Comparative Leaf Phenology of White Oak and Northern Red Oak
Abstract
:1. Introduction
2. Experimental Section
2.1. Tree Characterization
2.2. Phenological Development
2.3. Data Analysis
| Location | No. of Pairs | 2006 | 2007 | ||
|---|---|---|---|---|---|
| Red Oak | White Oak | Red Oak | White Oak | ||
| Height (m)/dbh cm | |||||
| Arboretum | 4 | 23.4 */57.1 * a | 17.7 */49.0 * | 24.3 */58.6 * | 18.5 */50.3 * |
| Crystal Lake | 5 | 23.7/75.9 | 21.6/69.3 | 23.9/77.7 | 22.5/71.6 |
| Illini Grove | 6 | 29.6/61.0 | 29.9/59.7 | 31.4/62.3 | 31.5/60.7 |
3. Results and Discussion
3.1. Tree Characterization
3.2. Phenological Development
| Location | Species | Growth Stage | ||
|---|---|---|---|---|
| Swollen Bud | Leaf Unfolding | Expanded Leaf | ||
| Julian d | ||||
| Arboretum | Red | 101.6 f a | 111.6 de | 124.4 b |
| White | 96.5 fg | 113.3 cd | 130.8 a | |
| Crystal Lake | Red | 94.6 g | 109.4 e | 126.2 b |
| White | 97.6 fg | 114.9 c | 129.7 a | |
| Illini Grove | Red | 100.4 f | 110.1 e | 125.5 b |
| White | 101.7 f | 113.5 cd | 130.1 a | |
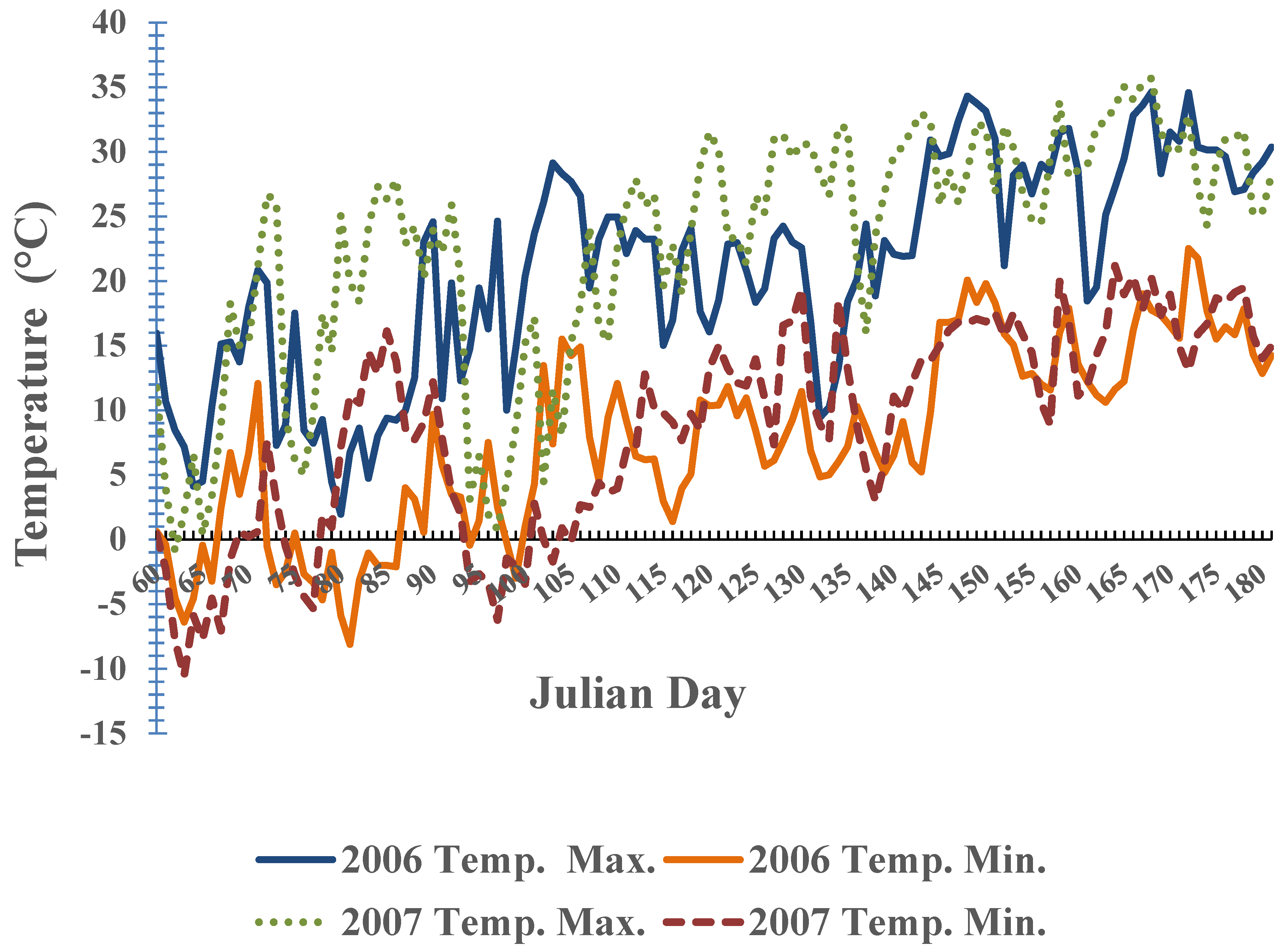
| Year | Species | Growth Stage | ||
|---|---|---|---|---|
| Swollen Bud | Leaf Unfolding | Expanded Leaf | ||
| Julian d | ||||
| 2006 | Red | 101.0 h a | 105.3 g | 123.2 c |
| White | 105.6 g | 109.2 f | 128.1 b | |
| 2007 | Red | 96.8 i | 115.4 e | 127.6 b |
| White | 91.5 i | 118.6 d | 132.3 a | |
| Location | Species | Duration Phase 1 a | Duration Phase 2 | ||
|---|---|---|---|---|---|
| No. of d | |||||
| 2006 | 2007 | 2006 | 2007 | ||
| Arboretum | Red | 5.3 d b | 14.8 a–c | 13.8 c | 11.8 c |
| White | 2.8 d | 30.8 a | 20.3 ab | 14.8 bc | |
| Crystal Lake | Red | 4.2 e | 25.4 ab | 21.4 bc | 12.2 d |
| White | 4.8 e | 28.6 a | 17.6 c | 11.7 d | |
| Illini Grove | Red | 3.7 c | 15.7 ab | 18.3 a | 12.5 b |
| White | 3.0 c | 18.5 ab | 19.0 a | 13.7 b | |
| Species | Year | Growth Stage | Growing Degree Days | Rainfall | Day length |
|---|---|---|---|---|---|
| r(p) | |||||
| Red | 2006 | Swollen bud | 0.96 (<0.0001) | 0.92 (<0.0001) | 1.0 (<0.0001) |
| Leaf unfolding | 0.99 (<0.0001) | 0.95 (0.0140) | 1.0 (0.0001) | ||
