Synthesis, Crystal Structures, and Magnetic Properties of Lanthanide (III) Amino-Phosphonate Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses of the Complexes
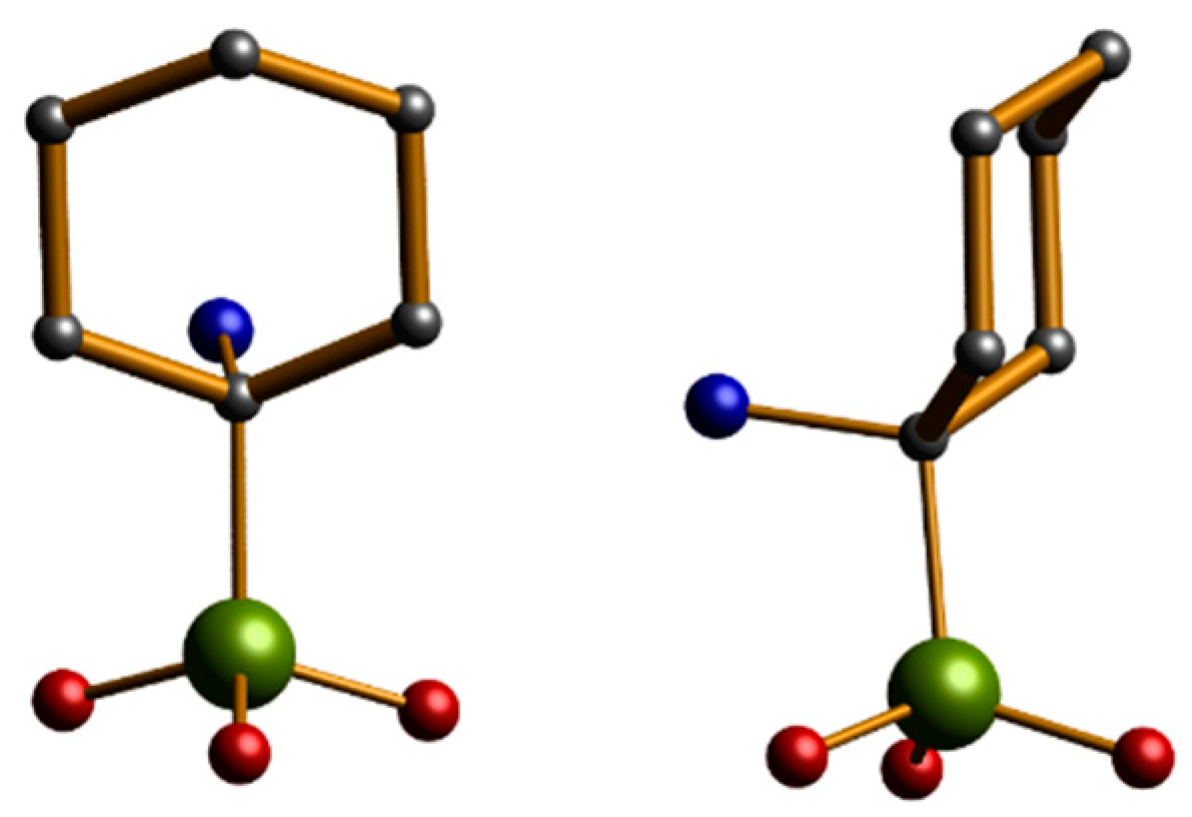
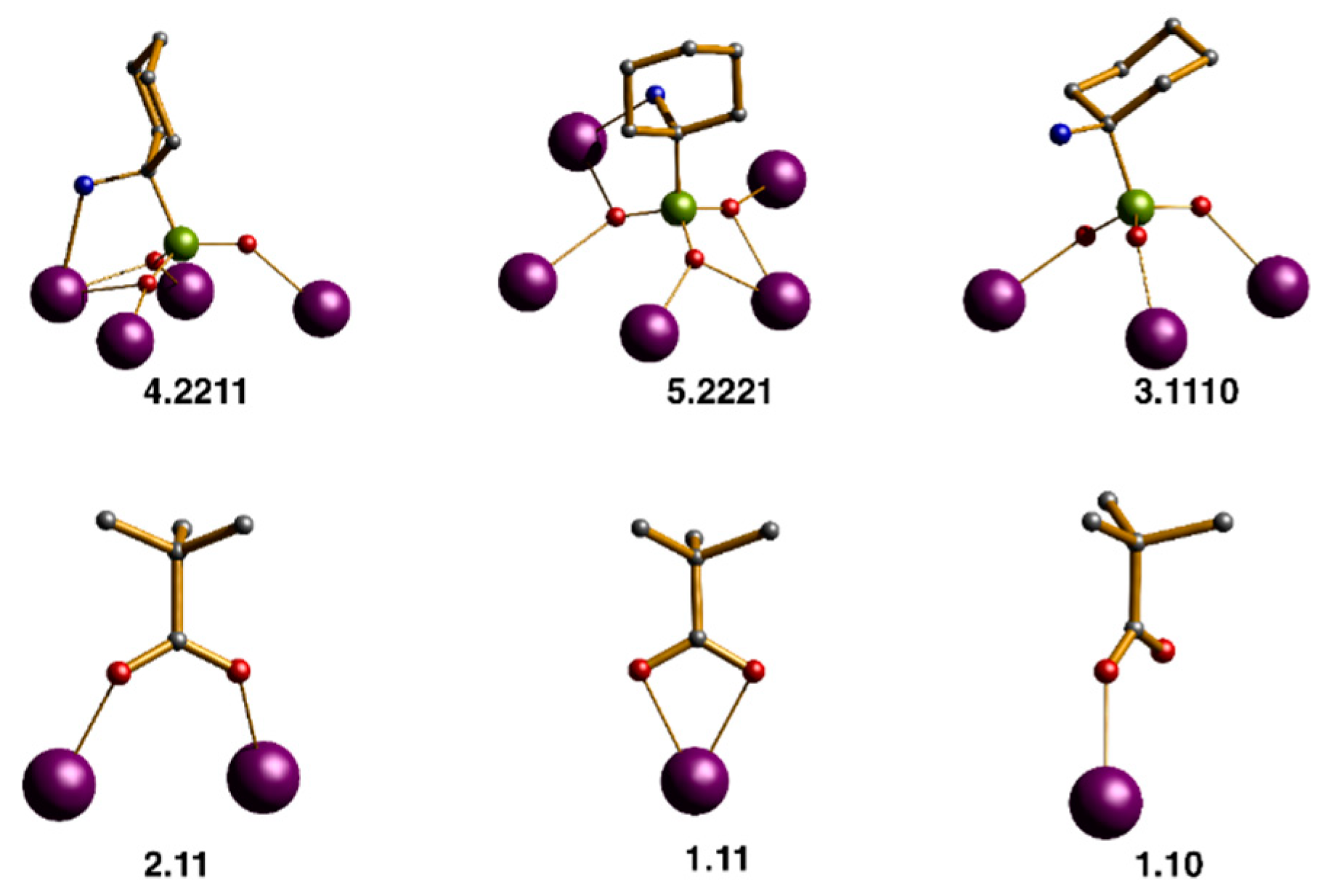
2.2. Description of the Structures
2.3. Magnetic Properties
3. Experimental Section
3.1. Starting Materials
3.2. Synthesis of [Ln10(μ3-OH)3(µ-OH) (CO3)2(O2CtBu)15(O3PC6H10NH2)3(O3PC6H10NH3)2(H2O)2] [Et2NH2] (Ln = Gd(III), 1 and Tb(III), 2)
3.3. Magnetic Measurements
3.4. Crystallographic Data Collection and Refinement
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Clearfield, A. Organically Pillared Micro- and Mesoporous Materials. Chem. Mater. 1998, 10, 2801–2810. [Google Scholar] [CrossRef]
- Groves, J.A.; Miller, S.R.; Warrender, S.J.; Mellot, D.-C.; Lightfoot, P.; Wright, P.A. The first route to large pore metal phosphonates. Chem. Commun. 2006, 31, 3305–3307. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhu, G.; Qiu, S.; Huang, K.; Yu, J.; Xu, R. Zn2[(S)-O3PCH2NHC4H7CO2]2. A Homochiral 3D Zinc Phosphonate with Helical Channels. Angew. Chem. Int. Ed. 2004, 43, 6482–6485. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.-B.; Hu, S.-M.; Wu, X.-T. Two new molecular zinc phosphonates with bright luminescence for sensing UV radiation. CrystEngComm 2013, 15, 8937–8940. [Google Scholar] [CrossRef]
- Huang, J.; Bao, S.-S.; Ling, L.-S.; Zhu, H.; Li, Y.-Z.; Pi, L.; Zheng, L.-M. A Racemic Polar Cobalt Phosphonate with Weak Ferromagnetism. Chem. Eur. J. 2012, 18, 10839–10842. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-Y.; Xu, H.-B.; Mao, J.-G. Rational Design of 0D, 1D, and 3D Open Frameworks Based on Tetranuclear Lanthanide(III) Sulfonate−Phosphonate Clusters. Inorg. Chem. 2006, 45, 9780–9788. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.-H.; Yi, F.-Y.; Li, P.-X.; Mao, J.-G. Synthesis, Crystal Structures, and Luminescent Properties of Two Series’ of New Lanthanide (III) Amino-Carboxylate-Phosphonates. Inorg. Chem. 2010, 49, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Zangana, K.H.; Moreno-Pineda, E.; Iñigo, V.-Y.; McInnes, J.L.; Winpenny, R.E.P. Linking Cr3 triangles through phosphonates and lanthanides: Synthetic, structural, magnetic and EPR studies. Dalton Trans. 