1. Introduction
Drought is a critical environmental stress that disrupts crop growth and performance that will be exacerbated as the climate changes [
1]. To avoid water deficits that reduce crop production, irrigation is applied, but water availability is the main limiting factor for productive agriculture in most arid and semi-arid regions. In Jordan, it has been estimated that crop irrigation utilizes about 70% of the total demand for water [
2]. As the population grows and demand increases, the water available to agriculture will decline. Irrigation below the evapotranspiration (ET) requirements of a crop is considered deficit irrigation. If the water available for irrigation declines, deficit irrigation may become more common [
3].
Plant species respond to water deficits through molecular changes, biochemical and physiological modifications, and ultimately morphological adaptations [
4]. Plants have developed three major drought-resistance strategies in their adaptation to drought stress, including drought tolerance, drought avoidance, and drought escape [
5]. Under water deficit conditions, most plant species close their stomata and modulate their leaf area (drought avoidance), adjusting the loss of water from the canopy, and may osmotically adjust (drought tolerance) [
6]. Drought escape, completing the life cycle prior to severe stress, is most common in annual plant species.
Apple (
Malus domestica Borkh.) is one of most widely grown and valuable deciduous fruit crops worldwide, and is extensively grown in the arid southern regions of Jordan. Several selection criteria have been proposed for identifying drought-tolerant genotypes based on their performance in stress and non-stress environments. Differences in canopy structure and water use efficiency (WUE) [
7], as well as in leaf water potential (Ψ
w), osmotic adjustment, net photosynthetic rate (Pn), and stomatal conductance (g
s) [
8], may be responsible to varying extents in determining how a genotype/rootstock combination responds to drought. A significant genotypic influence of apple rootstock tolerance to water stress has been reported [
8,
9,
10]. Considerable variation among different apple genotypes has also been identified for a range of physiological and biochemical characteristics including responses to water stress [
11,
12]. Vegetative growth is drastically reduced as a result of water deficit stress. This growth reduction has been attributed to reduced Pn, g
s, and leaf Ψ
w [
7]. In contrast, moderate water deficits have been shown to improve apple fruit quality with increased mean fruit weight, return bloom, flesh firmness, red skin color intensity [
13], and total soluble solids content [
11].
Deficit irrigation strategies can be useful for reducing water use and for managing fruit tree growth and yield [
14]. Deficit irrigation has reduced apple tree growth without reducing fruit yield in some studies [
15,
16,
17], but the effect may not be consistent year to year, as fruit yield has declined in other work [
18,
19]. As water deficit stress increases, negative impacts on yield are more likely, but finding the optimum deficit irrigation strategy may be challenging as environmental conditions (i.e., ambient temperature, relative humidity/vapor pressure deficit, sunny versus cloudy conditions) vary from season to season.
Numerous compounds have been studied as tools to alleviate water deficit stress by reducing the rate of leaf water loss. Foliar application of kaolin mineral particle film (PF) was reported to protect plants against drought stress [
20,
21,
22,
23]. Seasonal use in apple orchards increased Pn and T by reducing heat stress on leaves [
20,
21]. Abscisic acid (ABA) application reduces transpiration and stimulates osmotic adjustment in plants [
24]. Exogenous application of ABA has reduced T and enhanced drought tolerance in several plant species [
25,
26,
27,
28,
29]. Increasing the endogenous foliar ABA content in apple by inhibiting its hydroxylation improved physiological and biochemical aspects of tree drought tolerance [
30].
Many studies of the effects of drought stress and antitranspirant application have been conducted for a relatively short duration, only a few weeks [
12,
31,
32]. This approach can successfully expose incipient physiological and biochemical drought stress effects and drought tolerance responses. Fewer studies have looked at longer-term effects on physiological and biochemical responses. As trees develop drought avoidance modifications over longer periods, it is not clear if drought tolerance responses might also be adapting to the modified status. Whether deficit irrigation strategies are by choice to manage tree growth or are imposed by limited water availability, antitranspirants could be useful for alleviating some of the effects of drought stress. PF use in wine grapes has reduced the effects of moderate water stress during deficit irrigation [
33,
34], but there have been no reports examining this possibility with apple using either PF or ABA.
