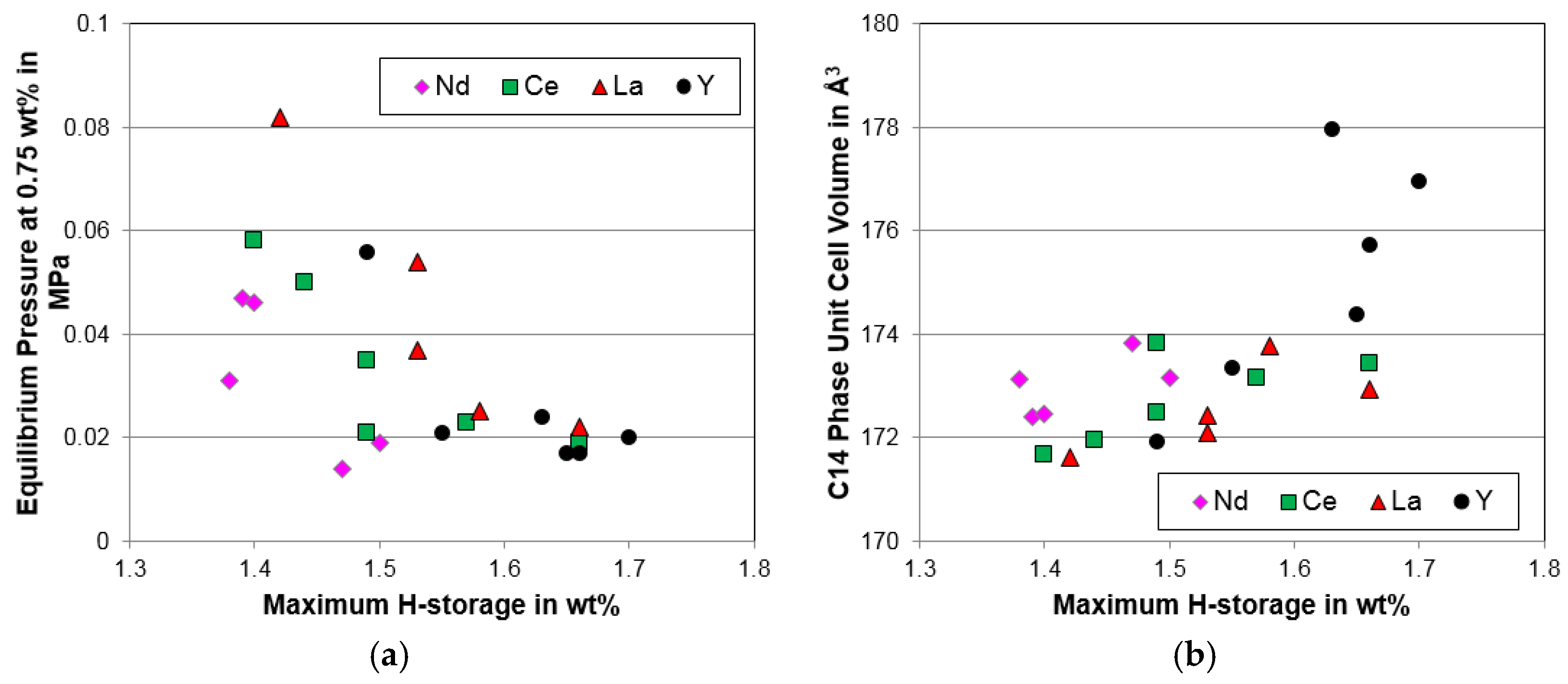
4.3. Pressure–Concentration–Temperature Analysis
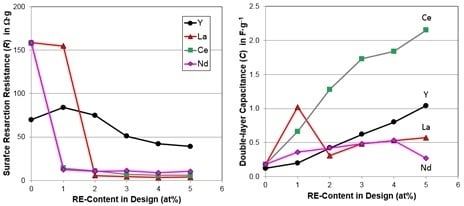
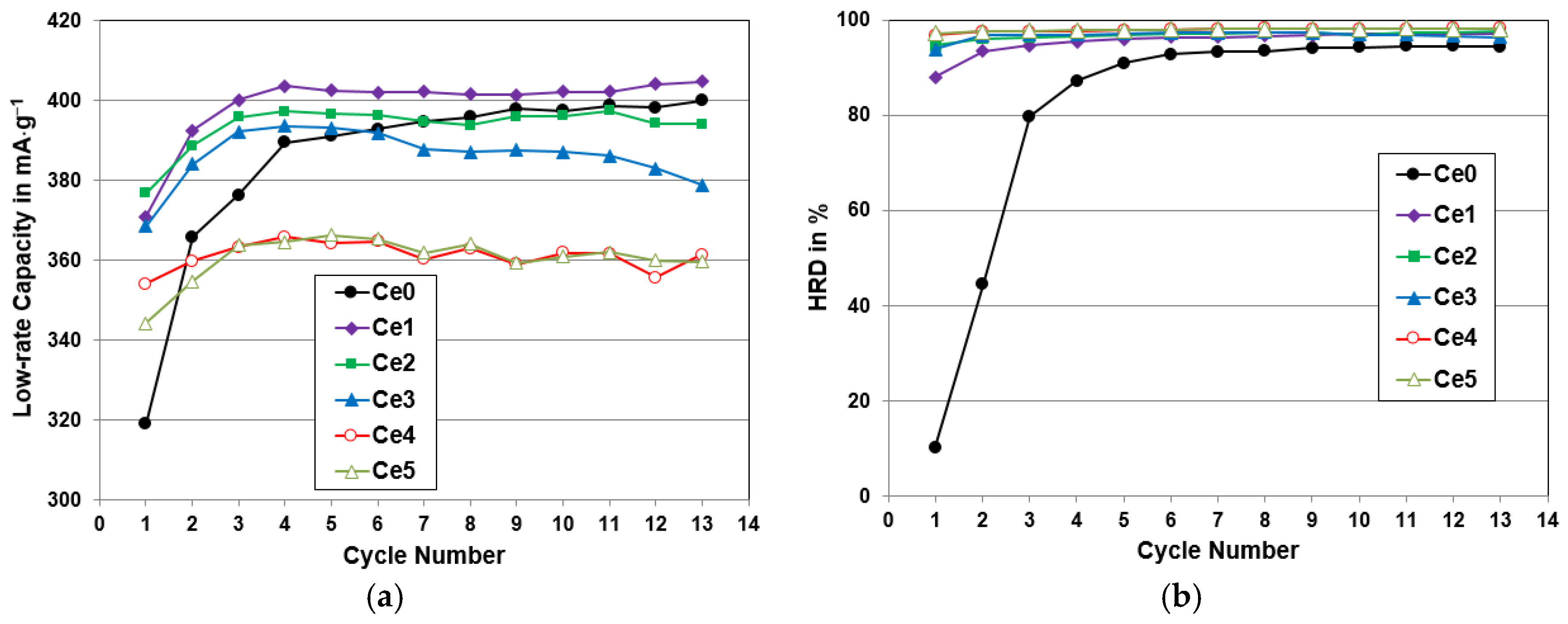
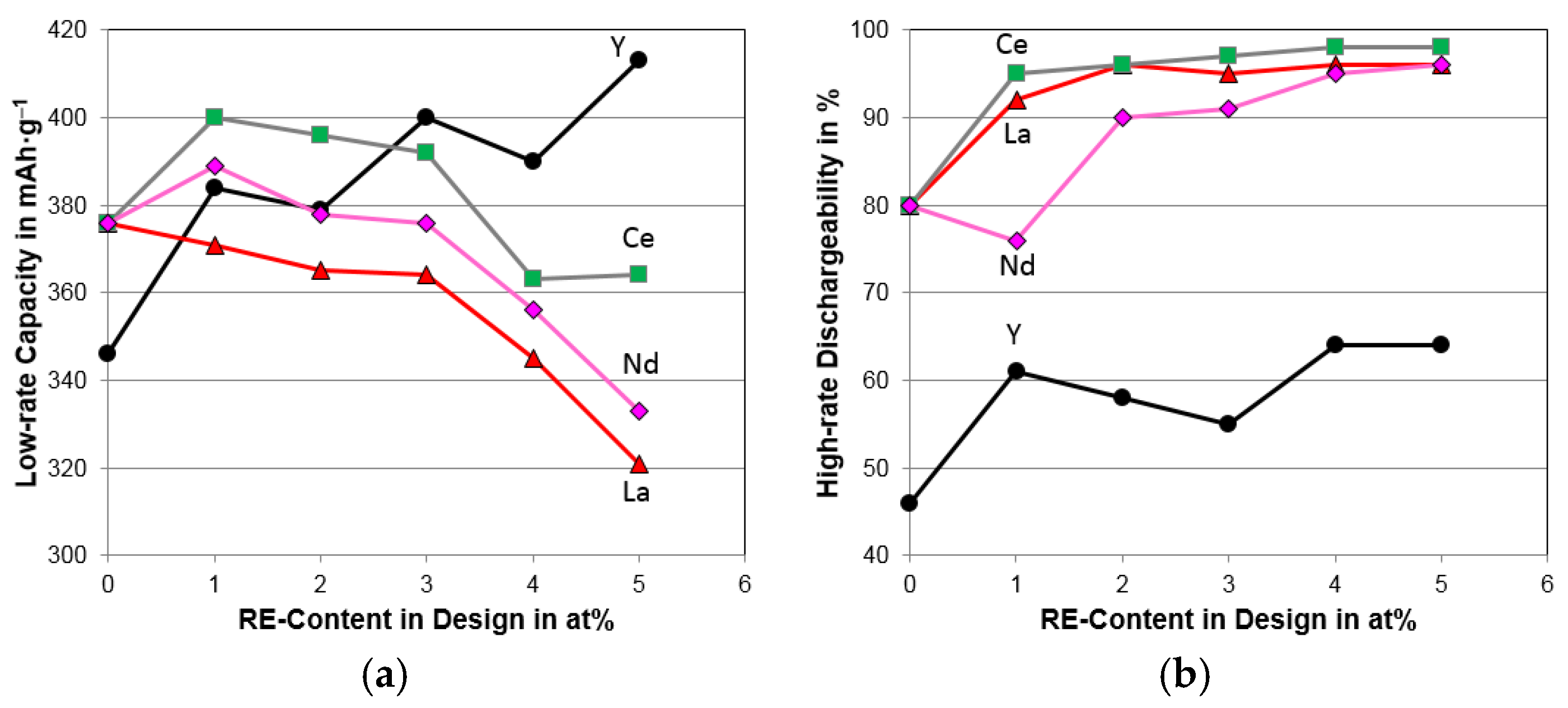
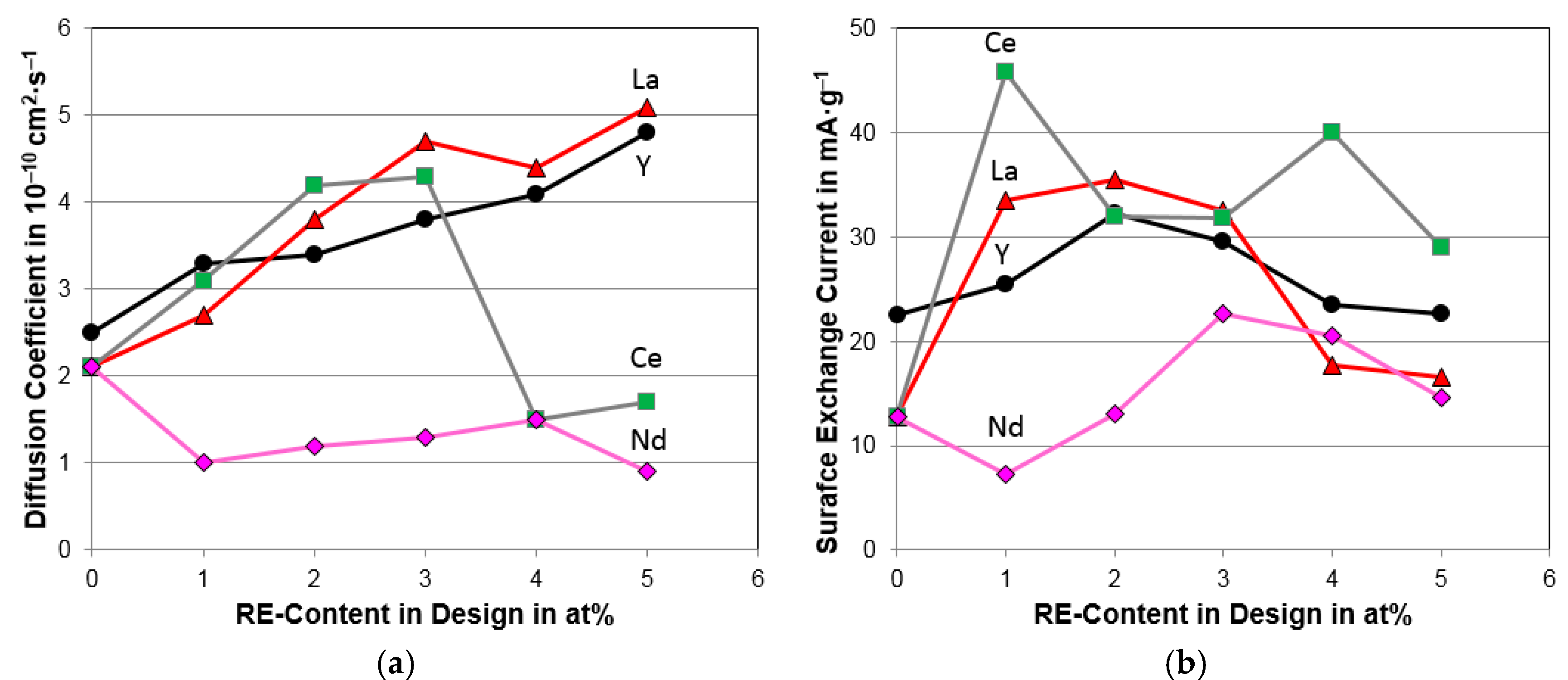
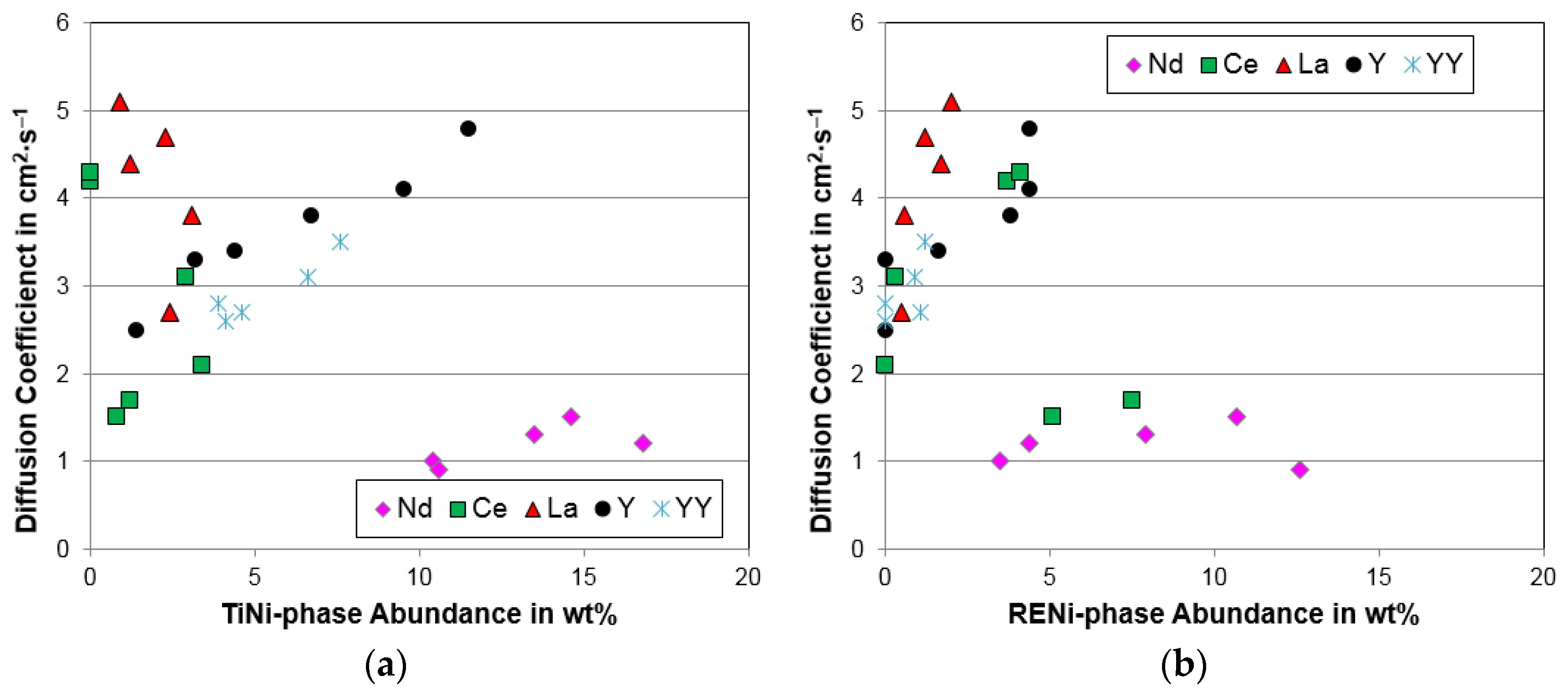
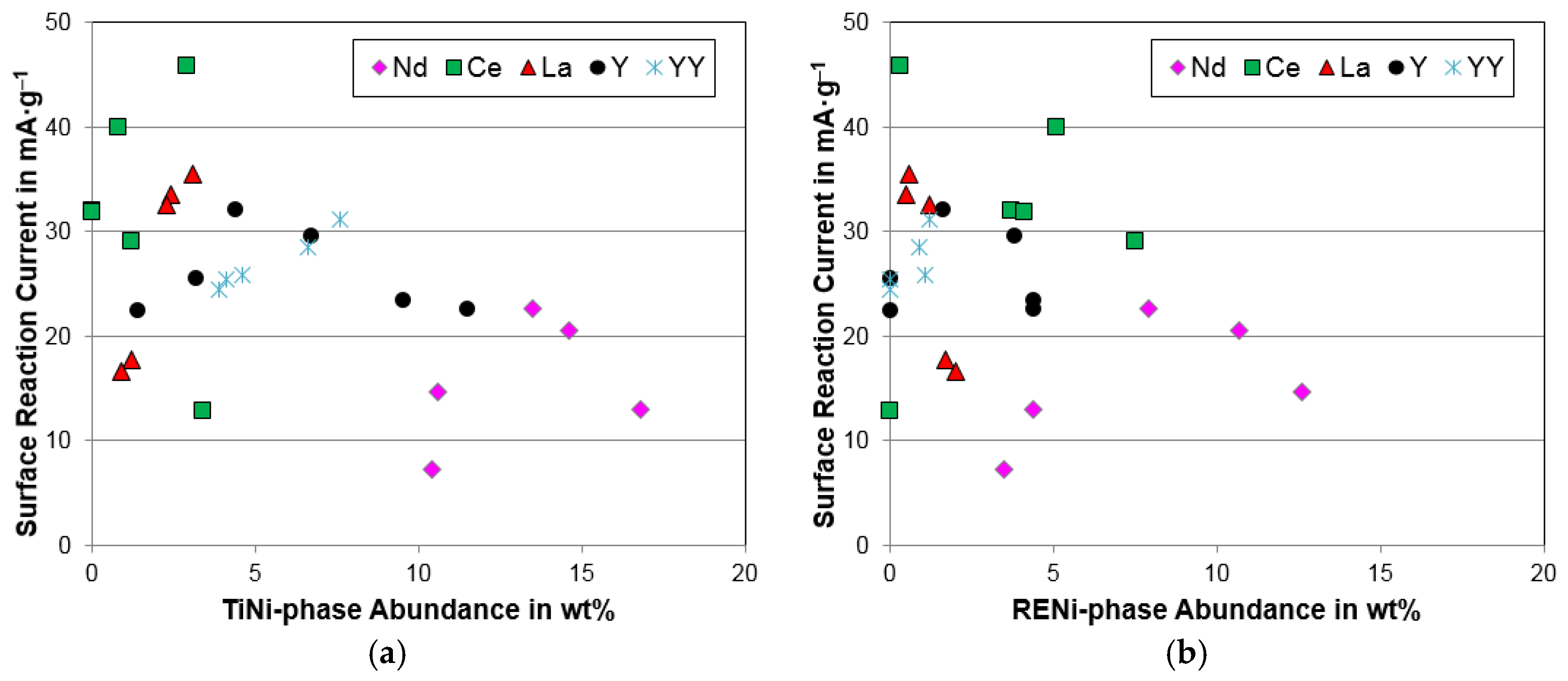
4.4. Electrochemical Analysis
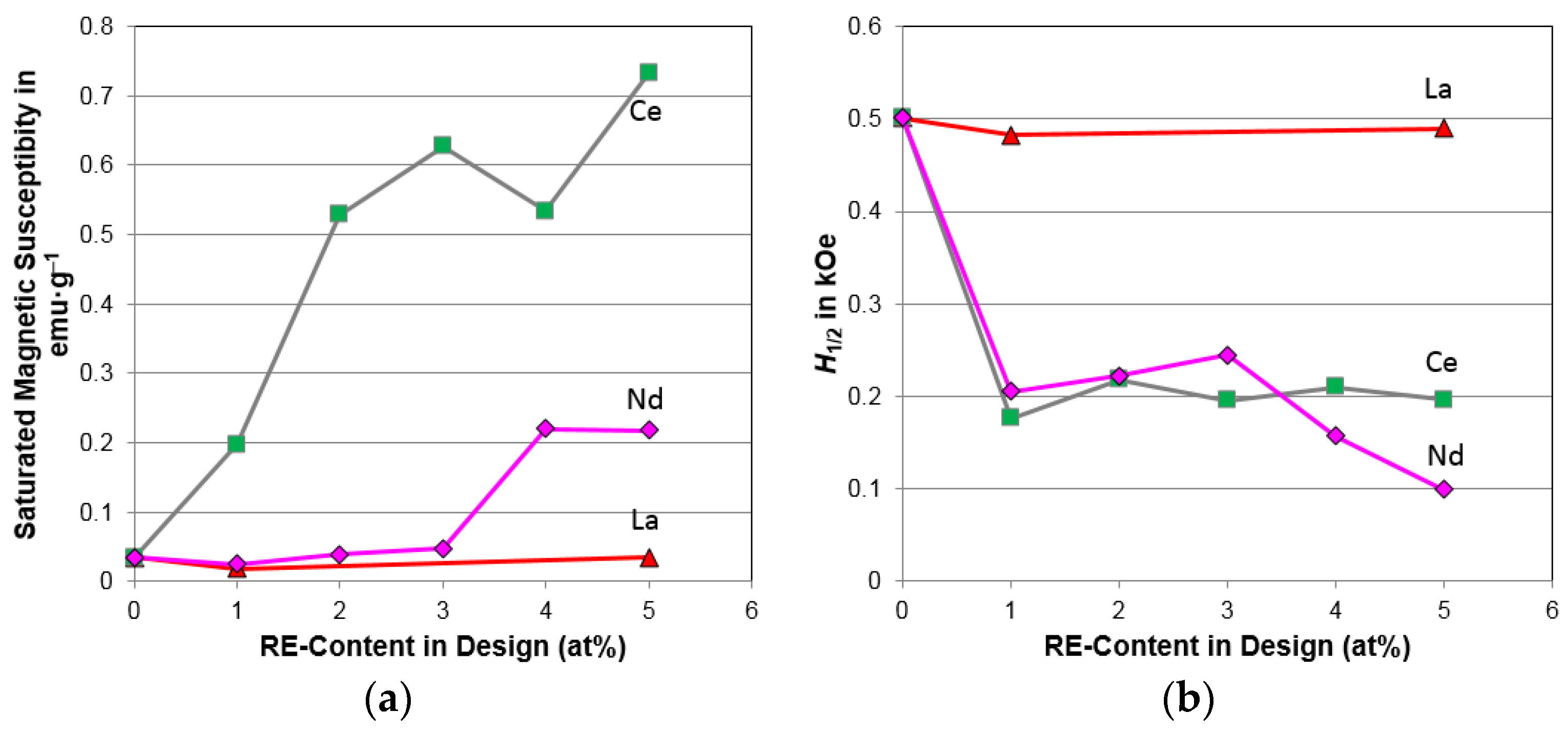
4.5. Magnetic Properties
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ni/MH | Nickel/metal hydride |
| MH | Metal hydride |
| RE | Rare earth |
| ICP-OES | Inductively coupled plasma-optical emission spectrometer |
| XRD | X-ray diffractometer |
| SEM | Scanning electron microscope |
| EDS | Energy dispersive spectroscopy |
| PCT | Pressure–concentration–temperature |
| H-storage | Hydrogen storage |
| M.S. | Magnetic susceptibility |
| M.P. | Melting point |
| HCP | Hexagonal closest-packed |
| DHCP | Double- c hexagonal close-packed |
| e/a | Average electron density |
| DOD | Degree of disorder |
| VC14 | Unit cell volume of the C14 phase |
| FWHM | Full-width at half-maximum |
| SEI | Secondary electron image |
| BEI | Back-scattering electron image |
| HRD | High-rate dischargeability |
| Δ Hh | Heat of hydride formation |
| Δ Sh | Change in entropy |
| M–H | Metal–hydrogen |
| SF | Slope factor |
| Pa | Absorption equilibrium pressure at 0.75 wt% H-storage |
| Pd | Desorption equilibrium pressure at 0.75 wt% H-storage |
| β | Hydride phase |