2014, 43, 13242–13249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Song, Y.; Okamura, T.; Hasegawa, Y.; Sun, W.Y.; Ueyama, N. Syntheses, Structures, Near-Infrared and Visible Luminescence, and Magnetic Properties of Lanthanide-Organic Frameworks with an Imidazole-Containing Flexible Ligand. Inorg. Chem. 2006, 45, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; Chastanet, G.; Sessoli, R.; Mallah, T.; Wernsdorfer, W.; Winpenny, R.E.P. Minor changes in phosphonate ligands lead to new hexa- and dodeca-nuclear Mn clusters. J. Mater. Chem. 2006, 16, 2576–2578. [Google Scholar] [CrossRef]
- Shanmugam, M.; Chastanet, G.; Mallah, T.; Sessoli, R.; Teat, S.J.; Timco, G.A.; Winpenny, R.E.P. Synthesis and Characterization of Mixed-Valent Manganese Phosphonate Cage Complexes. Chem. Eur. J. 2006, 12, 8777–8785. [Google Scholar] [CrossRef] [PubMed]
- Zangana, K.H.; Moreno-Pineda, E.; Winpenny, R.E.P. Single molecule magnet behaviour in a {Dy4P2} octahedron. Dalton Trans. 2015, 44, 12522–12525. [Google Scholar] [CrossRef] [PubMed]
- Zangana, K.H.; Moreno-Pineda, E.; Schnack, J.; Winpenny, R.E.P. Octametallic 4f-phosphonate horseshoes. Dalton Trans. 2013, 42, 14045–14048. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, A.; Sharma, C.V.K.; Zhang, B. Crystal Engineered Supramolecular Metal Phosphonates: Crown Ethers and Iminodiacetates. Chem. Mater. 2001, 13, 3099–3112. [Google Scholar] [CrossRef]
- Ngo, H.L.; Lin, W. Chiral Crown Ether Pillared Lamellar Lanthanide Phosphonates. J. Am. Chem. Soc. 2002, 124, 14298–14299. [Google Scholar] [CrossRef] [PubMed]
- Vojtíšek, P.; Cígler, P.; Kotek, J.; Rudovský, J.; Hermann, P.; Lukeš, I. Crystal Structures of Lanthanide(III) Complexes with Cyclen Derivative Bearing Three Acetate and One Methylphosphonate Pendants. Inorg. Chem. 2005, 44, 5591–5599. [Google Scholar] [CrossRef] [PubMed]
- Bligh, S.W.A.; Choi, N.; Geraldes, C.F.G.C.; Knoke, S.; McPartlin, M.; Sanganee, M.J.; Woodroffe, T.M. A novel hexaaza macrocycle with methylenephosphonate pendant arms: A potential useful chelate for biomedical applications. Dalton Trans. 1997, 21, 4119–4126. [Google Scholar] [CrossRef]
- Avecilla, F.; Peters, J.A.; Geraldes, C.F.G.C. X-ray Crystal Structure of a Sodium Salt of [Gd(DOTP)]5−: Implications for Its Second-Sphere Relaxivity and the 23Na NMR Hyperfine Shift Effects of [Tm(DOTP)]5−. Eur. J. Inorg. Chem. 2003, 23, 4179–4186. [Google Scholar] [CrossRef]
- Legendziewicz, J.; Gawryszewska, P.; Gałdecka, E.; Galdecki, Z. Novel polynuclear compound of europium with N-phosphonomethylglycine: Spectroscopy and structure. J. Alloys Compd. 1998, 275, 356–360. [Google Scholar] [CrossRef]