Results of container [
25] and parallel field [
11] experiments, in addition to those of other investigators [
35], clearly demonstrated that water deficit stress caused plants to reduce Pn and, ultimately, growth. The response was strongly affected by the rapidity, severity and duration of the water shortage. In view of these results, and the need to minimize water stress effects during deficit irrigation of apple, the objective of this work was to study responses of three apple genotypes, “Granny Smith”, “Royal Gala” and “Golden Delicious”, grafted on MM106 rootstock, all common in Jordan, to scheduled use of PF and ABA during deficit irrigation, hypothesizing that the antitranspirants would moderate water deficit stress effects on leaf water relations, gas exchange, chlorophyll fluorescence properties, mineral composition, and leaf production.
2. Materials and Methods
This study was carried out in a greenhouse located in the Department of Horticulture, College of Agriculture, University of Kentucky, Lexington, KY, USA, from November 2010 to June 2011. The greenhouse was 22 ± 6 °C with ambient light levels during the investigation. Uniform, one year-old, dormant “Granny Smith”, “Royal Gala” and “Golden Delicious” apple scions grafted on MM106 rootstock (Vaughn Nursery, McMinnville, TN, USA), were used. The leader shoots were pruned to a height of 90 cm, lateral branches were removed, the roots were pruned to a uniform length, and the trees were planted in 14.4 L black poly containers filled with Barky Beaver medium (composed of hardwood bark fines, pine park fines and peat moss, pH 5.5–6.5) (Barky Beaver Mulch & Soil Mix, Inc., Moss, TN, USA). The few floral buds present were removed when first evident during bud swell. The trees were irrigated uniformly, and were supplied with macro- and microelements (Peters Professional, Scotts-Sierra Horticultural Products Co., Marysville, OH, USA).
The trees were grown for two weeks prior to initiating the treatments. There were three replicate trees per irrigation level by antitranspirant treatment within each genotype, laid out in a randomized complete block design with three blocks (each on a separate greenhouse bench), each block with one replicate tree per treatment-genotype combination. Trees were subjected to one of the following irrigation treatments: Irrigation to runoff after 30%, 60%, or 80% depletion of available water (DAW). The irrigation treatments were derived after field capacity and wilting point were determined by generating a pressure-volume curve. Based on these two points, the total volume of available water (between field capacity and the wilting point) was calculated, and the weight derived. Each container was then weighed every two days, and when the depletion of available water was 30% ± 3%, 60% ± 3%, or 80% ± 3% of the total, each was irrigated to field capacity. Kaolin-based particle film (Surround WP Crop Protectant, Engelhard Corporation, Iselin, NJ, USA) and abscisic acid (ABA; ProTone SG, Valent BioSciences Corporation, Libertyville, IL, USA, 20% concentration, w/w), were used as antitranspirants. PF was applied at 6% w/v and ABA was applied at 5 mM as sprays to run off of half of the trees of each irrigation treatment on three dates: At 0, 30, and 60 days after the initiation of the study. Untreated trees at each % DAW constituted the controls. The trees were maintained under these conditions for 150 days.