| Expanded leaf | 1.0 (<0.0001) | 0.85 (<0.0001) | 1.0 (<0.0001) | ||
| Red | 2007 | Swollen bud | 0.97 (<0.0001) | 0.95 (<0.0001) | 1.0 (<0.0001) |
| Leaf unfolding | 0.93 (<0.0001) | 0.88 (<0.0001) | 1.0 (<0.0001) | ||
| Expanded leaf | 1.0 (<0.0001) | 0.49 (0.0728) | 1.0 (<0.0001) | ||
| White | 2006 | Swollen bud | 0.96 (<0.0001) | 0.94 (<0.0001) | 1.0 (<0.0001) |
| Leaf unfolding | 1.0 (0.0015) | 0.78 (0.2254) | 1.0 (0.0029) | ||
| Expanded leaf | 1.0 (<0.0001) | 0.89 (<0.0001) | 1.0 (<0.0001) | ||
| White | 2007 | Swollen bud | 0.96 (<0.0001) | 0.93 (<0.0001) | 1.0 (<0.0001) |
| Leaf unfolding | 0.95 (<0.0001) | 0.95 (<0.0001) | 1.0 (<0.0001) | ||
| Expanded leaf | 1.0 (<0.0001) | 0.61 (0.0212) | 1.0 (<0.0001) | ||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Samtani, J.B.; Masiunas, J.B.; Appleby, J.E. Injury on white oak seedlings from herbicide exposure simulating drift. HortScience 2008, 43, 2076–2080. [Google Scholar]
- Samtani, J.B.; Masiunas, J.B.; Appleby, J.E. White oak and northern red oak leaf injury from exposure to chloroacetanilide herbicides. HortScience 2010, 45, 696–700. [Google Scholar]
- Böger, P.; Matthes, B.; Schmalfuß, J. Towards the primary target of chloroacetamides—New findings pave the way. Pest Manag. Sci. 2000, 56, 497–508. [Google Scholar] [CrossRef]
- Von Wettstein-Knowles, P. Biosynthesis and genetics of waxes. In Waxes: Chemistry, Molecular Biology and Functions; Hamilton, R.J., Ed.; Oily Press: Dundee, Scotland, UK, 1995; pp. 91–130. [Google Scholar]
- Post-Beittenmiller, D. Biochemistry and molecular biology of wax production in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Heide, O.M. Daylength and thermal time responses of budburst during dormancy release in some northern deciduous trees. Phys. Plant. 1993, 88, 531–540. [Google Scholar] [CrossRef]
- Correia, O.A.; Martins, A.C.; Catarino, F.M. Comparative phenology and seasonal foliar nitrogen variation in Mediterranean species of Portugal. Ecol. Mediterr. 1992, 8, 7–18. [Google Scholar]
- Rathcke, B.; Lacey, E.P. Phenological patterns of terrestrial plants. Annu. Rev. Ecol. Syst. 1985, 16, 179–214. [Google Scholar] [CrossRef]
