Effect of Saccharomyces, Non-Saccharomyces Yeasts and Malolactic Fermentation Strategies on Fermentation Kinetics and Flavor of Shiraz Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation and Enumeration of Microorganisms
2.2. Wine Production
2.3. Juice and Wine Analyses
2.4. Sensory Evaluation
2.5. Data and Statistical Analysis
3. Results and Discussion
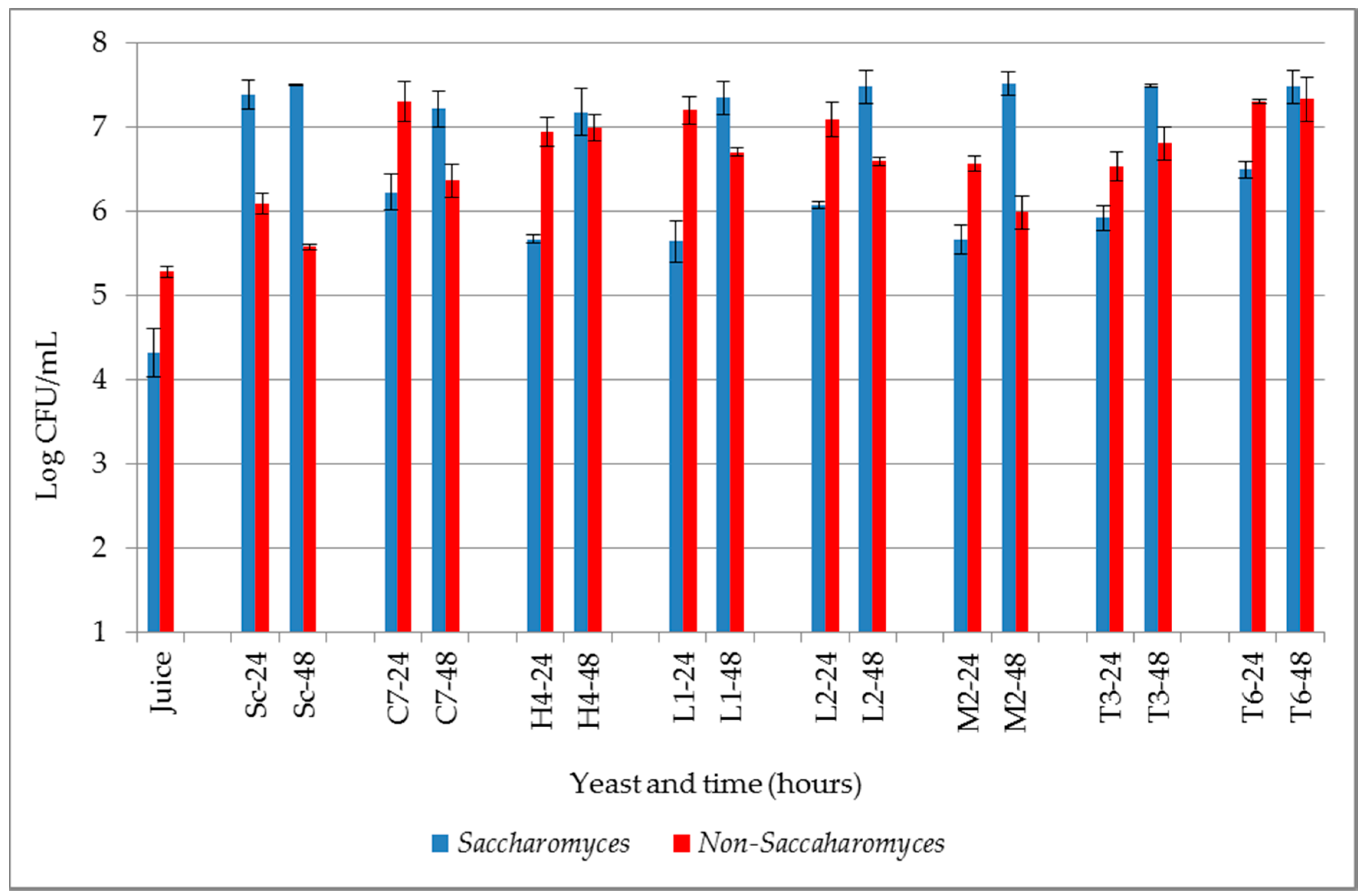
3.1. Fermentation Kinetics and Progress of MLF
3.1.1. Yeast Growth in Wines without MLF
3.1.2. LAB Growth
3.1.3 Progression of MLF
3.2. Standard Oenological Parameters
3.2.1. Wines without MLF
3.2.2. Wines that Underwent MLF
3.3. Flavor Compounds