| α | Metal phase or alloy matrix |
| R | Ideal gas constant |
| T | Absolute temperature |
| D | Bulk diffusion coefficient |
| Io | Surface exchange current |
| R | Surface charge-transfer resistance |
| C | Surface double-layer capacitance |
| Ms | Saturated magnetic susceptibility |
| H1/2 | Applied magnetic field strength corresponding to half of saturated magnetic susceptibility |
References
- Wikipedia, the Free Encyclopedia. Nickel–Metal Hydride Battery. Available online: https://en.wikipedia.org/wiki/Nickel%E2%80%93metal_hydride_battery (accessed on 14 April 2016).
- Zelinsky, M.; Koch, J.; Fetcenko, M. Heat Tolerant NiMH Batteries for Stationary Power. Available online: www.battcon.com/PapersFinal2010/ZelinskyPaper2010Final_12.pdf (accessed on 28 March 2016).
- Zelinsky, M.; Koch, J. Batteries and Heat—A Recipe for Success? Available online: www.battcon.com/PapersFinal2013/16-Mike%20Zelinsky%20-%20Batteries%20and%20Heat.pdf (accessed on 28 March 2016).
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef]
- Chang, S.; Young, K.; Nei, J.; Fierro, C. Reviews on the U.S. Patents regarding nickel/metal hydride batteries. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Koch, J.; Fetcenko, M.A. The role of Mn in C14 Laves phase multi-component alloys for NiMH battery application. J. Alloys Compd. 2009, 477, 749–758. [Google Scholar] [CrossRef]
