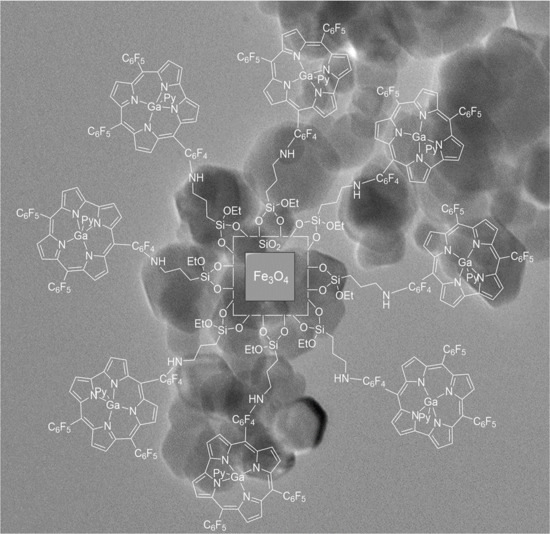
Magnetite–Corrole Hybrid Nanoparticles
Abstract
:1. Introduction
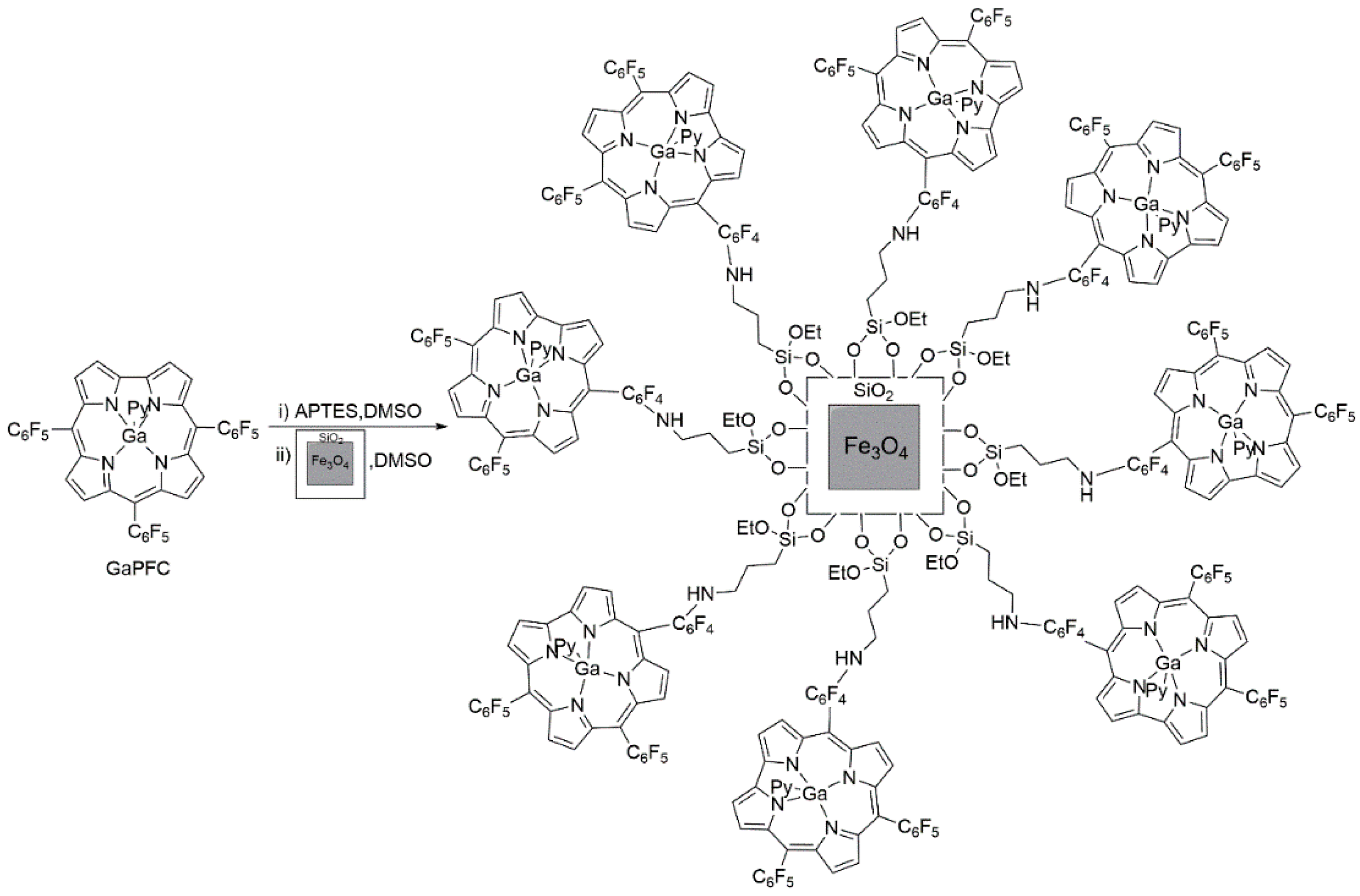
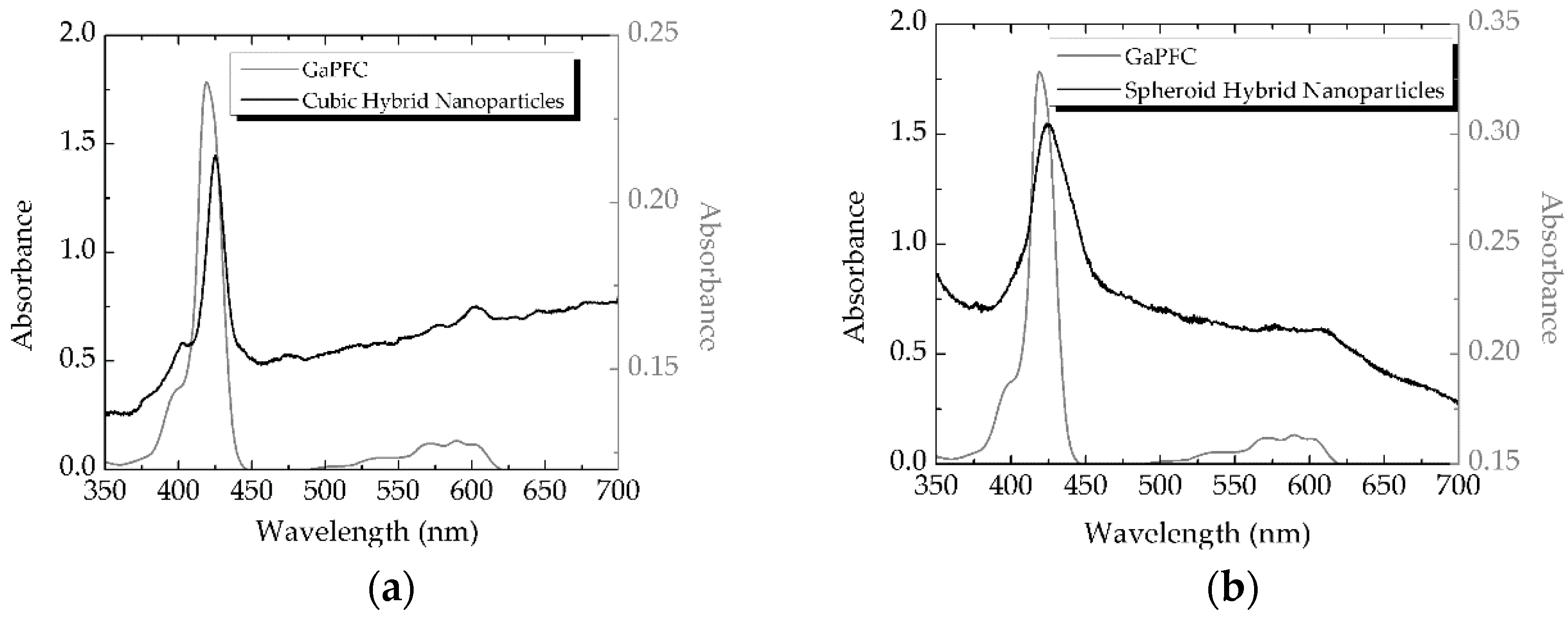
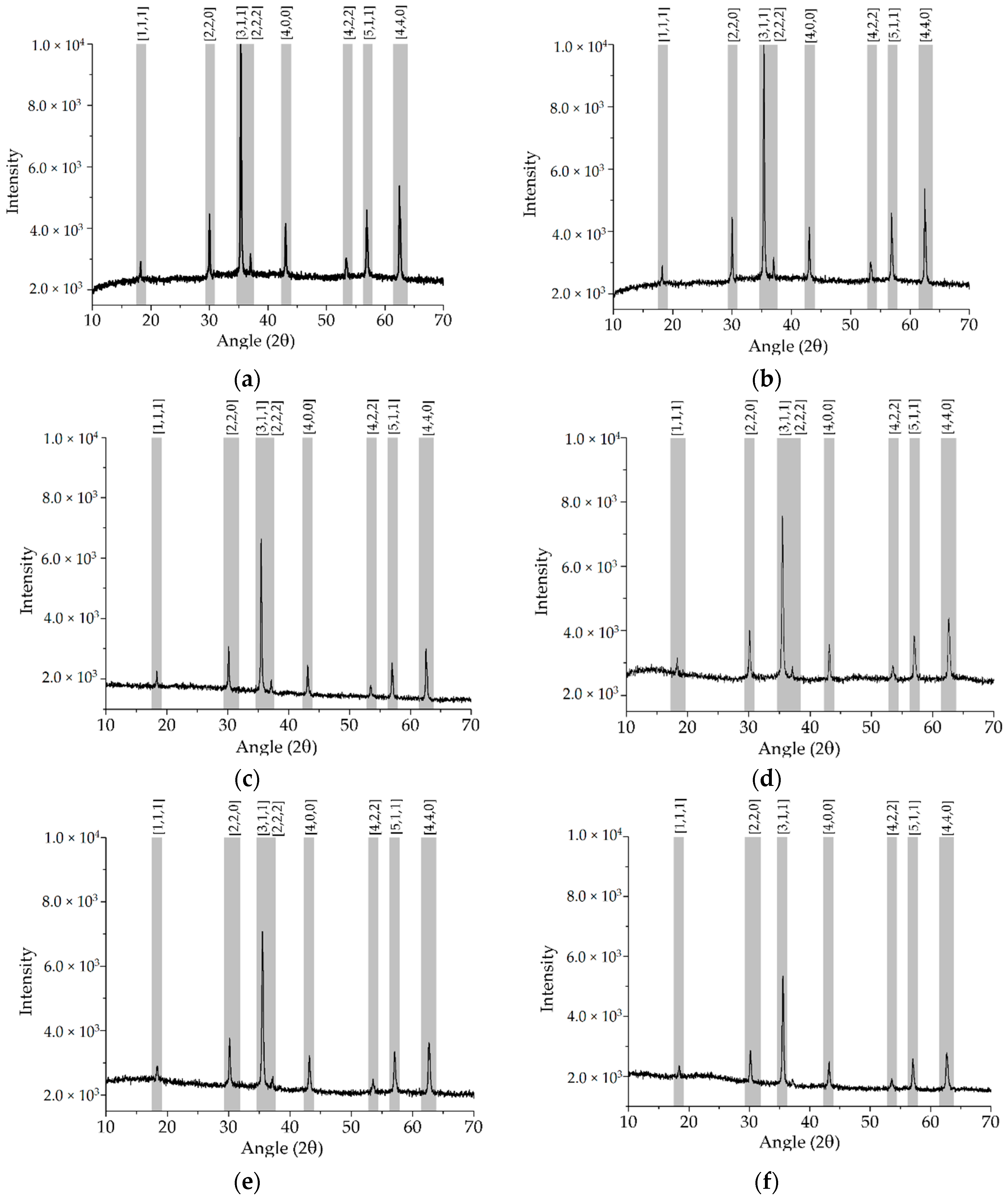
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Syntheses
3.3. Surface Functionalization
3.4. Instrumentation
4. Conclusions
Author Contributions
Funding
Acknowledgment
Conflicts of Interest
References
- Barata, J.F.B.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Cavaleiro, J.A.S. Strategies for corrole functionalization. Chem. Rev. 2017, 117, 3192–3253. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.I.M.; Oliveira, E.; Barata, J.F.B.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Lodeiro, C. Corroles as Anion Chemosensors: Exploiting Their Fluorescence Behaviour from Solution to Solid-Supported Devices. J. Mater. Chem. 2012, 22, 13811–13819. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Oliveira, E.; Barata, J.F.B.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Lodeiro, C. New gallium(III) Corrole Complexes as Colorimetric Probes for Toxic Cyanide Anion. Inorg. Chim. Acta 2014, 417, 148–154. [Google Scholar] [CrossRef]
- Sheng, X.; Zhao, H.; Du, L. Selectivity of Cobalt Corrole for CO vs. O2 and N2 in Indoor Pollution. Sci. Rep. 2017, 7, 14536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lai, W.; Cao, R. Chemical Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef] [PubMed]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2016, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed]
- Teo, R.D.; Hwang, J.Y.; Termini, J.; Gross, Z.; Gray, H.B. Fighting Cancer with Corroles. Chem. Rev. 2017, 117, 2711–2729. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.F.B.; Pinto, R.J.B.; Vaz Serra, V.I.R.C.; Silvestre, A.J.D.; Trindade, T.