- Gill, D.S.; Amthor, J.S.; Bormann, F.H. Leaf phenology, photosynthesis, and the persistence of saplings and shrubs in a mature northern hardwood forest. Tree Physiol. 1998, 18, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.E.; Debandi, G.O.; Peralta, I.E.; Palle, E.M. Comparative phenology and floral patterns in Larrea species (Zygophyllaceae) in the Monte desert (Mendoza, Argentina). J. Arid Environ. 1999, 43, 213–226. [Google Scholar] [CrossRef]
- Ahlgren, C.E. Phenological observations of nineteen native tree species in northeastern Minnesota. Ecology 1957, 38, 622–628. [Google Scholar] [CrossRef]
- Senn, J.; Hanhimäki, S.; Haukioja, E. Among-tree variation in leaf phenology and morphology and its correlation with insect performance in the mountain birch. Oikos 1992, 63, 215–222. [Google Scholar] [CrossRef]
- Ne’eman, G. Variation in leaf phenology and habit in Quercus ithaburensis, a Mediterranean deciduous tree. J. Ecol. 1993, 81, 627–634. [Google Scholar] [CrossRef]
- Yacine, A.; Lumaret, R. Spatial distribution of genotypes in a population of holm oak (Quercus ilex L.), gene flow and mating system. Genet. Sel. Evol. 1988, 20, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Crawley, M.J.; Akhteruzzaman, M. Individual variation in the phenology of oak trees and its consequences for herbivorous insects. Funct. Ecol. 1988, 2, 409–415. [Google Scholar] [CrossRef]
- Van Schaik, C.P.; Terborgh, J.W.; Wright, S.J. The phenology of tropical forests: Adaptive significance and consequences for primary consumers. Annu. Rev. of Ecol. and Syst. 1993, 24, 353–377. [Google Scholar] [CrossRef]
- Williams, R.J.; Myers, B.A.; Muller, W.J.; Duff, G.A.; Eamus, D. Leaf phenology of woody species in a north Australian tropical savanna. Ecology 1997, 78, 2542–2558. [Google Scholar] [CrossRef]
- Bullock, S.H.; Solís-Magallanes, J.A. Phenology of canopy trees of a tropical deciduous forest in Mexico. Biotropica 1990, 22, 22–35. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Soil Web Survey. Available online: http://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 25 August 2015).