- Young, K.; Reichman, B.; Fetcenko, M.A. Electrochemical properties of AB2 metal hydride alloys measured at −40 °C. J. Alloys Compd. 2013, 580, S349–S353. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Nei, J.; Reichman, B. Electrochemical properties of hypo-stoichiometric Y-doped AB2 metal hydride alloys at ultra-low temperature. J. Alloys Compd. 2015, 643, 17–27. [Google Scholar] [CrossRef]
- Huang, B.; Young, K.; BASF-Ovonic. Private Communication, 2016.
- Kim, S.; Lee, J.; Park, H. A study of the activation behavior of ZrCrNiLa metal hydride electrodes in alkaline solution. J. Alloys Compd. 1994, 205, 225–229. [Google Scholar] [CrossRef]
- Jung, J.; Lee, K.; Lee, J. The activation mechanism of Zr-based alloy electrodes. J. Alloys Compd. 1995, 226, 166–169. [Google Scholar] [CrossRef]
- Sun, D.; Latroche, M.; Percheron-Guégan, A. Effects of lanthanum or cerium on the equilibrium of ZrNi1.2Mn0.6V0.2Cr0.1 and its related hydrogenation properties. J. Alloys Compd. 1997, 248, 215–219. [Google Scholar] [CrossRef]
- Park, H.Y.; Cho, W.I.; Cho, B.W.; Lee, S.R.; Yun, K.S. Effect of fluorination on the lanthanum-doped AB2-type metal hydride electrodes. J. Power Sources 2001, 92, 149–156. [Google Scholar] [CrossRef]
- Park, H.Y.; Chang, I.; Cho, W.I.; Cho, B.W.; Jang, H.; Lee, S.R.; Yun, K.S. Electrode characteristics of the Cr and La dopes AB2-type hydrogen storage alloys. Int. J. Hydrog. Energy 2001, 26, 949–955. [Google Scholar] [CrossRef]
- Gao, M.; Miao, H.; Zhao, Y.; Liu, Y.; Pan, H. Effects of rare earth elements substitution for Ti on the structure and electrochemical properties of Fe-doped Ti-V-based hydrogen storage alloy. J. Alloys Compd. 2009, 484, 249–255. [Google Scholar] [CrossRef]
