Agronomic Management for Enhancing Plant Tolerance to Abiotic Stresses—Drought, Salinity, Hypoxia, and Lodging
Abstract
:1. Introduction
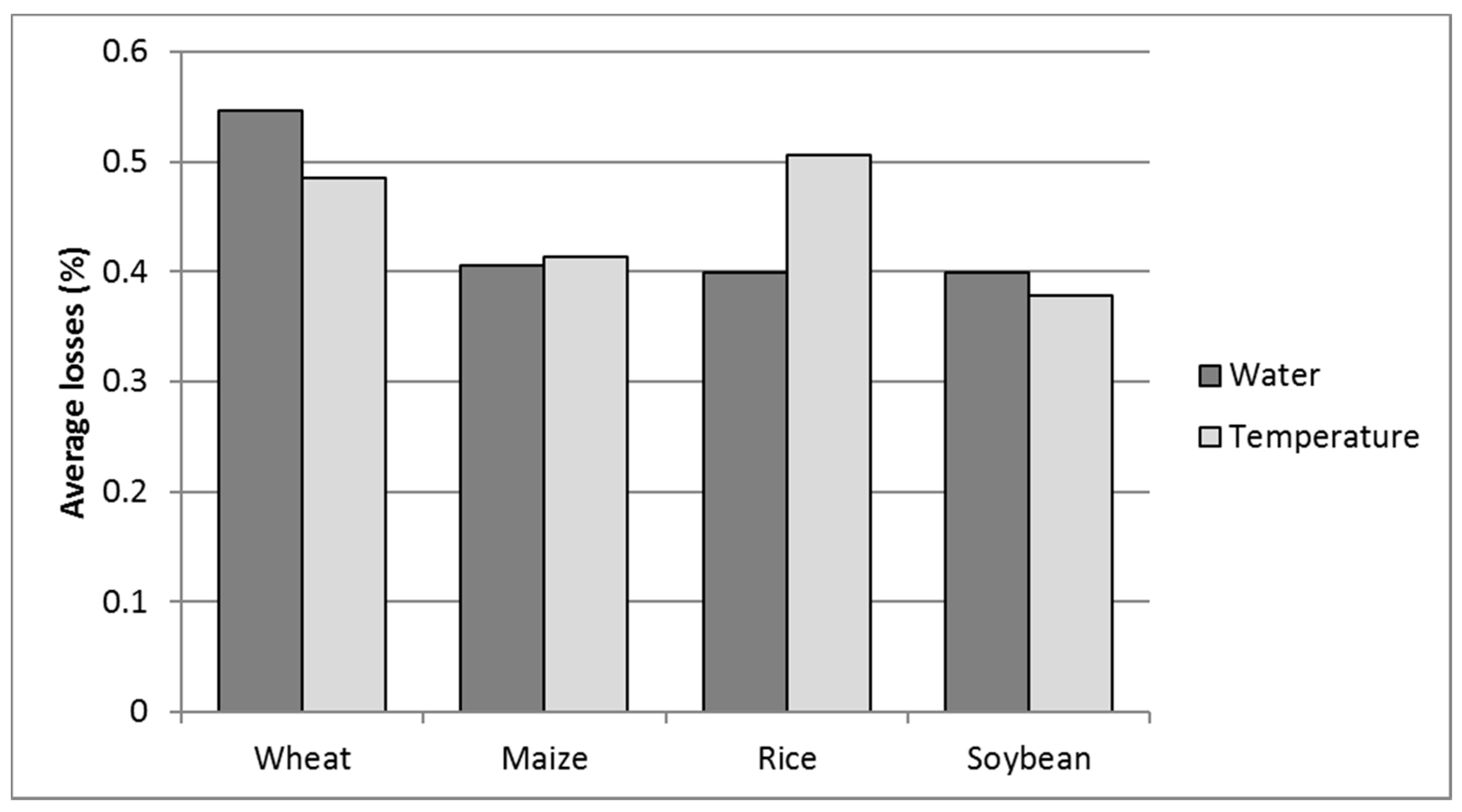
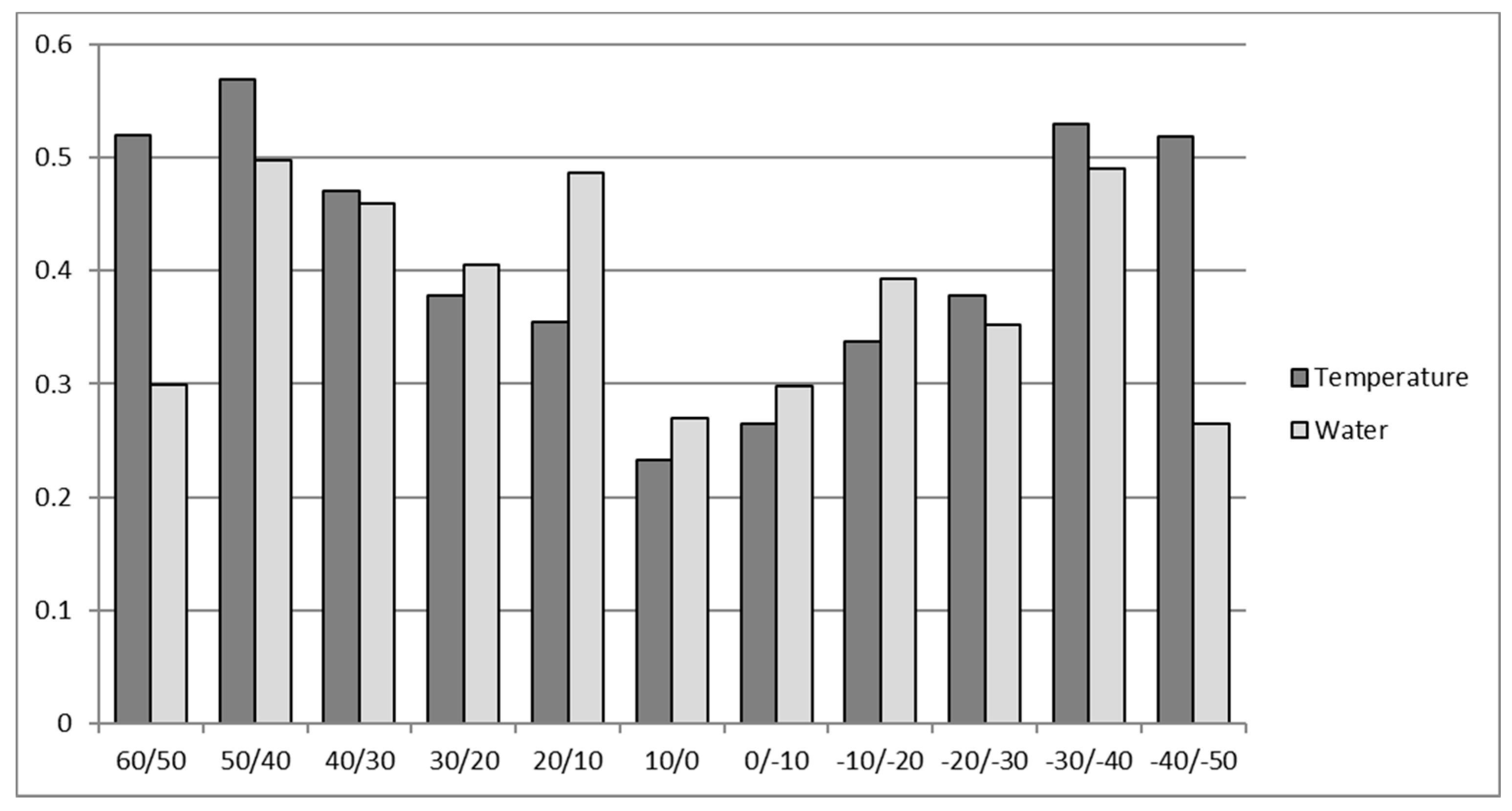
2. Role and Impact of Abiotic Limitations to Crop Yield
3. Soil Texture, Structure, and Field Hydraulic Arrangements
4. The Impacts of Individual Stress Factors on Crops
4.1. Hypoxia/Anoxia Stress
4.2. Drought Stress and Dry Farming
- -
- adopting hydraulic arrangements that slow down the speed of surface water runoff and allow water penetration into soil, refilling the underground water reservoir. The infiltration of surface water can also be enhanced by appropriate tillage (e.g., by surface tillage that increases the roughness of the soil). Different tillage methods have a different effect on soil porosity and water infiltration. Ploughing to the depth of 20 cm is the tillage system that gives the highest porosity and water storage capacity, while the lowest was obtained in no-tillage systems [44];
- -
- enhancing the drainage of infiltrated water in order to reach the entire profile explored by roots (e.g., by deep tillage carried out with rippers);
- -
- favoring seasonal flooding of soils by water courses;
- -
- catching runoff water and directing it towards compliant areas suitable for agriculture.
- -
- breaking waterproof or compacted layers by means of ripping or ploughing;
- -
- intervening with drainage or filled ditches to contain the winter rise of the water table, which imposes on crops a superficial root system, enhancing their sensitivity to summer drought.