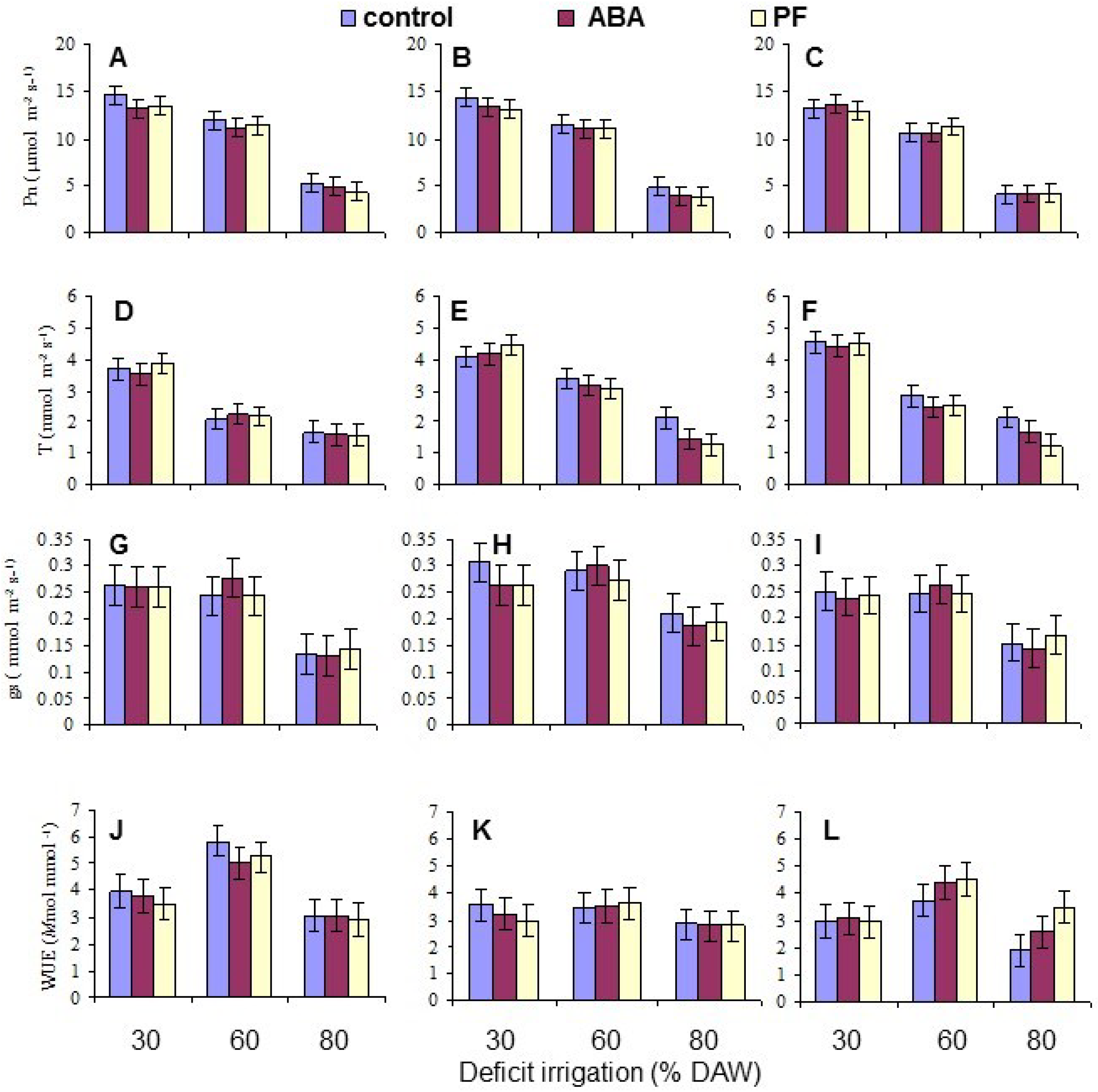
Gas exchange measurements were taken when the assigned DAW was reached at or after 120 days after beginning the deficit irrigation treatments, between 9 a.m. and 1 p.m. The exact measurement date for each tree varied as the rate and severity of depletion differed. Measurements of net photosynthetic (CO2 assimilation) rate (Pn), transpiration rate (T), and stomatal conductance (gs) were conducted on both the second and third intact, fully-expanded leaf from the apex of the terminal shoot of each replicate tree using a portable steady state infrared gas analyzer (LI-6400, LI-COR, Lincoln, NE, USA), and the means per tree derived for data analysis. The leaf surface area within the measurement chamber of the gas analyzer was 6 cm2, and the ambient CO2 concentration was adjusted to 352 µmol·mol−1. Water use efficiency (WUE) was calculated as the instantaneous ratio of Pn/T.