- Young, K.; Young, M.; Ouchi, T.; Reichman, B.; Fetcenko, M.A. Improvement in high-rate dischargeability, activation, and low-temperature performance in multi-phase AB2 alloys by partial substitution of Zr with Y. J. Power Sources 2012, 215, 279–287. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Ouchi, T.; Huang, B.; Reichman, B. Effects of La-addition to the structure, hydrogen storage, and electrochemical properties of C14 metal hydride alloys. Electrochim. Acta 2015, 174, 815–825. [Google Scholar] [CrossRef]
- Wong, D.F.; Young, K.; Nei, J.; Wang, L.; Ng, K.Y.S. Effects of Nd-addition on the structural, hydrogen storage, and electrochemical properties of C14 metal hydride alloys. J. Alloys Compd. 2015, 647, 507–518. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Li, F.; Ouchi, T. Structural, thermodynamics, and electrochemical properties of TixZr1–x(VNiCrMnCoAl)2 C14 Laves phase alloys. J. Alloys Compd. 2008, 464, 238–247. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Koch, J.; Morii, K.; Shimizu, T. Studies of Sn, Co, Al, and Fe additives in C14/C15 Laves alloys for NiMH battery application by orthogonal arrays. J. Alloy. Compd. 2009, 486, 559–569. [Google Scholar] [CrossRef]
- Wikipedia, the Free Encyclopedia. Rare Earth Element. Available online: https://en.wikipedia.org/wiki/Rare_earth_element (accessed on 14 April 2016).