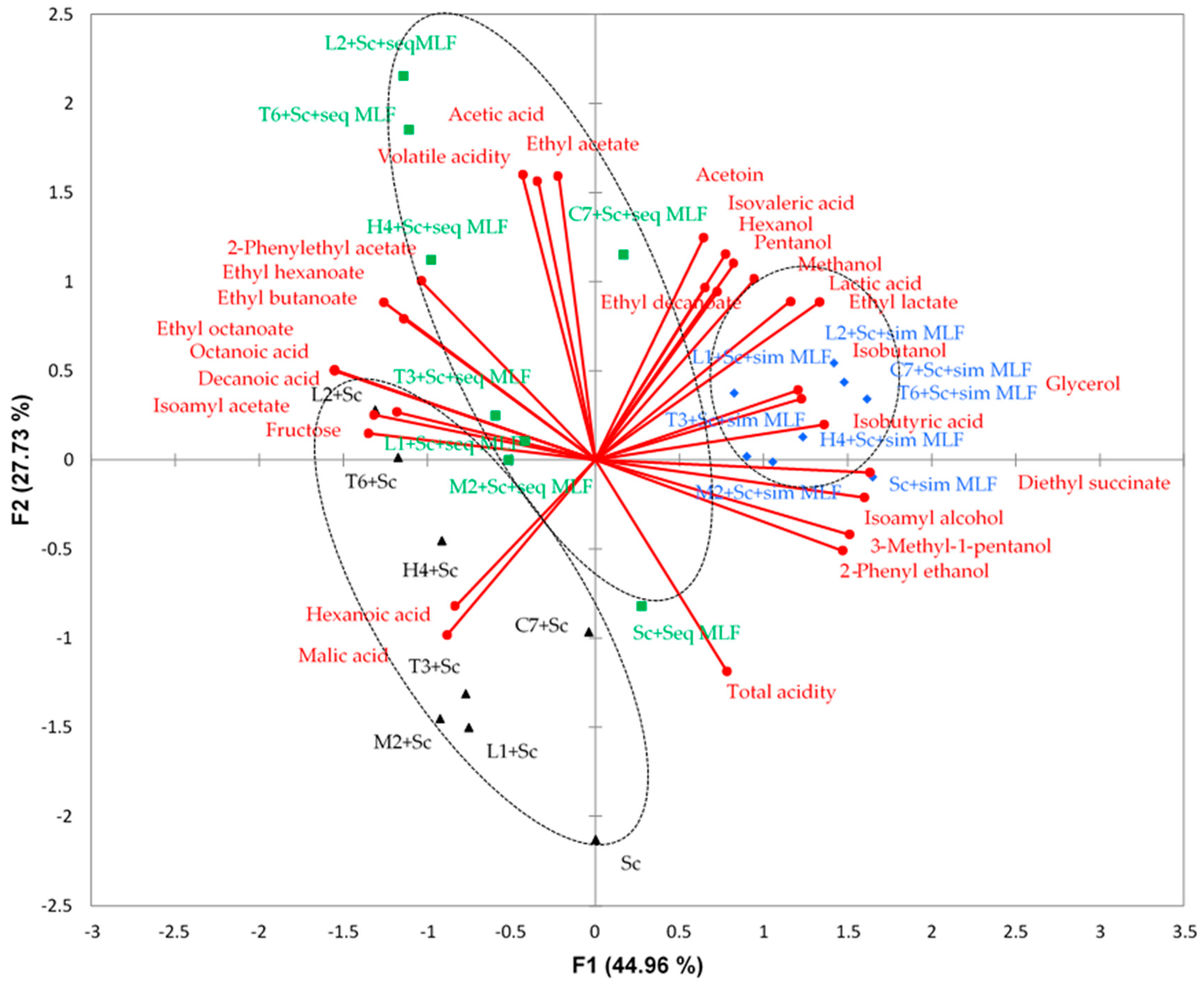
3.4. Multivariate Data Analysis of Wines
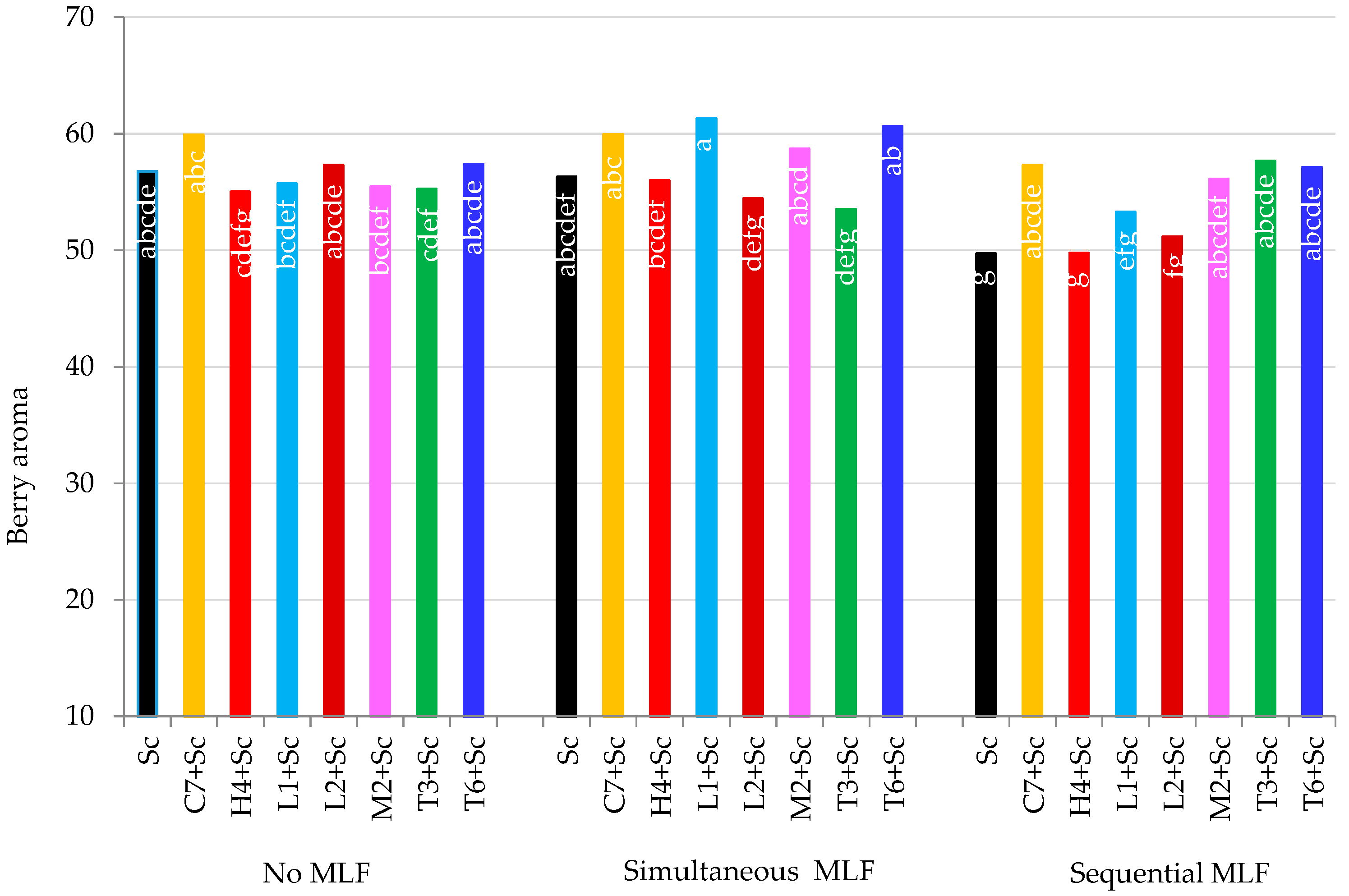
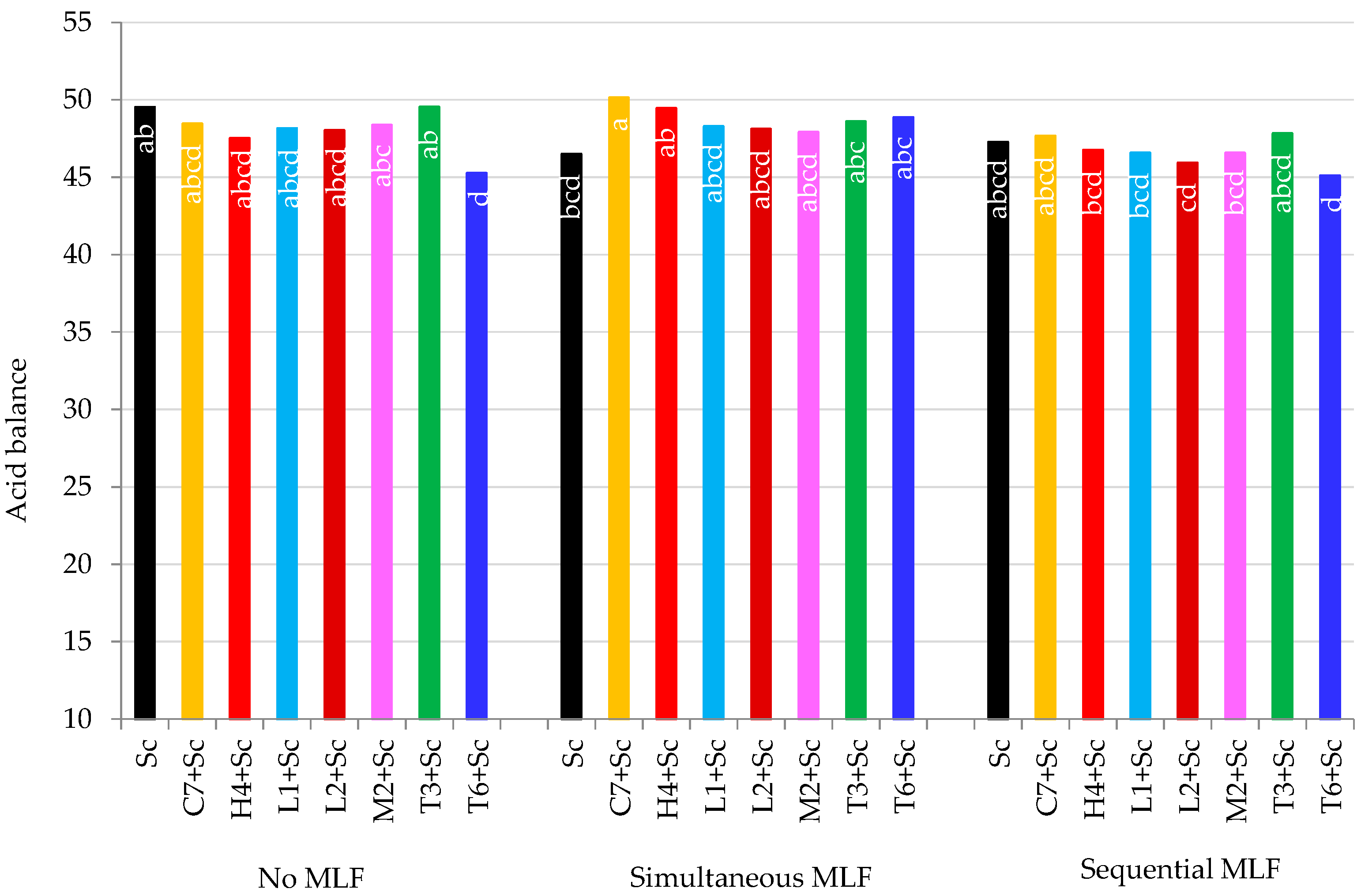
3.5. Sensory Evaluation
3.5.1. Berry Aroma
3.5.2. Acid Balance
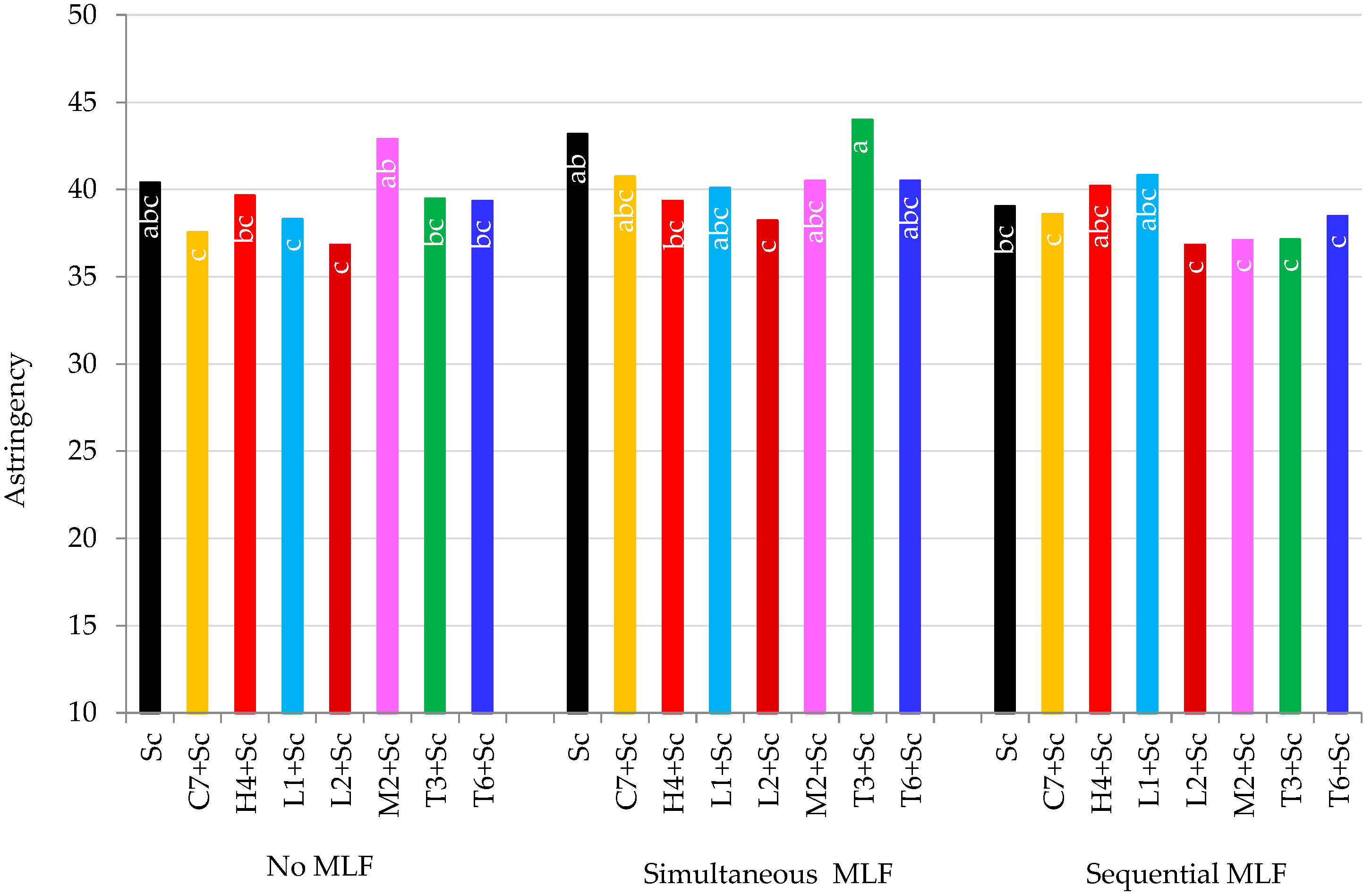
3.5.3. Astringency
3.6. Overall Effects
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Condurso, C.; Cincotta, F.; Tripodi, G.; Sparacio, A.; Giglio, D.M.L.; Sparla, S.; Verzera, A. Effects of cluster thinning on wine quality of Syrah cultivar (Vitis vinifera L.). Eur. Food Res. Technol. 2016, 242, 1719–1726. [Google Scholar] [CrossRef]