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Daina, S.; Sadocco, P.; Freire, C.S.R. Fluorescent Bioactive Corrole Grafted-Chitosan Films. Biomacromolecules 2016, 17, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Saltsman, I.; Mahammed, A.; Gross, Z.; Roder, B. Inhibition of Green Algae Growth by Corrole-Based Photosensitizers. J. Appl. Microbiol. 2015, 118, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.D.; Hwang, J.Y.; Wagner, S.; Alonso-Valenteen, F.; Hanson, C.; Taguiam, J.M.; Polo, R.; Harutyunyan, I.; Karapetyan, G.; Sorasaenee, K.; et al. A Corrole Nanobiologic Elicits Tissue-Activated MRI Contrast Enhancement and Tumor-Targeted Toxicity. J. Control. Release 2015, 217, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Samaroo, D.; Perez, E.; Aggarwal, A.; Wills, A.; O’Connor, N. Strategies for Delivering Porphyrinoid-Based Photosensitizers in Therapeutic Applications. Ther. Deliv. 2014, 5, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Preuß, A.; Saltsman, I.; Mahammed, A.; Pfitzner, M.; Goldberg, I.; Gross, Z.; Roder, B. Photodynamic Inactivation of Mold Fungi Spores by Newly Developed Charged Corroles. J. Photochem. Photobiol. B 2014, 133, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Pribisko, M.; Palmer, J.; Grubbs, R.H.; Gray, H.B.; Termini, J.; Lim, P. Cellular Uptake and Anticancer Activity of Carboxylated Gallium Corroles. Proc. Natl. Acad. Sci. USA 2016, 113, E2258–E2266. [Google Scholar] [CrossRef] [PubMed]
- Aviv-Harel, I.; Gross, Z. Aura of Corroles. Chem. Eur. J. 2009, 15, 8382–8394. [Google Scholar] [CrossRef] [PubMed]
- Haber, A.; Angel, I.; Mahammed, A.; Gross, Z. Combating Diabetes Complications by 1-Fe, a Corrole-Based Catalytic Anti-oxidant. J. Diabetes Complicat. 2013, 27, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.F.B.; Zamarron, A.