- Gałdecka, E.; Gałdecki, Z.; Gawryszewska, P.; Legendziewicz, J. Structure of a novel polynuclear europium compound with N-phosphonomethylglycine: Heptaaquaperchloratodi-μ4-N-phosphonomethylglycine-dieuropium(III) triperchlorate monohydrate, [Eu2(HO3PCH2NH2CH2CO2)2(H2O)7(ClO4)]·3ClO4·H2O. New J. Chem. 2000, 24, 387–391. [Google Scholar] [CrossRef]
- Tang, S.-F.; Song, J.-L.; Mao, J.-G. Syntheses, Crystal Structures, and Characterizations of a Series of New Layered Lanthanide Carboxylate-Phosphonates. Eur. J. Inorg. Chem. 2006, 2006, 2011–2019. [Google Scholar] [CrossRef]
- Yue, Q.; Yang, J.; Li, G.-H.; Li, G.-D.; Chen, J.-S. Homochiral Porous Lanthanide Phosphonates with 1D Triple-Strand Helical Chains: Synthesis, Photoluminescence, and Adsorption Properties. Inorg. Chem. 2006, 45, 4431–4439. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-F.; Song, J.-L.; Li, X.-L.; Mao, J.-G. Luminescent Lanthanide(III) Carboxylate−Phosphonates with Helical Tunnels. Cryst. Growth Des. 2006, 6, 2322–2326. [Google Scholar] [CrossRef]
- Głowiak, T.; Huskowska, E.; Legendziewicz, J. Preparation and X-ray crystal structure determination of an octahedral polymeric lutetium compound with ciliatine; {Lu(PO3HCH2CH2NH3)3(ClO4)3·3D2O}n. Polyhedron 1991, 10, 175–178. [Google Scholar] [CrossRef]
- Groves, J.A.; Wright, P.A.; Lightfoot, P. Two Closely Related Lanthanum Phosphonate Frameworks Formed by Anion-Directed Linking of Inorganic Chains. Inorg. Chem. 2005, 44, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Comby, S.; Scopelliti, R.; Imbert, D.; Charbonnière, L.; Ziessel, R.; Bünzli, J.C.G. Dual Emission from Luminescent Nonalanthanide Clusters. Inorg. Chem. 2006, 45, 3158–3160. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.-K.; Li, Y.-Z.; Song, Y.; Zheng, L.-M. Three-, Two-, and One-Dimensional Metal Phosphonates Based on [Hydroxy(4-pyridyl)methyl]phosphonate: M{(4-C5H4N)CH(OH)PO3}(H2O) (M = Ni, Cd) and Gd{(4-C5H4N)CH(OH)P(OH)O2}3·6H2O. Inorg. Chem. 2005, 44, 3599–3604. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.M.; Bingamin, I.; Rapko, B.M.; Fox, J.; Duesler, E.N.; Paine, R.T. Hydrogen Bonded Framework Structures Constructed from 2-(Pyridyl N-oxide) Methylphosphonic Acid Ligands and Erbium(III). Inorg. Chem. 2004, 43, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.