- Chuine, I.; Cour, P. Climatic determinants of budburst seasonality in four temperate-zone tree species. New Phytol. 1999, 143, 339–349. [Google Scholar] [CrossRef]
- Saxton, A.M. A macro for converting mean separation output to letter groupings in Proc Mixed. In Proceedings of The 23rd SAS User’s Group International, SAS Institute, Cary, NC, USA; 1998; pp. 1243–1246. [Google Scholar]
- Fritts, H.C. The relation of radial growth to maximum and minimum temperatures in three tree species. Ecology 1959, 40, 261–265. [Google Scholar] [CrossRef]
- Tardif, J.C.; Conciatori, F. A comparison of ring-width and event-year chronologies derived from white oak (Quercus alba) and northern red oak (Quercus rubra), southwest Quebec, Canada. Dendrochronologia 2006, 23, 133–138. [Google Scholar] [CrossRef]
- Pan, C.; Tajchman, S.J.; Kochenderfer, J.N. Dendroclimatological analysis of major species of the central Appalachians. Forest Ecol. Manage. 1997, 98, 77–87. [Google Scholar] [CrossRef]
- Illinois State Water Survey. Local Climatological Data, Champaign-Urbana, IL-118740, March through June 2006 and 2007. Available online: http://www.sws.uiuc.edu/atmos/statecli/ (accessed on 27 July 2015).
- LeBlanc, D.C.; Stahle, D.W. Radial growth responses of four oak species to climate in eastern and central north America. Can. Journal. For. Res. 2015, 45, 793–804. [Google Scholar] [CrossRef]
- Vitasse, Y.; Delzon, S.; Dufrêne, E.; Pontailler, J.-Y.; Louvet, J.-M.; Kremer, A.; Michalet, R. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. and Forest Meteorol. 2009, 149, 735–744. [Google Scholar] [CrossRef]
- Medel, G.; Medel, F.; Huber, A.; McConchie, C. Phenological development and growing degree days in Gevuina avellana Mol. Acta. Hortic. 2014, 1052, 355–361. [Google Scholar] [CrossRef]
- Ballestrini, C.; Tezara, W.; Herrera, A. Environmental drivers of leaf phenology in trees of the tropical species Ficus obtusifolia. Braz. J. Plant Physiol. 2011, 23, 113–122. [Google Scholar] [CrossRef]
- Patrícia, L.; Morellato, C.; Talora, D.C.; Takahasi, A.; Bencke, C.C.; Romera, E.C.; Zipparro, V.B. Phenology of Atlantic rain forest trees: A comparative study. Biotropica 2000, 32, 811–823. [Google Scholar] [CrossRef]
- Grogan, J.; Schulze, M. The impact of annual and seasonal rainfall patterns on growth and phenology of emergent tree species in southeastern Amazonia, Brazil. Biotropica 2012, 44, 331–340. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samtani, J.B.; Appleby, J.E.; Masiunas, J.B. Comparative Leaf Phenology of White Oak and Northern Red Oak. Horticulturae 2015, 1, 44-54. https://doi.org/10.3390/horticulturae1010044
Samtani JB, Appleby JE, Masiunas JB. Comparative Leaf Phenology of White Oak and Northern Red Oak. Horticulturae. 2015; 1(1):44-54. https://doi.org/10.3390/horticulturae1010044
Chicago/Turabian StyleSamtani, Jayesh B., James E. Appleby, and John B. Masiunas. 2015. "Comparative Leaf Phenology of White Oak and Northern Red Oak" Horticulturae 1, no. 1: 44-54. https://doi.org/10.3390/horticulturae1010044