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Tomé, A.C.; Cavaleiro, J.A.S.; Röder, B.; Juarranz, A.; Sanz-Rodriiguez, F. Photodynamic effects induced by meso-tris(pentafluorophenyl)corrole and its cyclodextrin conjugates on cytoskeletal components of HeLa cells. Eur. J. Med. Chem. 2015, 92, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, C.; Sadtler, B.; Fernandez, G.; Dara, L.; Nguyen, C.; Alonso-Valenteen, F.; Medina-Kauwe, L.; Moats, R.; Lewis, N.; Grubbs, R.; et al. Cellular uptake and cytotoxicity of a near-IR fluorescente corrole-TiO2 nanoconjugate. J. Inorg. Biochem. 2014, 140, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, K.; Giribabu, L.; Salvatori, P.; de Angelis, F. Triphenylamine-functionalized corrole sensitizers for solar-cell applications. Phys. Status Solidi A 2015, 212, 194–202. [Google Scholar] [CrossRef]
- Barata, J.F.B.; Daniel-da-Silva, A.L.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Trindade, T. Corrole-silica hybrid particles: Synthesis and effects on singlet oxygen generation. RSC Adv. 2013, 3, 274–280. [Google Scholar] [CrossRef]
- Lemon, C.M.; Nocera, D.G. Comparison of self-assembled and micelle encapsulated QD chemosensor constructs for biological sensing. Faraday Discuss. 2015, 185, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, C.B.; Cerize, N.N.P.; Morais, P.C.; Ré, M.I.; Tedesco, A.C. Biocompatible Magnetic Microscopheres for Use in PDT and Hyperthermia. J. Nanosci. Nanotechnol. 2012, 12, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nanotoday 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Neamtu, M.; Nadejde, C.; Hodoroaba, V.; Schneider, R.; Panne, U. Singlet oxygen generation potential of porphyrin-sensitized magnetite nanoparticles: Synthesis, characterization and photocatalytic application. Appl. Catal. B 2018, 232, 553–561. [Google Scholar] [CrossRef]
- Mak, C.; Pericas, M.; Fagadar-Cosma, E. Functionalization of A3B-type porphyrin with Fe3O4 MNPs. Supramolecular assemblies, gas sensor and catalytic applications. Catal. Today 2018, 306, 268–275. [Google Scholar] [CrossRef]