- -
- adopting management practices useful for promoting a granular structure with a good equilibrium between macro- and microporosity;
- -
- increasing soil organic matter with organic fertilizers or green manure (the positive effect of organic matter on the soil reservoir is more significant in sandy soils or clay soils with low-quality clays—kaolinite). Organic matter increases the water storage ability of soils. Therefore, higher organic matter concentration means higher water availability for a crop [45];
- -
- adjusting soil texture by mixing surface horizons with excess of sand with lower layers richer in clay (the presupposition for this activity is a suitable analysis of the soil profile and horizon distribution);
- -
- adopting fallow techniques (field plowed and harrowed but left unseeded for one year) aimed at accumulating water in the soil during the “rest” period. For example, the biennial rotation fallow—wheat can be a solution for crop areas where yearly rainfall is insufficient for continuous cultivation. In this context, while the naked fallow should be avoided due to erosion problems and negative impacts on humus content, the most rational form of fallow is that of early autumn plowing (before the rainy season) and superficial work (harrowing) during the following spring and summer, whenever the soil appears encrusted or covered by weeds that waste a relevant quantity of water.
- -
- minimizing tillage works (minimum tillage, sod seeding) in order to limit water evaporation from clods exposed to air. Superficial tillage usually limits water losses through evaporation since the capillarity is interrupted and water does not reach the soil surface. Water evaporation can be 70% higher in untilled soils compared with conventional ones [45].
- -
- breaking the soil surface layer or soil crust by means of light soil work (harrows or weeders) in order to eliminate soil cracks that increase the exchange surface with the atmosphere and to interrupt the continuity between soil and atmosphere, reducing water flow towards this latter.
- -
- implementing rational control of weeds, which are strong competitors for water. This is very important during the early stages of the crop cycle and the most critical phases in terms of the water deficit;
- -
- reducing evaporation and transpiration loss through shading, windbreaks, mulching, and anti-hail nets. For example, on tendon-grapevines in Apulia (Italy), plastic films and/or anti-hail nets are used as cover in order to anticipate or delay the harvest and to reduce respiratory losses thanks to the shading effect and the limitation of the transpirational flow. Mulching with plastic or biodegradable films is commonly used in vegetable production to reduce water losses by evaporation and irrigation requirements [46,47]. A reduction of 45% of water need has been demonstrated with combined drip irrigation and mulching, in comparison with overhead sprinkler systems [48].
- -
- using antitranspirants (mostly restricted to nurseries, to avoid excessive transpiration in newly transplanted crops). Antitranspirants are wax or plastic compounds that create a film on the leaves, covering the stomata. The effect is the reduction of water losses by transpiration and the reduction of photosynthesis, which means lower water use and improved tolerance of crops to drought stress [49]. Recent studies report that an antitranspirant sprayed on soybeans under a regular or low irrigation rate was able to improve WUE, acting on stomata and leaf gas exchange [50].
- -
- adopting increased distances between rows and along the row in sowing and transplanting, reducing plant density and competition among plants;
- -
- performing pruning or leaf removal in order to reduce the leaf area index;
- -
- implementing a rational use of fertilizers. In this context, organic fertilization is generally useful for positive effects on soil water retention, and phosphate fertilization is often useful because it stimulates radicle growth, while nitrogen fertilization should be limited to avoid increasing concentrations of the circulating solution with greater difficulty in water supply for plants;
- -
- delivering the water supply strictly needed to restore the useful water soil reservoir;
- -
- choosing more efficient irrigation techniques (considering efficiency to be the ratio between water transpired by a crop to water distributed into the field, the mean efficiency is about 80–90% for drip, subsurface, center pivot or linear irrigation, 60–70% for sprinkler irrigation, and 30–40% for surface irrigation).
- -
- selecting species and varieties so that the stage of maximum sensitivity to water stress does not coincide with the period of maximum dryness for the selected environment;
- -
- choosing early sowing that enhances deep soil colonization by roots and in some species/varieties anticipates harvest. In this sense, autumn sowing is preferable for winter crops (wheat, barley, oat, canola, etc.) while early spring sowing is preferable for summer crops. This choice is obviously suitable only for zones that are not prone to frost risk or where early sowing is compatible with the harvest of previous crops;
- -
- using biostimulants that can improve root development or enhance the biosynthesis of osmotic compounds [51]. These metabolites are able to improve crop tolerance and include plant hormones (abscisic acid) proline, sugars, amino acids, etc. The application of biostimulants can be carried out by soil drench or spray.