-M.; Rapko, B.M.; Fox, J.; Binyamin, I.; Pailloux, S.; Duesler, E.N.; Paine, R.T. A Three-Dimensional Framework Structure Constructed from 2-(2-Pyridyl-N-oxide) Ethylphosphonic Acid and Nd(III). Inorg. Chem. 2006, 45, 3741–3745. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.-M.; Zeng, X.-R.; Fang, X.-N.; Li, X.-F.; Liu, D.-S. Synthesis, crystal structure and fluorescent characterization of a novel lanthanide tetraphosphonate with a layered structure. Inorg. Chim. Acta 2006, 359, 1589–1593. [Google Scholar] [CrossRef]
- Nash, K.L.; Rogers, R.D.; Ferraro, J.; Zhang, J. Lanthanide complexes with 1-hydroxyethane-1,1-diphosphonic acid: Solvent organization and coordination geometry in crystalline and amorphous solids. Inorg. Chem. Acta 1998, 269, 211–223. [Google Scholar] [CrossRef]
- Gan, X.-M.; Binyamin, I.; Pailloux, S.; Duesler, E.N.; Paine, R.T. Formation of a layered framework structure based upon 4-methyl-2,6-bis(methylphosphonic acid) phenol. Dalton Trans. 2006, 32, 3912–3917. [Google Scholar] [CrossRef] [PubMed]
- Wharmby, M.T.; Miller, S.R.; Groves, J.A.; Margiolaki, I.; Ashbrooka, S.E.; Wright, P.A. Yttrium bisphosphonate STA-13: A racemic phosphonate metal organic framework with permanent microporosity. Dalton Trans. 2010, 39, 6389–6391. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Manseki, K.; Hisashi, O. d–f Heteronuclear complexes: synthesis, structures and physicochemical aspects. Coord. Chem. Rev. 2001, 221, 379–414. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Sun, Y.-H.; Liu, Q.-Y.; Xie, Y.-R.; Wen, H.-R. Octanuclear aluminum(III) and iron(III) phosphonate cages encapsulating two Na(I) ions. Inorg. Chem. 2009, 48, 7015–7017. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.A.; Gil-Hernández, B.; Abu-Shandi, K.; Sanchiz, J.; Janiak, C. Iron, copper and zinc ammonium-1-hydroxyalkylidene-diphosphonates with zero-, one- and two-dimensional covalent metal–ligand structures extended into three-dimensional supramolecular networks by charge-assisted hydrogen-bonding. Polyhedron 2010, 29, 2537–2545. [Google Scholar] [CrossRef]
- Li, J.T.; Guo, L.R.; Shen, Y.; Zheng, L.M. LiF-assisted crystallization of zinc 4-carboxyphenylphosphonates with pillared layered structures. CrystEngComm 2009, 11, 1674–1678. [Google Scholar] [CrossRef]
- Moreno-Pineda, E.; Tuna, F.; Pritchard, R.G.; Regan, A.C.; Winpenny, R.E.P.; McInnes, E.J.L. Molecular amino-phosphonate cobalt–lanthanide clusters. Chem. Commun. 2013, 49, 3522–3524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-M.; Zangana, K.H.; Kostopoulos, A.K.; Tong, M.-L.; Winpenny, R.E.P. A pseudo-icosahedral cage {Gd12} based on aminomethylphosphonate. Dalton Trans. 2016, 45, 9041–9044. [Google Scholar] [CrossRef] [PubMed]