- Carvalho, C.M.B.; Alves, E.; Costa, L.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Cunha, Â.; Lin, Z.; Rocha, J. Functional Cationic Nanomagnet–Porphyrin Hybrids for the Photoinactivation of Microorganisms. ACS Nano 2010, 4, 7133–7140. [Google Scholar] [CrossRef] [PubMed]
- Bakhshayesh, S.; Dehghani, H. Synthesis of magnetite-porphyrin nanocomposite and its application as novel magnetic adsorbent for removing heavy cations. Mat. Res. Bull. 2013, 48, 2614–2624. [Google Scholar] [CrossRef]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Photodynamic properties and photoinactivation of microorganisms mediated by 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin covalently linked to silica-coated magnetite nanoparticles. J. Photochem. Photobiol. A 2017, 346, 452–461. [Google Scholar] [CrossRef]
- Alves, E.; Rodrigues, J.; Faustino, M.; Neves, M.; Cavaleiro, J.; Lin, Z.; Cunha, Â.; Nadais, M.; Tomé, J.; Almeida, A. A new insight on nanomagnet-porphyrin hybrids for photodynamic inactivation of microorganisms. Dyes Pigments 2014, 110, 80–88. [Google Scholar] [CrossRef]
- Rabbani, M.; Rafiee, F.; Ghafuri, H.; Rahimi, R. Synthesis of Fe3O4 nanoparticles via a fast and facile mechanochemical method: Modification of surface with porphyrin and photocatalytic study. Mater. Lett. 2016, 166, 247–250. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, S.; Pang, L.; Chen, P.; Zhu, G. Porphyrin-based magnetic nanocomposites for efficient extraction of polycyclic aromatic hydrocarbons from water samples. J. Chromatogr. A 2018, 1540, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gross, Z.; Golubkov, G.; Simkhovich, L. Structural, electrochemical, and photophysical properties of gallium(III) 5,10,15-tris(pentafluorophenyl)corrole. Angew. Chem. 2000, 39, 4048–4051. [Google Scholar]