4.2.1. Precision Farming and Variable-Rate Irrigation
4.2.2. Grafting as an Agronomic Tool to Improve Drought Tolerance
4.3. Salinity Stress
4.4. Lodging
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B.; Gruissem, W.; Russell, L.J. (Eds.) Biochemistry and Molecular Biology of Plants, 2nd ed.; Wiley: Hoboken, NJ, USA, 2015; 1280p, ISBN 978-0-470-71421-8. [Google Scholar]
- Boogaard, H.; Wolf, J.; Supit, I.; Niemeyer, S.; van Ittersum, M. A regional implementation of WOFOST for calculating yield gaps of autumn-sown wheat across the European Union. Field Crops Res. 2013, 143, 130–142. [Google Scholar] [CrossRef]
- Singh, R.; Van Dam, J.C.; Feddes, R.A. Water productivity analysis of irrigated crops in Sirsa district, India. Agric. Water Manag. 2006, 82, 253–278. [Google Scholar] [CrossRef]
- Mariani, L. Carbon plants nutrition and global food security. Eur. Phys. J. Plus 2017, 132, 69. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. Centenary review. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Hillel, D. Introduction to Environmental Soil Physics; Elsevier: Amsterdam, The Netherlands, 2013; 495p. [Google Scholar]
- Rich, S.M.; Watt, M. Soil conditions and cereal root system architecture: Review and considerations for linking Darwin and Weaver (Darwin review). J. Exp. Bot. 2013, 64, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Passioura, J.B. Soil conditions and plant growth. Plant Cell Environ. 2002, 25, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.J.; Norman, D.W.; Dixon, J. Sustainable Dryland Cropping in Relation to Soil Productivity—FAO Soils Bulletin 72; Food & Agriculture Organization of the United Nations (FAO): Rome, Italy, 1995. [Google Scholar]
- Valentine, T.A.; Hallett, P.D.; Binnie, K.; Young, M.W.; Squire, G.R.; Hawes, C.; Bengough, A.G. Soil strength and macropore volume limit root elongation rates in many UK agricultural soils. Ann. Bot. 2012, 110, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Bonciarelli, F. Fondamenti di Agronomia Generale; Edagricole: Bologna, Italy, 1989; 292p. [Google Scholar]
- Mariani, L.; Cola, G.; Parisi, S. Dimensioning of field ditches in function of heavy and frequent precipitations. In Proceedings of the AIAM 2013 17th Annual Meeting of the Italian Agrometeorological Association Agrometeorology for Environmental and Food Security, Florence, Italy, 4–6 June 2013. (supplement to the Italian Journal of Agrometeorology 2013, 101–102). [Google Scholar]
- Pagliai, M.; Vignozzi, N.; Pellegrini, S. Soil structure and the effect of management practices. Soil Tillage Res. 2004, 79, 131–143. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Carmeis Filho, A.C.A.; Crusciol, C.A.C.; Guimarães, T.M.; Calonego, J.C.; Mooney, S.J. Correction: Impact of amendments on the physical properties of soil under tropical long-term no till conditions. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Mulumba, N.L.; Lal, R. Mulching effects on selected soil physical properties. Soil Tillage Res. 2008, 98, 106–111. [Google Scholar] [CrossRef]
- Wagner, L.E.; Ambe, N.M.; Barnes, P. Tillage-induced soil aggregate status as influenced by water content. Trans. ASAE 1992, 35, 499–504. [Google Scholar] [CrossRef]
- Liu, Z.; Rong, Q.; Zhou, W.; Liang, G. Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS ONE 2017, 12, e0172767. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; Gonzalez, J.L. Influence of organic amendments on soil structure and soil loss under simulated rain. Soil Tillage Res. 2017, 93, 197–205. [Google Scholar] [CrossRef]
- Wu, S.F.; Wu, P.T.; Feng, H.; Bu, C.F. Influence of amendments on soil structure and soil loss under simulated rainfall China’s loess plateau. Afr. J. Biotechnol. 2010, 9, 6116–6121. [Google Scholar]
- Su, L.; Wang, Q.; Wang, C.; Shan, Y. Simulation Models of Leaf Area Index and Yield for Cotton Grown with Different Soil Conditioners. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.J.; Norman, D.W.; Dixon, J. Physical aspects of crop productivity. In Sustainable Dryland Cropping in Relation to Soil Productivity, FAO Soils Bulletin 72; Food & Agriculture Organization of the United Nations (FAO): Rome, Italy, 1995; Chapter 2; Available online: http://www.fao.org/docrep/V9926E/v9926e04.htm (accessed on 22 September 2017).
- Mohamadi, M.A.; Kavian, A. Effects of rainfall patterns on runoff and soil erosion in field plots. Int. Soil Water Conserv. Res. 2015, 3, 273–281. [Google Scholar] [CrossRef]
- NSW DPI Soils Advisory Office. Soil Erosion Solutions, Fact Sheet 1: Types of Erosion. 2017. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/255153/fact-sheet-1-types-of-erosion.pd (accessed on 3 September 2017).