- Aboshyan, S.L.; Cantuel, M.; Petoud, S.; Hauser, A.; Piguet, C. Optical sensitization and upconversion in discrete polynuclear chromium–lanthanide complexes. Coord. Chem. Rev. 2012, 256, 1644–1663. [Google Scholar] [CrossRef]
- Mao, J.-G. Structures and luminescent properties of lanthanide phosphonates. Coord. Chem. Rev. 2007, 251, 1493–1520. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Moreno-Pineda, E.; Helliwell, M.; Winpenny, R.E.P. Mn(II) -Gd(III) phosphonate cages with a large magnetocaloric effect. Chem. Eur. J. 2012, 18, 4161–4165. [Google Scholar] [CrossRef] [PubMed]
- Konar, S.; Clearfield, A. Synthesis and characterization of high nuclearity iron(III) phosphonate molecular clusters. Inorg. Chem. 2008, 47, 5573–5579. [Google Scholar] [CrossRef] [PubMed]
- Goura, J.; Bag, P.; Mereacre, V.; Powell, A. K.; Chandrasekhar, V. Molecular iron(III) phosphonates: Synthesis, structure, magnetism, and Mössbauer studies. Inorg. Chem. 2014, 53, 8147–8154. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.R.; Schmitt, W.; Evans, R.C. Lighting Up Two-Dimensional Lanthanide Phosphonates: Tunable Structure—Property Relationships toward Visible and Near-Infrared Emitters. J. Phys. Chem. 2014, 118, 10291–10301. [Google Scholar] [CrossRef]
- Song, J.-L.; Lei, C.; Mao, J.-G. Syntheses, Crystal Structures, and Luminescent Properties of Novel Layered Lanthanide Sulfonate−Phosphonates. Inorg. Chem. 2004, 43, 5630–5634. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-L.; Mao, J.-G. New Types of Blue, Red or Near IR Luminescent Phosphonate-Decorated Lanthanide Oxalates. Eur. J. Inorg. Chem. 2005, 11, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.-M.; Mao, J.-G. Introducing a Second Ligand: New Route to Luminescent Lanthanide Polyphosphonates. Cryst. Growth Des. 2006, 6, 964–968. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Evangelisti, M.; Winpenny, R.E.P. Co–Gd phosphonate complexes as magnetic refrigerants. Chem. Sci. 2011, 2, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-Z.; Evangelisti, M.; Winpenny, R.E.P. Large Magnetocaloric Effect in a Wells–Dawson Type {Ni6Gd6P6} Cage. Angew. Chem. Int. Ed. 2011, 50, 3692–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.