- Girginova, P.I.; Daniel-da-Silva, A.L.; Lopes, C.B.; Figueira, P.; Otero, M.; Amaral, V.S.; Pereira, E.; Trindade, T. Silica coated magnetite particles for magnetic removal of Hg2+ from water. J. Colloid Interface Sci. 2010, 345, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Silva, R.; Pinto da Costa, J.; Vitorino, R.; Daniel-da-Silva, A.L. Magnetic chelating nanoprobes for enrichment and selective recovery of metalloproteases from human saliva. J. Mater. Chem. B 2015, 3, 238–249. [Google Scholar] [CrossRef]
- Tavares, D.S.; Daniel-da-Silva, A.L.; Lopes, C.B.; Silva, N.J.O.; Amaral, V.S.; Rocha, J.; Pereira, E.; Trindade, T. Efficient sorbents based on magnetite coated with siliceous hybrid shells for removal of mercury ions. J. Mater. Chem. A 2013, 1, 8134–8143. [Google Scholar] [CrossRef]
- Gryko, D.T.; Koszarna, B. Refined methods for the synthesis of meso-substituted A3- and trans-A2B-corroles. Org. Biomol. Chem. 2003, 1, 3–10. [Google Scholar] [CrossRef]

| 536 cm−1 | 551–558 cm−1 | 773–785 cm−1 | 1002–1028 cm−1 | 1400–1600 cm−1 | 700–1500 cm−1 | |
|---|---|---|---|---|---|---|
| Cuboid and spheroid Fe3O4 | ν(Fe–O) | - | - | - | - | - |
| Cuboid and spheroid Fe3O4@SiO2 | - | ν(Fe–O) | ν(Si–O–Si) | ν(Si–O–Si) | - | - |
| Cuboid and spheroid Magnetite–corrole Hybrid nanoparticles | - | ν(Fe–O) | ν(Si–O–Si) | ν(Si–O–Si) | n(C–H), n(C–C), n(C–N) | n(C–H), n(C–C), n(C–N) |
| Photosensitizer | DPiBF Decay (%) |
|---|---|
| GaPFC (0.008mg/mL) | 80 |
| Cuboid hybrid nanoparticles (0.231 mg/mL) | 15 |
| Spheroid hybrid (0.242 mg/mL) | 25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.A.; Trindade, T.; Barata, J.F.B. Magnetite–Corrole Hybrid Nanoparticles. Magnetochemistry 2018, 4, 37. https://doi.org/10.3390/magnetochemistry4030037
Pereira RA, Trindade T, Barata JFB. Magnetite–Corrole Hybrid Nanoparticles. Magnetochemistry. 2018; 4(3):37. https://doi.org/10.3390/magnetochemistry4030037
Chicago/Turabian StylePereira, Rute A., Tito Trindade, and Joana F. B. Barata. 2018. "Magnetite–Corrole Hybrid Nanoparticles" Magnetochemistry 4, no. 3: 37. https://doi.org/10.3390/magnetochemistry4030037