- Taguas, E.V.; Guzman, E.; Guzman, G.; Vanwalleghem, T.; Gomez, J.A. Characteristics and Importance of Rill and Gully Erosion. Cuadenos de Investigacion Geografica 2015, 41, 107–126. [Google Scholar] [CrossRef]
- Soracco, C.G.; Lozano, L.A.; Villarreal, R.; Palancar, T.C.; Collazo, D.J.; Sarli, G.O.; Filgueira, R.R. Effects of compaction due to machinery traffic on soil pore configuration. Rev. Bras. Ciênc. Solo 2015, 39. [Google Scholar] [CrossRef]
- Dagesse, D.F. Freezing cycle effects on water stability of soil aggregates. Can. J. Soil Sci. 2013, 93, 473–483. [Google Scholar] [CrossRef]
- Tennis, E.S.; Dolferus, R.; Ellis, M.; Rahman, M.; Wu, Y.; Hoeren, F.U.; Grover, A.; Ismond, K.P.; Good, A.G.; Peacock, W.J. Molecular strategies for improving waterlogging tolerance in plants. J. Exp. Bot. 2000, 51, 89–97. [Google Scholar]
- Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Linkemer, G.; Board, J.E.; Musgrave, M.E. Waterlogging effects on growth and yield components in late-planted soybean. Crop Sci. 1998, 38, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Bailey-Serres, J. Plant responses to hypoxia—Is survival a balancing act? Trends Plant Sci. 2004, 9, 449–456. [Google Scholar] [CrossRef] [PubMed]
- MacEwan, R.J.; Gardner, W.K.; Ellington, A.; Hopkins, D.G.; Bakker, A.C. Tile and mole drainage for control of waterlogging in duplex soils of south-eastern Australia. Aust. J. Exp. Agric. 1992, 32, 865–878. [Google Scholar] [CrossRef]
- Huang, B.; Johnson, J.W.; Nesmith, S.; Bridges, D.C. Growth physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. J. Exp. Bot. 1994, 45, 193–202. [Google Scholar] [CrossRef]
- Allègre, A.; Silvestre, J.; Morard, P.; Kallerhoff, J.; Pinelli, E. Nitrate reductase regulation in tomato roots by exogenous nitrate: A possible role in tolerance to long-term root anoxia. J. Exp. Bot. 2004, 55, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Hill, R.D. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: An alternative to the classic fermentation pathways. J. Exp. Bot. 2004, 55, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Serraj, R.; Sinclair, T.R. Osmolyte accumulation: Can it really help increase crop yield under drought conditions? Plant Cell Environ. 2002, 25, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Widtsoe, J.A. Dry Farming, a System of Agriculture for Countries under a Low Rainfall; The Mcmillan Company: New York, NY, USA, 1920; p. 501. Available online: http://archive.org/details/dryfarmingasyst01widtgoog (accessed on 15 September 2017).
- Lipiec, J.; Kuś, J.; Słowińska-Jurkiewicz, A.; Nosalewicz, A. Soil porosity and water infiltration as influenced by tillage methods. Soil Tillage Res. 2006, 89, 210–220. [Google Scholar] [CrossRef]
- Bond, J.J.; Willis, W.O. Soil water evaporation: Surface residue rate and placement effects. Soil Sci. Soc. Am. J. 1969, 33, 445–448. [Google Scholar] [CrossRef]
- Gupta, S.; Larson, W.E. Estimating soil water retention characteristics from particle size distribution, organic matter percent, and bulk density. Water Resour. Res. 1979, 15, 1633–1635. [Google Scholar] [CrossRef]
- Lament, W.J. Plastic mulches for the production of vegetable crops. HortTechnology 1993, 3, 35–39. [Google Scholar]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Clough, G.H.; Locascio, S.J.; Olson, S.M. Continuous use of polyethylene mulched beds with overhead or drip irrigation for successive vegetable production. In Proceedings of the 20th National Agriculture Plastics Congress, Portland, OR, USA, 25–27 August 1987; pp. 57–61. [Google Scholar]
- Davenport, D.; Hagan, R.; Martin, P. Antitranspirants uses and effects on plant life. Calif. Agric. 1969, 23, 14–16. [Google Scholar]
- Shasha, J.I.; Ling, T.O.N.G.; Fusheng, L.I.; Hongna, L.U.; Sien, L.I.; Taisheng, D.U.; Youjie, W.U. Effect of a new antitranspirant on the physiology and water use efficiency of soybean under different irrigation rates in an arid region. Front. Agric. Sci. Eng. 2017, 4, 155–164. [Google Scholar]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Application of biostimulants for improving yield and quality of vegetables and floricultural crops. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Alvino, A.; Marino, S. Remote sensing for irrigation of horticultural crops. Horticulturae 2017, 3, 40. [Google Scholar] [CrossRef]