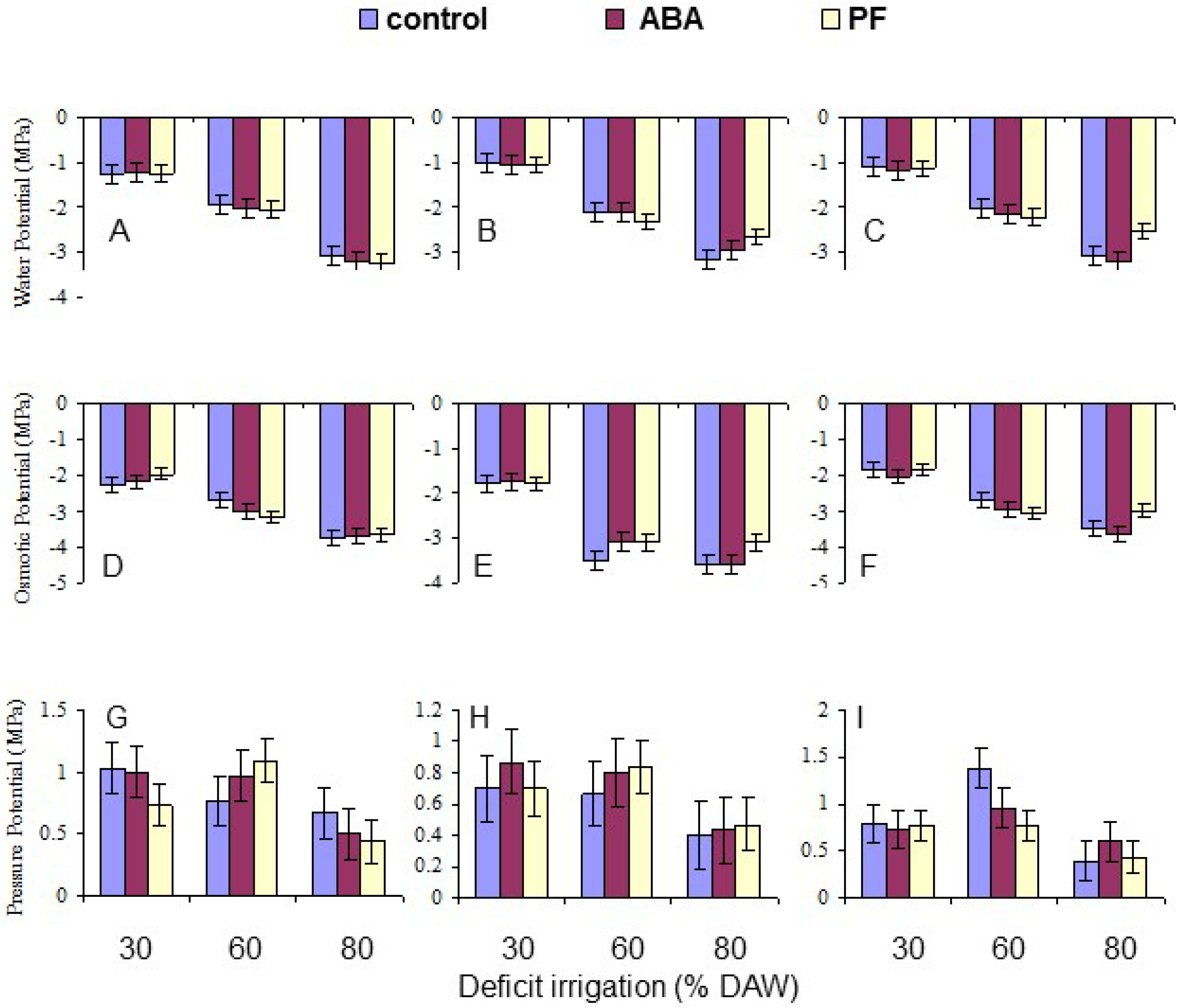
Leaf water potential (Ψ
w) of two recent fully expanded, mature leaves on each replicate tree was measured using a pressure bomb (Model 610, PMS Instruments, Corvallis, OR, USA) as described by Zhang and Archbold [
36]. Measurements were taken between 9 a.m. and 2 p.m. when the assigned DAW was reached as described above, at or after 120 days after beginning the deficit irrigation treatments. Each leaf was cut at the petiole base and immediately placed in the pressure chamber with about 5 mm of the cut end of the petiole protruding through the compressible rubber gland used to seal the chamber. Compressed N
2 was released into the chamber until the sap returned to the cut end of the xylem, and the pressure required was recorded. For measuring leaf osmotic potential (Ψ
π), the leaves were then sealed between two layers of parafilm and frozen at −20 °C. Leaf discs with a diameter of the sample cups were taken with a cork borer, placed into a sample cup, and thawed for 1 h inside the sealed cup at room temperature. The cup was then placed in a chilled mirror dewpoint sensor (Model WP4-T, Decagon Devices, Pullman, WA, USA). Each sample was processed for ~20 min at room temperature. Leaf pressure potential (Ψ
p) was calculated as the difference between Ψ
w and Ψ
π. The means per tree of each component of water potential were derived for data analysis.