-Z.; Evangelisti, M.; Tuna, F.; Winpenny, R.E.P. Co–Ln Mixed-Metal Phosphonate Grids and Cages as Molecular Magnetic Refrigerants. J. Am. Chem. Soc. 2012, 134, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangana, K.H.; Moreno-Pineda, E.; McInnes, E.J.L.; Schnack, J.; Winpenny, R.E.P. Centred nine-metal rings of lanthanides. Chem. Commun. 2014, 50, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Coxall, R.A.; Harris, S.G.; Henderson, D.K.; Parsons, S.; Tasker, P.A.; Winpenny, R.E.P. Inter-ligand reactions: In situ formation of new polydentate ligands. Dalton Trans. 2000, 14, 2349–2356. [Google Scholar] [CrossRef]
- Moreno-Pineda, E.; Lorusso, G.; Zangana, K.H.; Palacios, E.; Schnack, J.; Evangelisti, M.; Winpenny, R.E.P.; McInnes, E.J.L. Observation of the influence of dipolar and spin frustration effects on the magnetocaloric properties of a trigonal prismatic {Gd7} molecular nanomagnet. Chem. Sci. 2016, 7, 4891–4895. [Google Scholar] [CrossRef]
- Bochkarev, M.N.; Fedorova, E.A.; Radkov, Y.F.; Khorshev, S.Y.; Kalinina, G.S.; Razuvaev, G.A. Carbon dioxide fixaion by lathanide complexes. J. Organomet. Chem. 1983, 258, C29–C33. [Google Scholar] [CrossRef]
- Tang, X.-L.; Wang, W.-H.; Dou, W.; Jiang, J.; Liu, W.-S.; Qin, W.-W.; Zhang, G.-L.; Zhang, H.-R.; Yu, K.-B.; Zheng, L.-M. Olive-Shaped Chiral Supramolecules: Simultaneous Self-Assembly of Heptameric Lanthanum Clusters and Carbon Dioxide Fixation. Angew. Chem. Int. Ed. 2009, 48, 3499–3502. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhao, L.; Guo, Y.-N.; Guo, Y.; Tang, J.; Liu, Z. Quadruple-CO32− bridged octanuclear dysprosium(III) compound showing single-molecule magnet behavior. Chem. Commun. 2012, 48, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Zangana, K.H.; Moreno-Pineda, E.; Winpenny, R.E.P. Tetrametallic lanthanide(III) phosphonate cages: Synthetic, structural and magnetic studies. Dalton Trans. 2014, 43, 17101–17107. [Google Scholar] [CrossRef] [PubMed]
- Fomina, I.G.; Kiskin, M.A.; Martynov, A.G.; Aleksandrov, G.G.; Dobrokhotova, Zh.V.; Gorbunova, Y.G.; Shvedenkov, Y.G.; Tsivadze, A.Y.; Novotortsev, V.M. Lanthanum(III), Samarium(III), Europium(III), and Thulium(III) Binuclear Acetates and Pivalates: Synthesis, Structure, Magnetic Properties, and Solid-Phase Thermolysis. Russ. J. Inorg. Chem. 2004, 49, 1463–1474. [Google Scholar]