- Dukes, M.D.; Perry, C. Uniformity testing of variable-rate center pivot irrigation control systems. Precis. Agric. 2006, 7, 205. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Edelstein, M.; Ben-Hur, M.; Plaut, Z. Grafted melons irrigated with fresh or effluent water tolerate excess boron. J. Am. Soc. Hortic. Sci. 2007, 132, 484–491. [Google Scholar]
- Edelstein, M.; Plaut, Z.; Ben-Hur, M. Sodium and chloride exclusion and retention by non-grafted and grafted melon and Cucurbita plants. J. Exp. Bot. 2011, 62, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, F.; Syvertsen, J.P.; Gimeno, V.; Botia, P.; Perez-Perez, J.G. Responses to flooding and drought stress by two citrus rootstock seedlings with different water-use efficiency. Biol. Plant 2007, 130, 532–542. [Google Scholar] [CrossRef]
- Satisha, J.; Prakash, G.S.; Bhatt, R.M.; Sampath Kumar, P. Physiological mechanisms of water use efficiency in grape rootstocks under drought conditions. Int. J. Agric. Res. 2007, 2, 159–164. [Google Scholar]
- Sanders, P.L.; Markhart, A.H., III. Interspecific grafts demonstrate root system control of leaf water status in water stressed Phaseolus. J. Exp. Bot. 1992, 43, 1563–1567. [Google Scholar] [CrossRef]
- Serraj, R.; Sinclair, T.R. Processes contributing to N2-fixation insensitivity to drought in the soybean cultivar Jackson. Crop Sci. 1996, 36, 961–968. [Google Scholar] [CrossRef]
- Clearwater, M.J.; Lowe, R.G.; Hofstee, B.J.; Barclay, C.; Mandemaker, A.J.; Blattmann, P. Hydraulic conductance and rootstock effects in grafted vines of kiwifruit. J. Exp. Bot. 2004, 55, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Colla, G.; Rea, E. Yield, mineral composition, water relations, and water use efficiency of grafted mini-watermelon plants under deficit irrigation. HortScience 2008, 43, 730–736. [Google Scholar]
- Allario, T.; Brumos, J.; Colmenero-Flores, J.M.; Iglesias, D.J.; Pina, J.A.; Navarro, L.; Talon, M.; Ollitrault, P.; Morillon, R.; Morillon, R. Tetraploid Rangpur lime rootstock increases drought tolerance via enhanced constitutive root abscisic acid production. Plant Cell Environ. 2013, 36, 856–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, I.; Strever, A.; Myburgh, P.A.; Deloire, A. The interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Aust. J. Grape Wine Res. 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Lv, X.; Ahammed, G.J.; Xia, X.; Zhou, J.; Zhou, Y. Grafting cucumber onto luffa improves drought tolerance by increasing ABA biosynthesis and sensitivity. Sci. Rep. 2016, 6, 20212. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, N.M.; Shashidhar, V.R.; James, R.A.; Munns, R. Stomatal control in tomato with ABA-deficient roots: Response of grafted plants to soil drying. J. Exp. Bot. 2002, 53, 1503–1514. [Google Scholar] [PubMed]
- Corbineau, F.; Xia, Q.; Bailly, C.; El-Maarouf-Bouteau, H. Ethylene, a key factor in the regulation of seed dormancy. Front. Plant Sci. 2014, 5, 539. [Google Scholar] [CrossRef] [PubMed]
- Mustilli, A.C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L. Effects of salinity and sodicity on plant growth. Ann. Rev. Phytopathol. 1975, 13, 295–312. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- FAO. Annex 1. Crop Salt Tolerance Data. Available online: http://www.fao.org/docrep/005/y4263e/y4263e0e.htm (accessed on 12 September 2017).
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R.; Panneerselvam, R. Water deficit stress mitigation by calcium chloride in Catharanthus roseus: Effects on oxidative stress. proline metabolism and indole alkaloid accumulation. Colloids Surf. B Biointerfaces 2007, 60, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, Z.Z.; Zhou, X.F.; Yin, H.B.; Li, X.; Xin, X.F.; Gong, Z. Overexpression of SOS (Salt Overly Sensitive) genes increases salt tolerance in transgenic Arabidopsis. Mol. Plant 2009, 2, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Azcon, R.; Gomez, M. Alleviation of salt stress by arbuscular-mycorrhizal Glomus species in Lactuca sativa plants. Physiol. Plant. 1996, 98, 767–772. [Google Scholar] [CrossRef]
- Berry, P.M. Understanding and reducing lodging in cereals. Adv. Agron. 2004, 84, 217–271. [Google Scholar]
- Tams, A.R.; Mooney, S.J.; Berry, P.M. The Effect of Lodging in Cereals on Morphological Properties of the Root-Soil Complex. In Proceedings of the SuperSoil 2004: 3rd Australian New Zealand Soils Conference, Sydney, Australia, 5–9 December 2004; Available online: http://www.regional.org.au/au/asssi/supersoil2004/s9/oral/1998_tamsa.htm (accessed on 20 September 2017).