- Yaroslavtsev, A.; Menushenkov, A.; Chernikov, R.; Clementyev, E.; Lazukov, V.; Zubavichus, Y.; Veligzhanin, A.; Efremova, N.; Gribanov, A.; Kuchin, A. Ce valence in intermetallic compounds by means of XANES spectroscopy. Z. Kristallogr. 2010, 225, 482–486. [Google Scholar] [CrossRef]
- Gschneidner, K.A. Rare-Earth Element. Available online: http://www.britannica.com/science/rare-earth-element (accessed on 14 April 2016).
- Young, K.; Wong, D.F.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 1 Structural, hydrogen storage and electrochemical properties. J. Power Sources 2015, 277, 426–432. [Google Scholar] [CrossRef]
- Yasuoka, S.; Ishida, J. Effects of cerium (Ce) on the hydrogen absorption-desorption characteristics of RE-Mg-Ni hydrogen absorbing alloy. J. Power Sources 2016. submitted for publication. [Google Scholar]
- Young, K.; Chao, B.; Liu, Y.; Nei, J. Microstructures of the oxides on the activated AB2 and AB5 metal hydride alloys surface. J. Alloys Compd. 2014, 606, 97–104. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 74th ed.; CRC Press Inc.: Boca Raton, FL, USA, 1993; pp. 6–22. [Google Scholar]
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States—A Summary of Domestic Deposits and a Global Perspective; Scientific Investigations Report 2010-5220; U.S. Geological Survey: Reston, VA, USA, 2010.
- Gakkai, N.K. Hi Kagaku Ryouronteki Kinzoku Kagobutu; Maruzen: Tokyo, Japan, 1975; p. 296. [Google Scholar]
- Griessen, R.; Riesterer, T. Heat of Formation Models. In Hydrogen in Intermetallic Compounds I.; Schlapbach, L., Ed.; Springer-Verlag Berlin Heidelberg: Berlin, Germany, 1988. [Google Scholar]
- Courcot, E.; Rebillat, F.; Teyssandier, F. Thermochemical Stability of Rare Earth Sesquioxides under a Most Environment. In Design, Development, and Applications of Engineering Ceramics and Composites: Ceramic Transaction; Singh, D., Zhu, D., Zhou, Y., Eds.; John Wiley & Sons: New York, NY, USA, 2010; Volume 215. [Google Scholar]
- Cordfunke, E.H.P.; Konings, R.J.M.; Ouweltjes, W. The standard enthalpies of formation of hydroxides IV. La(OH)3 and LaOOH. J. Chem. Thermodyn. 1990, 22, 449–452. [Google Scholar] [CrossRef]
- Fang, Z.; Thanthiriwatte, K.S.; Dixon, D.A.; Andrews, L.; Wang, X. Properties of cerium hydroxides from matrix infrared spectra and electronic structure calculations. Inorg. Chem. 2015, 55, 1702–1714. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, C.; Brown, P.L. Hydrolysis of Metal Ions; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2016; p. 265. [Google Scholar]
- Merli, L.; Fuger, J. Thermochemistry of a few neptunium and neodymium oxides and hydroxides. Radiochim. Acta 1994, 66–67, 109–113. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibrium in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Powder Diffraction File (PDF) Database; MSDS No. 00-014-0481; International Centre for Diffraction Data: Newtown Square, PA, USA, 2011.
- Massalski, T.B. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Du, Z.; Yang, L.; Ling, G. Thermodynamic assessment of the Ce-Ni system. J. Alloys Compd. 2004, 375, 186–190. [Google Scholar] [CrossRef]
- Boer, F.R.; Boom, R.; Mattens, W.C.M.; Miedema, A.R.; Niessen, A.K. Cohesion in Metals Transition Metal Alloys; North-Holland: Amsterdam, The Netherlands, 1988; p. 302. [Google Scholar]
- Chen, N.; Lu, W.; Yang, J.; Li, G. Support Vector Machine in Chemistry; World Scientific Pub. Co.: Singapore, 2004; p. 148. [Google Scholar]
- Colinet, C.; Pasturel, A. Enthalpies of formation of RNi5 compounds. Inorg. Chim. Acta 1984, 94, 66–67. [Google Scholar] [CrossRef]
- Hussain, A.; Ende, A.V.; Kim, J.; Jung, I. Critical thermodynamic evaluation and optimization of the Co-Nd, Cu-Nd and Nd-Ni system. Calphad 2013, 41, 26–41. [Google Scholar] [CrossRef]
- Osumi, Y. Suiso Kyuzou Goukin; Agune Co. Ltd.: Tokyo, Japan, 1993; p. 54. [Google Scholar]
- Lundin, C.E.; Lynch, F.E.; Magg, C.B. A correlation between the interstitial hole sizes in intermetallic compounds and the thermodynamic properties of the hydrides formed from those compounds. J. Less Common Met. 1977, 56, 19–37. [Google Scholar] [CrossRef]
- Luo, Q.; Chen, S.; Zhang, J.; Li, L.; Chou, K.; Li, Q. Experimental investigation and thermodynamic assessment of Nd-H and Nd-Ni-H systems. Calphad 2015, 51, 282–291. [Google Scholar] [CrossRef]
- Thoma, D.J.; Perepezko, J.H. A geometric analysis of solubility ranges in Laves phases. J. Alloys Compd. 1995, 224, 330–341. [Google Scholar] [CrossRef]
- Crystal Structures of CrB and FeB. Available online: http://www.hardmaterials.de/html/crb__feb.html (accessed on 14 April 2016).