Chlorophyll fluorescence, as a measure of injury to the photosynthetic apparatus, was obtained when the assigned DAW was reached, as described above, using a Plant Efficiency Analyzer (PEA; Hansatech Instruments Ltd., Kings Lynn, UK). Chlorophyll fluorescence (as the Fv/Fm ratio) was measured on three dark-adapted leaves from each replicate tree. Dark-adaptation was achieved by covering the leaves for 30 min under plastic clips provided with the PEA. The mean per tree was derived for data analysis.
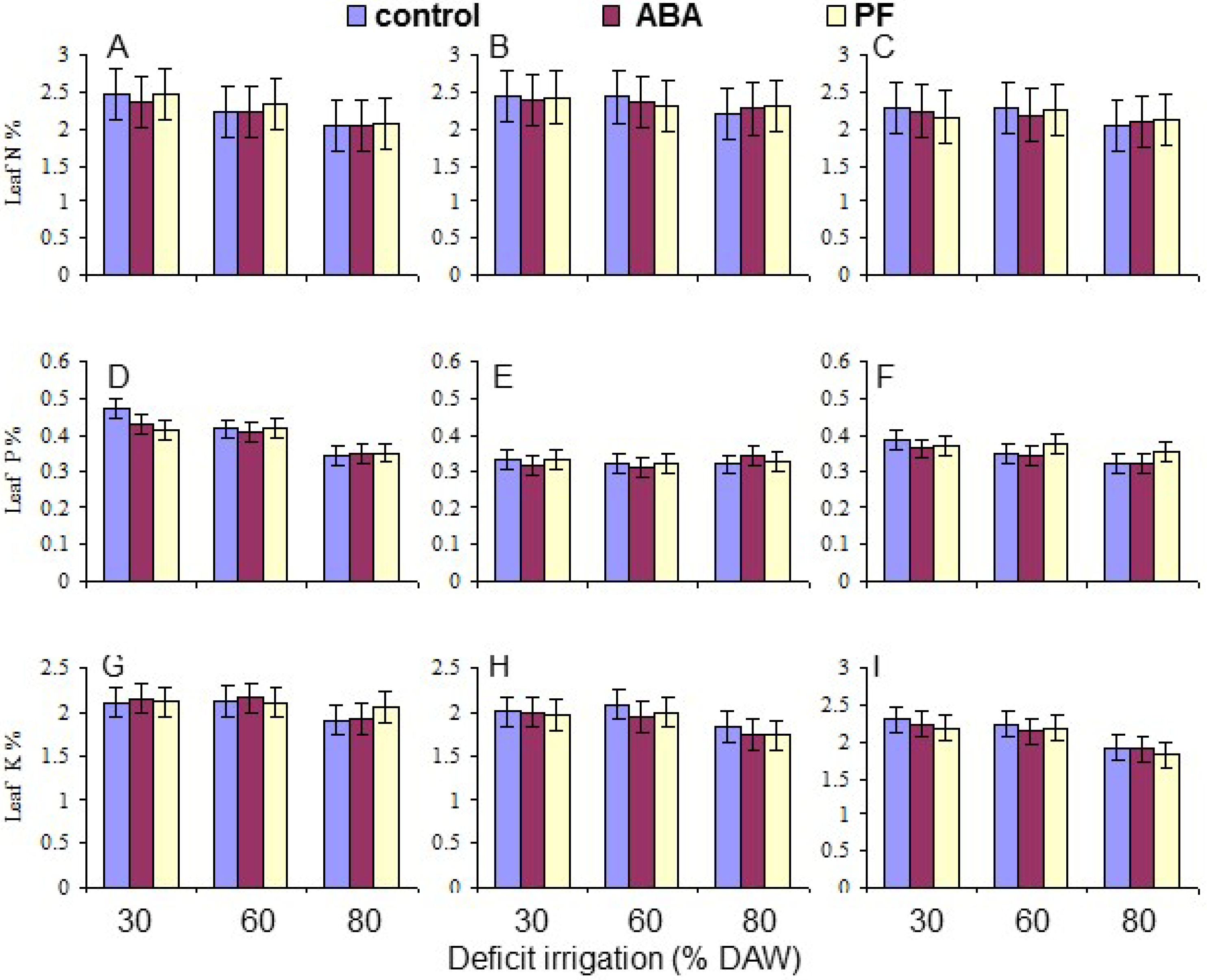
Two leaves were randomly collected from each replicate tree, when the assigned DAW was reached. The leaves were oven dried at 70 °C until a constant weight was obtained, combined, and ground to pass through a 20 mesh screen. Leaf mineral composition (N, P, K, Ca, and Mg) was determined as % dry weight on samples collected at 120 days as described by Tandon [
37].
The number of new leaves produced on each replicate tree during the course of the study was measured 150 days after beginning the deficit irrigation treatments as a measure of the treatment effects on tree growth.
Using means per replicate tree, the data were subjected to analysis of variance using MSTATC software. Significant effects and interactions were determined at a P ≤ 0.05, and means were compared using the Least Significant Difference (LSD) test.
4. Discussion
Overall, the water deficit stress at increasing % DAW reduced Pn, T, leaf Ψ
w and Ψ
π, the Fv/Fm ratio, and leaf N, P and K contents. WUE increased for two of the genotypes from 30% to 60% DAW because Pn did not change, but g
s declined, and it decreased in all genotypes from 60% to 80% DAW. Apart from genotypic variation, deficit irrigation had the most important effect. PF affected leaf Pn but not the other gas exchange traits, suggesting it did not alter leaf physiology or development. The lack of response to ABA was likely due to the fact that only three applications were made to the trees. ABA metabolism would occur in leaves shortly after each application [