- Bean, A.; Alperi, R.W.; Federer, C.A. A method for categorizing shelterbelts porosity. Agric. Meteorol. 1975, 14, 417–429. [Google Scholar] [CrossRef]
- Campi, P.; Palumbo, A.D.; Mastrorilli, M. Effect of tree windbreaks on microcliamte and wheat productivity in a Mediterrranean environment. Eur. J. Agron. 2009, 30, 220–227. [Google Scholar] [CrossRef]

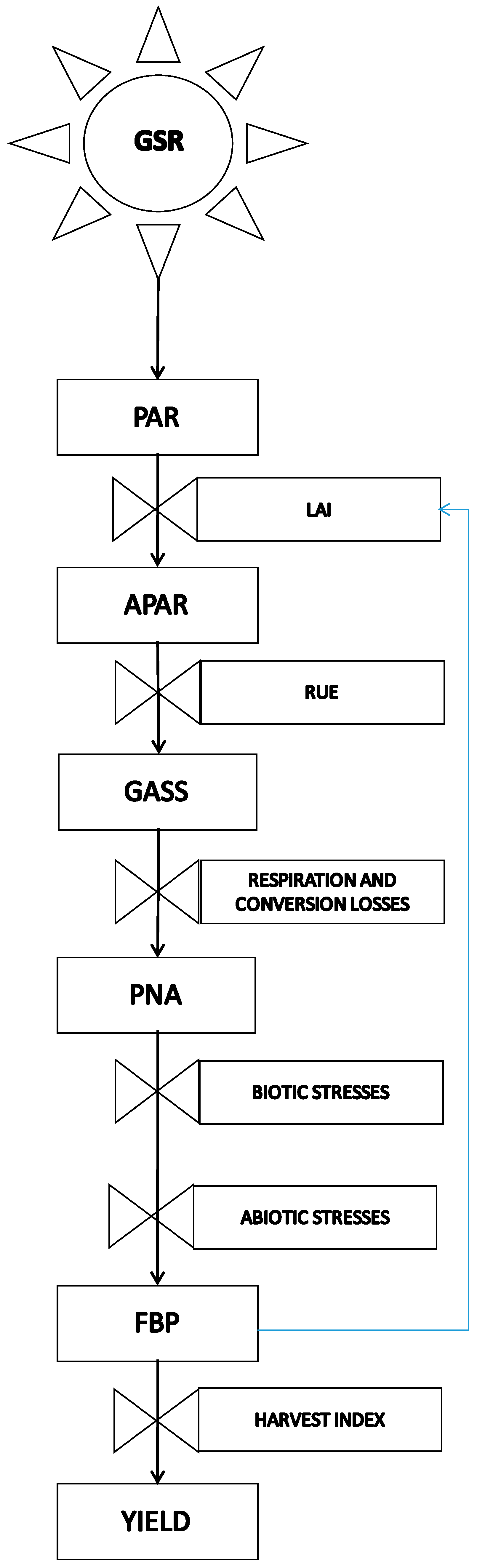
| Maize (Class 700 FAO) Yield in Field Conditions on the Po Plain (Italy) | Total Biomass Production (t·ha−1) | Harvest Index (%) | Grain Yield (t·ha−1) |
|---|---|---|---|
| Potential net assimilation (PNA) | 45.1 | 0.6 | 27.1 |
| Ordinary farmer objective in field conditions | 21.7 | 0.6 | 13 |
| Highest yield reachable in field conditions | 30 | 0.6 | 18 |
| Structure Conservation and Improvement Factors | Structure Degradation Factors |
|---|---|
| Self-healing capacity of soils [20] Organic or inorganic soil mulching [22] Minimum tillage and no tillage [19,21] Tillage at moisture content at which the largest number of small aggregates is produced [23] Amendments: - organic matter (e.g., manure, slurry, crop residues) [24,25] - soil conditioners (polymers) [26,27] - mixing of two or more soil layers in order to reach a more equilibrated texture [28] | Impact of rain and irrigation drops on bare soil surface [29] Runoff effect of rain and irrigation [29] Heavy traffic of agricultural machinery with high-volume pneumatics [28,32] Clods’ exposure to freezing–thawing cycles [33] |
| Vegetable Crops | Soil Salinity | Salinity in Irrigation Water | Tolerance | ||||
|---|---|---|---|---|---|---|---|
| Threshold (CEe) (dS·m−1) | Slope (%/dS·m−1) | Yield 0% (dS·m−1) | Threshold (CEi) (dS·m−1) | Slope (%/dS·m−1) | Yield 0% (dS·m−1) | ||
| Artichoke (Cynara scolymus L.) | 4.8 | 10.9 | 14 | 2.7 | 14.4 | 9.6 | MT |
| Asparagus (Asparagus officinalis L.) | 4.1 | 2 | 54.1 | 2.7 | 3.0 | 36 | T |
| Bean (Faseolus vulgaris L.) | 1 | 19 | 6.3 | 0.7 | 28.5 | 4.2 | S |
| Broad bean (Vicia faba L.) | 1.6 | 9.6 | 12 | 1.1 | 14.5 | 8 | MS |
| Broccoli (Brassica oleracea var. italica Plenck) | 2.8 | 9.2 | 13.7 | 1.9 | 13.8 | 9.2 | MS |
| Brussels sprouts (Brassica oleracea var. gemmifera DC.) | - | - | - | - | - | MS | |
| Cabbage hood (Brassica oleracea var. capitata L.) | 1.8 | 9.7 | 12.1 | 1.2 | 14.6 | 8.1 | MS |
| Carrot (Daucus carota L.) | 1 | 14 | 8.1 | 0.7 | 21.0 | 5.5 | S |