- Matar, S.F.; Nakhl, M.; Alam, A.F.A.; Ouaini, N.; Chevalier, B. YNi and its hydrides: Phase stabilities, electronic structures and chemical bonding properties from first principles. Chem. Phys. 2010, 377, 109–114. [Google Scholar] [CrossRef]
- Maeland, A.; Andresen, A.F.; Videm, K. Hydrides of lanthanum-nickel compounds. J. Less Common. Met. 1976, 45, 347–350. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; Bouten, P.C.P.; Miedema, A.R. Hydrides formed from inter metallic compounds of two transition metals: A special class of ternary alloy. Rep. Prog. Phys. 1982, 45, 939–1039. [Google Scholar] [CrossRef]
- Bobet, J.L.; Grigorova, E.; Chevalier, B.; Khrussanova, M.; Peshev, P. Hydrogenation of CeNi: Hydride formation, structure and magnetic properties. Intermetallics 2006, 14, 208–212. [Google Scholar] [CrossRef]
- Kolomiets, A.V.; Miliyanchuk, K.; Galadzhun, Y.; Havela, L.; Vejpravova, J. PrNi and CeNi hydrides with extremely high H-density. J. Alloys Compd. 2005, 402, 95–97. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; Ng, K.Y.S. Determination of C14/C15 phase abundance in Laves phase alloys. Mater. Chem. Phys. 2012, 136, 520–527. [Google Scholar] [CrossRef]
- Young, K. Stoichiometry in Inter-Metallic Compounds for Hydrogen Storage Applications. In Stoichiometry and Materials Science—When Numbers Matter; Innocenti, A., Kamarulzaman, N., Eds.; Intech: Rijeka, Croatia, 2012. [Google Scholar]
- Young, K.; Nei, J.; Ouchi, T.; Fetcenko, M.A. Phase abundances in AB2 metal hydride alloys and their correlations to various properties. J. Alloys Compd. 2011, 509, 2277–2284. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Lin, X.; Reichman, B. Effects of Zn-addition to C14 metal hydride alloys and comparisons to Si, Fe, Cu, Y, and Mo-additives. J. Alloys Compd. 2016, 655, 50–59. [Google Scholar] [CrossRef]
- Scholtus, N.A.; Hall, W.K. Hysteresis in the palladium-hydrogen system. J. Chem. Phys. 1963, 39, 868–870. [Google Scholar] [CrossRef]
- Makenas, B.J.; Birnbaum, H.K. Phase changes in the niobium-hydrogen system I: Accommodation effects during hydride precipitation. Acta Metall. 1980, 28, 979–988. [Google Scholar] [CrossRef]
- Balasubramaniam, R. Accommodation effects during room temperature hydrogen transformations in the niobium-hydrogen system. Acta Metall. Mater. 1993, 41, 3341–3349. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Fetcenko, M.A. Pressure-composition-temperature hysteresis in C14 Laves phase alloys: Part 1. Simple ternary alloys. J. Alloys Compd. 2009, 480, 428–433. [Google Scholar] [CrossRef]
- Young, K.; Chao Pawlik, D.; Shen, H.T. Transmission electron microscope studies in the surface oxide on the La-containing AB2 metal hydride alloy. J. Alloys Compd. 2016, 672, 356–365. [Google Scholar] [CrossRef]
- Züttle, A.; Meli, F. Electrochemical and surface properties of Zr(VxNi1−x)2 alloys as hydrogen absorbing electrodes in alkaline electrolyte. J. Alloys Compd. 1994, 203, 235–241. [Google Scholar] [CrossRef]
- Young, K.; Huang, B.; Regmi, R.K.; Lawes, G.; Liu, Y. Comparisons of metallic clusters imbedded in the surface of AB2, AB5, and A2B7 alloys. J. Alloys Compd. 2010, 506, 831–840. [Google Scholar] [CrossRef]
- Chang, S.; Young, K.; Ouchi, T.; Meng, T.; Nei, J.; Wu, X. Studies on incorporation of Mg in Zr-based AB2 metal hydride alloys. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Nei, J. Effects of vanadium/nickel contents in Laves phase-related body-centered-cubic solid solution metal hydride alloys. Batteries 2015, 1, 34–53. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Nei, J. Microstructures of the activated Si-containing AB2 metal hydride alloy surface by transmission electron microscope. Batteries 2016, 2. [Google Scholar] [CrossRef]






| Properties of RE, RENi, and RENi5 | Y | La | Ce | Pr | Nd |
|---|---|---|---|---|---|
| Atomic number | 39 | 57 | 58 | 59 | 60 |
| Content in earth crust (ppm) [28] | 33 | 39 | 66.5 | 9.2 | 41.5 |
| Outer shell electron configuration | 5s24d1 | 6s25d1 | 6s25d14f1 | 6s25d14f2 | 6s25d14f3 |
| Electronegativity | 1.22 | 1.10 | 1.12 | 1.13 | 1.14 |
| Ionic radius (Å) | 1.04 (Y3+) | 1.17 (La3+) | 1.15 (Ce3+); 1.01 (Ce4+) | 1.13 (Pr3+) | 1.12 (Nd3+) |
| Atomic radius in Laves phase alloy (Å) [29] | 1.990 | 3.335 | 2.017 | 2.013 | 2.013 |