50], and measurements reported here were taken 60 or more days after the last application, so ABA effects on stomatal closure or other physiological parameters would likely have subsided much earlier. More frequent ABA application might produce beneficial effects on gas exchange responses and WUE under stress conditions [
27]. Nonetheless, there were no long-term effects from the ABA applications in the present work.
Under conditions of water scarcity, irrigation should be scheduled at a level of no less than 60% DAW, as this optimized WUE and maintained growth, as indicated by the leaf number, while detrimental effects on leaf gas exchange and growth occurred at 80% DAW. Even though there was a residue of kaolin PF on the leaves throughout the period of the study because the trees were only watered at their base, and the residue was never washed off as may occur in environments with periodic rainfall, there were no sustained effects on any measured trait other than leaf Pn mitigating the impact of reduced water availability. This was also evident in tree growth (as indicated by leaf production) which did not respond to PF. The effects of PF film on apple have been most notable in environments with high vapor pressure deficits and temperature, and with irrigated trees [
20,
21,
22,
23], quite different than the conditions of the present work. Although the trees in the present work were young and had limited root development and container volume which may have impacted the results, the application of PF to orchard-grown walnut (
Juglans regia) also failed to mitigate the effects of water stress [
43], so it is likely to have a similar impact on mature apple trees in the field.