| Cauliflower (Brassica oleracea L. var. botrytis L.) | - | - | - | - | - | - | MS |
| Celery (Apium graveolens L. var. dulce [Mill.] Pers.) | 1.8 | 6.2 | 17.9 | 1.2 | 9.3 | 12 | MS |
| Cowpea (Vigna unguiculata [L.] Walpers subsp. unguiculata) | 4.9 | 12 | 13.2 | 3.3 | 18.2 | 8.8 | MT |
| Cucumber (Cucumis sativus L.) | 2.5 | 13 | 10.2 | 1.7 | 19.5 | 6.8 | MS |
| Eggplants (Solanum melongena L.) | 1.1 | 6.9 | 15.6 | 0.7 | 10.3 | 10.4 | MS |
| Funnel (Foeniculum vulgare Miller var. azoricum [Mill.] Thell.) | 1.5 | 15 | 8.2 | 1.1 | 18.0 | 6.7 | MS |
| Garlic (Allium sativum L.) | 1.7 | 10 | 11.7 | 1.1 | 14.9 | 7.8 | MS |
| Lettuce (Lactuca sativa L.) | 1.3 | 13 | 9 | 0.9 | 19.5 | 6 | MS |
| Melon (Cucumis melo L.) | 1 | 8.4 | 12.9 | 0.7 | 12.7 | 8.6 | MS |
| Onion (Allium cepa L.) | 1.2 | 16 | 7.5 | 0.8 | 24.0 | 5 | S |
| Pea (Pisum sativum L.) | - | - | - | - | - | - | S |
| Pepper (Capsicum annuum L.) | 1.5 | 14 | 8.6 | 1.0 | 21.0 | 5.8 | MS |
| Potato (Solanum tuberosum L.) | 1.7 | 12 | 10 | 1.1 | 18.0 | 6.7 | MS |
| Radish (Raphanus sativus L.) | 1.2 | 13 | 8.9 | 0.8 | 19.5 | 5.9 | MS |
| Rapa (Brassica rapa L. var. rapa) | 0.9 | 9 | 12 | 0.7 | 13.5 | 8.1 | MS |
| Spinach (Spinacia oleracea L.) | 2 | 7.6 | 15.2 | 1.3 | 11.4 | 10.1 | MS |
| Straberry (Fragaria x ananassa Duch.) | 1 | 33 | 4.0 | 0.7 | 49.5 | 2.7 | S |
| Swiss chard (Beta vulgaris L. var. conditiva Alef.) | 4 | 9 | 15.1 | 2.7 | 13.5 | 10.1 | MT |
| Tomato (Lycopersicon esculentum Mill.) | 2.5 | 9.9 | 12.6 | 1.7 | 15.0 | 8.4 | MS |
| Water melon (Citrullus lanatus [Thunberg] Matsumura et Nakai) | - | - | - | - | - | - | MS |
| Zucchini (Cucurbita pepo L.) | 4.7 | 9.4 | 15.3 | 3.1 | 14.1 | 10.2 | MT |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariani, L.; Ferrante, A. Agronomic Management for Enhancing Plant Tolerance to Abiotic Stresses—Drought, Salinity, Hypoxia, and Lodging. Horticulturae 2017, 3, 52. https://doi.org/10.3390/horticulturae3040052
Mariani L, Ferrante A. Agronomic Management for Enhancing Plant Tolerance to Abiotic Stresses—Drought, Salinity, Hypoxia, and Lodging. Horticulturae. 2017; 3(4):52. https://doi.org/10.3390/horticulturae3040052
Chicago/Turabian StyleMariani, Luigi, and Antonio Ferrante. 2017. "Agronomic Management for Enhancing Plant Tolerance to Abiotic Stresses—Drought, Salinity, Hypoxia, and Lodging" Horticulturae 3, no. 4: 52. https://doi.org/10.3390/horticulturae3040052