| Crystal structure at 25°C | HCP | DHCP | DHCP | DHCP | DHCP |
| Melting point (°C) | 1522 | 918 | 798 | 931 | 1021 |
| Temperature when vapor pressure reaches 0.001 Pa (°C) | 1220 | 1301 | 1290 | 1083 | 995 |
| Oxidation potential (V) | −2.37 | −2.37 | −2.335 | −2.353 | −2.246 |
| Heat of hydride formation (kJ·mol−1) [30] | −114 | −97 | −103 | −104 | −106 |
| Heat of formation of RE(OH)3 (kJ·mol−1) | −937.6 [31] | −1415.5 [32] | −1014.5 [33] | −1419 [34] | −1403.6 [35] |
| Solubility represented by the value of A in Equation (1) [36] | 19.86 | 23.02 | 22.15 | 22.50 | 21.25 |
| Crystal structure of RENi | FeB-(Pnma) | CrB-(Cmcm) | CrB-(Cmcm) | CrB-(Cmcm) | CrB-(Cmcm) |
| Unit cell volume of RENi (Å3) [37] | 162.6 | 183.9 | 174.6 | 174.5 | 172.6 |
| Melting point of RENi (°C) | 1070 [38] | 715 [38] | 680 [39] | 730 [38] | 780 [38] |
| Heat of formation of RENi (kJ·mol−1) | −37 [40] | −24.8 [41] | −30.3 [41] | −28.1 [41] | −25.0 [41] |
| Crystal structure of RENi5 [34] | CaCu5 | CaCu5 | CaCu5 | CaCu5 | CaCu5 |
| Unit cell volume of RENi5 (Å3) [34] | 81.7 | 86.8 | 82.8 | 84.8 | 84.3 |
| Heat of formation of RENi5 (kJ·mol−1) | −204.6 [42] | −158.9 [42] | −199 [36] | −160.6 [36] | −151.2 [43] |
| Plateau pressure at 20 °C of RENi5 (MPa) | 30 [44] | 0.15 [44] | 4.8 [45] | 1.2 [44] | 0.62 [44] |
| Heat of formation of RENi5H6 (kJ·mol−1) | - | −30.1 [44] | −14.2 [45] | −30.5 [45] | −29.4 [45] |
| Alloy | Source | Ti | Zr | V | Cr | Mn | Co | Ni | Ce | Al | e/a | B/A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce0 | Design | 12.0 | 22.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 0.0 | 0.4 | 6.771 | 1.87 |
| ICP | 11.9 | 22.9 | 10.0 | 7.5 | 8.0 | 7.1 | 32.2 | 0.0 | 0.4 | 6.773 | 1.87 | |
| Ce1 | Design | 12.0 | 21.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 1.0 | 0.4 | 6.771 | 1.87 |
| ICP | 11.9 | 21.3 | 10.3 | 7.7 | 8.0 | 7.0 | 32.3 | 1.0 | 0.5 | 6.780 | 1.92 | |
| Ce2 | Design | 12.0 | 20.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 2.0 | 0.4 | 6.771 | 1.87 |
| ICP | 12.0 | 20.6 | 10.3 | 6.8 | 8.2 | 7.1 | 32.6 | 2.0 | 0.4 | 6.792 | 1.89 | |
| Ce3 | Design | 12.0 | 19.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 3.0 | 0.4 | 6.771 | 1.87 |
| ICP | 12.0 | 19.4 | 10.2 | 7.5 | 7.8 | 7.1 | 32.6 | 3.0 | 0.4 | 6.793 | 1.91 | |
| Ce4 | Design | 12.0 | 18.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 4.0 | 0.4 | 6.771 | 1.87 |
| ICP | 11.9 | 18.4 | 10.2 | 7.6 | 8.0 | 7.0 | 32.5 | 3.9 | 0.5 | 6.789 | 1.92 | |
| Ce5 | Design | 12.0 | 17.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 5.0 | 0.4 | 6.771 | 1.87 |
| ICP | 12.1 | 17.6 | 10.3 | 7.6 | 7.2 | 7.1 | 32.8 | 4.9 | 0.4 | 6.790 | 1.89 |
| Structural Properties | Ce0 | Ce1 | Ce2 | Ce3 | Ce4 | Ce5 |
|---|---|---|---|---|---|---|
| a, C14 (Å) | 4.9739 | 4.9703 | 4.9678 | 4.9616 | 4.9566 | 4.9542 |
| c, C14 (Å) | 8.1134 | 8.1067 | 8.1018 | 8.0905 | 8.0824 | 8.0763 |
| a/c, C14 (Å) | 0.61305 | 0.61311 | 0.61317 | 0.61326 | 0.61326 | 0.61342 |
| VC14 (Å3) | 173.83 | 173.44 | 173.16 | 172.48 | 171.96 | 171.67 |
| FWHM C14 (103) | 0.237 | 0.216 | 0.217 | 0.23 | 0.245 | 0.255 |
| C14 crystallite size (Å) | 482 | 554 | 551 | 503 | 458 | 434 |
| a, C15 (Å) | 7.0121 | 7.003 | 6.9973 | 6.9886 | 6.989 | 6.9831 |
| C14 abundance (%) | 85.4 | 79.9 | 78.8 | 79.9 | 73.2 | 78.1 |
| C15 abundance (%) | 11.2 | 16.9 | 17.5 | 16 | 20.9 | 13.2 |
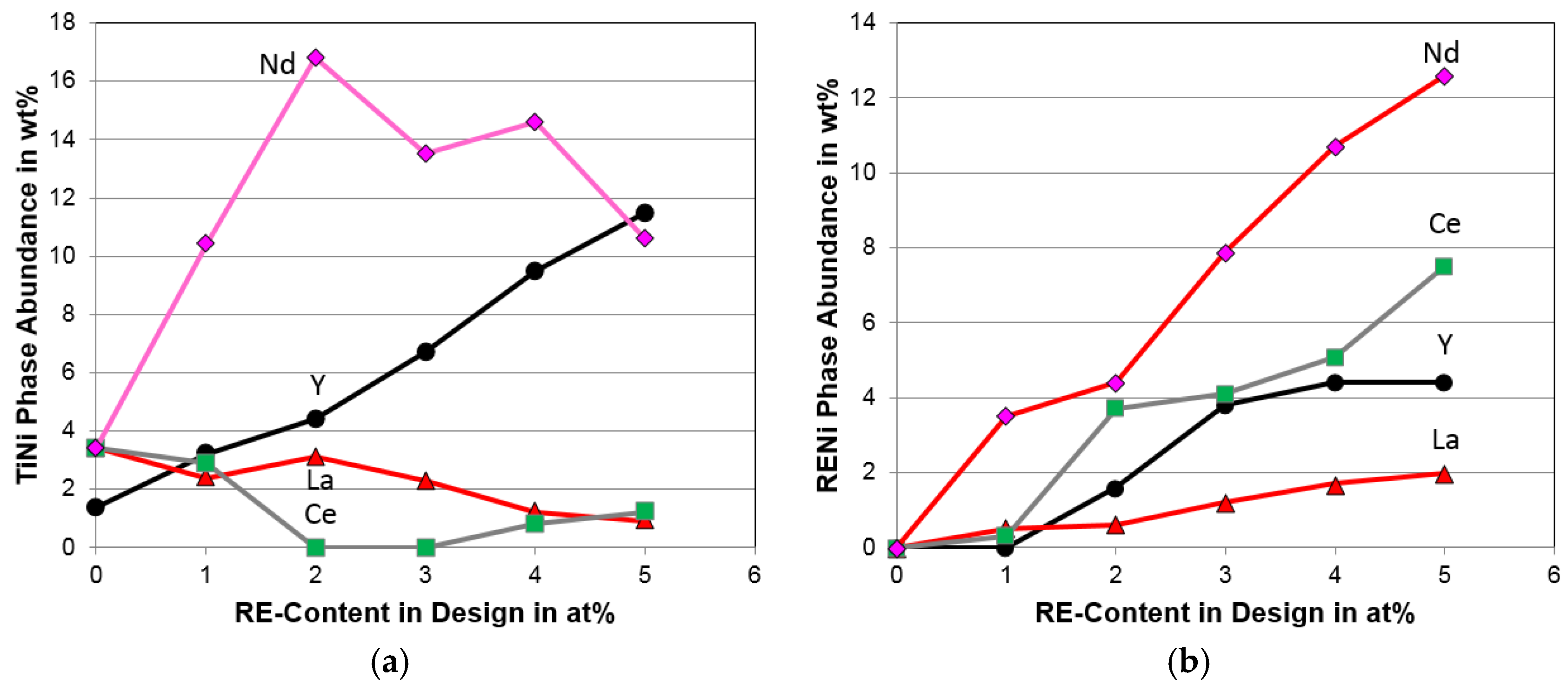
| TiNi abundance (%) | 3.4 | 2.9 | 0.0 | 0.0 | 0.8 | 1.2 |
| CeNi abundance (%) | 0.0 | 0.3 | 3.7 | 4.1 | 5.1 | 7.5 |
| Alloy | Location | Ti | Zr | V | Ni | Co | Mn | Cr | Al | Ce | B/A | e/a | Phase |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce1 | Figure 5a-1 | 0.9 | 3.1 | 1.7 | 3.8 | 1.0 | 1.0 | 0.0 | 0.1 | 88.4 | 0.08 | 3.44 | Ce |
| Figure 5a-2 | 13.5 | 26.8 | 0.7 | 54.8 | 1.6 | 1.2 | 0.3 | 0.3 | 0.8 | 1.39 | 7.41 | Zr7Ni10 | |
| Figure 5a-3 | 11.9 | 22.8 | 6.9 | 41.7 | 5.5 | 6.3 | 3.9 | 0.6 | 0.4 | 1.85 | 7.10 | C15 | |
| Figure 5a-4 | 10.6 | 22.3 | 12.4 | 28.4 | 7.7 | 8.9 | 9.3 | 0.5 | 0.0 | 2.04 | 6.67 | C14 | |
| Figure 5a-5 | 24.7 | 17.2 | 1.9 | 44.2 | 6.0 | 2.9 | 1.1 | 0.5 | 1.6 | 1.20 | 7.06 | TiNi | |
| Ce2 | Figure 5b-1 | 2.7 | 1.8 | 1.2 | 41.8 | 0.8 | 1.0 | 0.0 | 0.2 | 50.5 | 0.82 | 6.08 | CeNi |
| Figure 5b-2 | 13.4 | 27.0 | 0.6 | 55.1 | 1.6 | 1.1 | 0.3 | 0.3 | 0.6 | 1.40 | 7.42 | Zr7Ni10 | |