- Zoan, T.A.; Kuzmina, N.P.; Frolovskaya, S.N.; Rykov, A.N.; Mitrofanova, N.D.; Troyanov, S.I.; Pisarevsky, A.P.; Martynenko, L.I.; Korenev, Y.M. Synthesis, structure and properties of volatile lanthanide pivalates. J. Alloys Compd. 1995, 225, 396–399. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C17, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourthis, L.J.; Gildea, R.L.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sluis, P.V.D.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Cryst. 1990, A46, 194–201. [Google Scholar] [CrossRef]
- Evangelisti, M.; Berchin, E.K. Recipes for enhanced molecular cooling. Dalton Trans. 2010, 39, 4672–4676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serre, C.; Stock, N.; Bein, T.; Ferey, G. Synthesis and Characterization of a New Three-Dimensional Lanthanide Carboxyphosphonate: Ln4(H2O)7[O2C−C5H10N−CH2-PO3]4(H2O)5. Inorg. Chem. 2004, 43, 3159–3163. [Google Scholar] [CrossRef] [PubMed]



| Compound b | Yield a | Elemental Analysis: Found (Calculated) | ||||
|---|---|---|---|---|---|---|
| C | H | Ln | P | N | ||
| 1 (MeCN)5 | 45% | 32.50 (32.45) | 5.15 (5.20) | 35.15 (35.10) | 3.45 (3.46) | 3.48 (3.44) |
| 2 (MeCN)8 | 39% | 33.06 (33.02) | 5.21 (5.24) | 34.38 (34.40) | 3.38 (3.35) | 4.22 (4.25) |
| 1 | 2 | |
|---|---|---|
| Formula a | C134H249.5Gd10N17.5 O59 P5 | C149H270Tb10N25O59 P5 |
| Fw | 4366.81 | 4463.60 |
| T/K | 150(1) | 150(1) |
| Cryst system | monoclinic | monoclinic |
| space group | P21/n | P21/n |
| a/Å | 17.9933(3) | 18.0239(3) |
| b/Å | 33.9737(5) | 34.0856(4) |
| c/Å | 28.6063(4) | 28.6516(4) |
| α/° | 90 | 90 |
| β/° | 90.883(2) | 91.006(2) |
| γ/° | 90 | 90 |
| V/Å3 | 17484.9(5) | 17599.6(4) |
| Z | 2 | 4 |
| ρ calcd/g cm−3 | 1.659 | 1.678 |
| μ (Mo Kα)/mm−1 | 3.855 | 4.083 |
| R1(I > 2σ)(I)) a | 0.0516 | 0.0714 |
| wR2 a | 0.1283 | 0.1459 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangana, K.H. Synthesis, Crystal Structures, and Magnetic Properties of Lanthanide (III) Amino-Phosphonate Complexes. Magnetochemistry 2018, 4, 29. https://doi.org/10.3390/magnetochemistry4030029
Zangana KH. Synthesis, Crystal Structures, and Magnetic Properties of Lanthanide (III) Amino-Phosphonate Complexes. Magnetochemistry. 2018; 4(3):29. https://doi.org/10.3390/magnetochemistry4030029
Chicago/Turabian StyleZangana, Karzan H. 2018. "Synthesis, Crystal Structures, and Magnetic Properties of Lanthanide (III) Amino-Phosphonate Complexes" Magnetochemistry 4, no. 3: 29. https://doi.org/10.3390/magnetochemistry4030029