| Figure 5b-3 | 11.8 | 22.8 | 6.9 | 41.0 | 5.7 | 7.2 | 3.6 | 0.6 | 0.3 | 1.86 | 7.09 | C15 | |
| Figure 5b-4 | 10.4 | 22.2 | 13.1 | 26.6 | 7.8 | 9.6 | 9.9 | 0.4 | 0.0 | 2.07 | 6.60 | C14 | |
| Figure 5b-5 | 28.8 | 14.0 | 1.5 | 41.9 | 7.4 | 3.1 | 0.7 | 0.5 | 2.1 | 1.16 | 6.98 | TiNi | |
| Ce3 | Figure 5c-1 | 0.4 | 0.4 | 0.7 | 49.5 | 0.3 | 0.5 | 0.0 | 0.3 | 47.9 | 1.05 | 6.53 | CeNi |
| Figure 5c-2 | 13.6 | 26.0 | 0.5 | 55.8 | 1.4 | 1.0 | 0.2 | 0.3 | 1.1 | 1.42 | 7.44 | Zr7Ni10 | |
| Figure 5c-3 | 12.1 | 22.6 | 6.8 | 41.3 | 5.7 | 6.9 | 3.7 | 0.6 | 0.3 | 1.86 | 7.10 | C15 | |
| Figure 5c-4 | 11.9 | 21.3 | 13.1 | 26.0 | 8.0 | 9.2 | 9.8 | 0.5 | 0.2 | 1.99 | 6.56 | C14 | |
| Figure 5c-5 | 25.7 | 13.3 | 1.2 | 44.3 | 6.5 | 3.4 | 0.5 | 1.0 | 4.1 | 1.26 | 7.06 | TiNi | |
| Ce4 | Figure 5d-1 | 1.1 | 1.3 | 1.0 | 49.0 | 0.7 | 0.9 | 0.0 | 0.0 | 46.0 | 1.07 | 6.55 | CeNi |
| Figure 5d-2 | 13.7 | 24.7 | 0.5 | 55.6 | 1.5 | 1.2 | 0.2 | 0.3 | 2.3 | 1.43 | 7.43 | Zr7Ni10 | |
| Figure 5d-3 | 12.3 | 22.4 | 6.6 | 41.2 | 5.6 | 7.1 | 3.8 | 0.6 | 0.4 | 1.85 | 7.10 | C15 | |
| Figure 5d-4 | 11.7 | 20.5 | 13.7 | 26.5 | 8.2 | 9.2 | 9.8 | 0.4 | 0.1 | 2.10 | 6.61 | C14 | |
| Figure 5d-5 | 28.3 | 12.4 | 2.4 | 41.8 | 7.3 | 3.7 | 1.5 | 0.7 | 2.0 | 1.22 | 7.02 | TiNi | |
| Ce5 | Figure 5e-1 | 0.5 | 0.7 | 1.1 | 48.6 | 0.5 | 0.6 | 0.0 | 0.3 | 47.7 | 1.04 | 6.49 | CeNi |
| Figure 5e-2 | 13.6 | 24.0 | 0.6 | 55.4 | 1.6 | 1.2 | 0.3 | 0.3 | 3.0 | 1.43 | 7.42 | Zr7Ni10 | |
| Figure 5e-3 | 12.5 | 21.6 | 6.5 | 41.7 | 5.8 | 6.9 | 3.9 | 0.6 | 0.5 | 1.89 | 7.13 | C15 | |
| Figure 5e-4 | 12.4 | 19.5 | 13.3 | 27.9 | 8.2 | 8.2 | 10.0 | 0.3 | 0.2 | 2.12 | 6.66 | C14 | |
| Figure 5e-5 | 28.8 | 11.2 | 2.3 | 41.8 | 7.8 | 3.8 | 1.2 | 0.9 | 2.1 | 1.25 | 7.03 | TiNi |
| Gaseous Phase Properties | Ce0 | Ce1 | Ce2 | Ce3 | Ce4 | Ce5 |
|---|---|---|---|---|---|---|
| Maximum capacity @30 °C (wt%) | 1.49 | 1.66 | 1.57 | 1.49 | 1.44 | 1.4 |
| Reversible capacity @30 °C (wt%) | 1.22 | 1.42 | 1.34 | 1.35 | 1.29 | 1.22 |
| Desorption pressure @30 °C (MPa) | 0.021 | 0.019 | 0.023 | 0.035 | 0.05 | 0.058 |
| SF @30 °C (%) | 78 | 72 | 76 | 76 | 78 | 73 |
| Hysteresis @30 °C | 0.21 | 0.05 | 0.13 | 0.08 | 0.08 | 0.02 |
| Maximum capacity @60 °C (wt%) | 1.38 | 1.49 | 1.44 | 1.38 | 1.3 | 1.25 |
| Reversible capacity @60 °C (wt%) | 1.2 | 1.35 | 1.28 | 1.27 | 1.19 | 1.14 |
| Desorption pressure @60 °C (MPa) | 0.1 | 0.08 | 0.096 | 0.13 | 0.2 | 0.23 |
| SF @60 °C (%) | 80 | 78 | 79 | 74 | 73 | 72 |
| Hysteresis @60 °C | 0.13 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 |
| −ΔHh (kJ·mol−1) | 41.6 | 40.2 | 40 | 39.1 | 38.3 | 38.5 |
| −ΔSh (J·mol−1·K) | 125 | 119 | 120 | 120 | 121 | 122 |
| Electrochemical and Magnetics Properties | Ce0 | Ce1 | Ce2 | Ce3 | Ce4 | Ce5 |
|---|---|---|---|---|---|---|
| 3rd cycle capacity @50 mA·g−1 (mAh·g−1) | 300 | 378 | 381 | 380 | 355 | 355 |
| 3rd cycle capacity @4 mA·g−1 (mAh·g−1) | 376 | 400 | 396 | 392 | 363 | 364 |
| HRD (%) | 80 | 95 | 96 | 97 | 98 | 98 |
| Number of activation cycle(s) to reach 92% HRD | 6 | 2 | 1 | 1 | 1 | 1 |
| Diffusion coefficient, D (10−10 cm2·s−1) | 2.1 | 3.1 | 4.2 | 4.3 | 1.5 | 1.7 |
| Surface reaction current, Io (mA·g−1) | 12.8 | 45.8 | 32 | 31.8 | 40 | 29 |
| Charge-transfer resistance, R @−40 °C (Ω·g) | 158.55 | 14.13 | 10.89 | 6.88 | 5.76 | 5.84 |
| Double-layer capacitance, C @−40 °C (F·g−1) | 0.18 | 0.66 | 1.28 | 1.73 | 1.84 | 2.15 |
| RC product @−40 °C (s) | 28.4 | 9.39 | 13.94 | 11.93 | 12.41 | 12.54 |
| Saturated magnetic susceptibility, Ms (emu·g−1) | 0.0353 | 0.197 | 0.529 | 0.627 | 0.534 | 0.733 |
| Applied field at M.S. = ½ Ms, H1/2 (kOe) | 0.500 | 0.177 | 0.218 | 0.196 | 0.210 | 0.196 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, K.-H.; Ouchi, T.; Nei, J.; Moghe, D. The Importance of Rare-Earth Additions in Zr-Based AB2 Metal Hydride Alloys. Batteries 2016, 2, 25. https://doi.org/10.3390/batteries2030025
Young K-H, Ouchi T, Nei J, Moghe D. The Importance of Rare-Earth Additions in Zr-Based AB2 Metal Hydride Alloys. Batteries. 2016; 2(3):25. https://doi.org/10.3390/batteries2030025
Chicago/Turabian StyleYoung, Kwo-Hsiung, Taihei Ouchi, Jean Nei, and Dhanashree Moghe. 2016. "The Importance of Rare-Earth Additions in Zr-Based AB2 Metal Hydride Alloys" Batteries 2, no. 3: 25. https://doi.org/10.3390/batteries2030025