- Mayr, C.M.; Geue, J.P.; Holt, H.E.; Pearson, W.P.; Jeffery, D.W.; Francis, I.L. Characterization of the key aroma compounds in Shiraz wine by quantitation, aroma reconstitution, and omission studies. J. Agric. Food Chem. 2014, 62, 4528–4536. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A. Handbook of Enology, 2nd ed.; The Microbiology of Wine and Vinifications; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 1. [Google Scholar]
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The next generation of malolactic fermentation starter cultures—An overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavor. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Granchi, L.; Bosco, M.; Messini, A.; Vincenzini, M. Rapid detection and quantification of yeast species during spontaneous wine fermentation by PCR–RFLP analysis of the rDNA ITS region. J. Appl. Microbiol. 1999, 87, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Valera, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Whitener, M.E.B.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; du Toit, M.; Vrhovsek, U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: A targeted approach. LWT Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Whitener, M.E.B.; Stanstrup, J.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Austr. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Whitener, M.E.B.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 1–25. [Google Scholar] [CrossRef]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging trends in the application of malolactic fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavor. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Lerm, E.; Engelbrecht, L.; Du Toit, M. Malolactic fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212. [Google Scholar]
- Bartowsky, E.J. Oenococcus oeni and malolactic fermentation—Moving into the molecular arena. Aust. J. Grape Wine Res. 2005, 11, 174–187. [Google Scholar] [CrossRef]
- Gammacurta, M.; Lytra, G.; Marchal, A.; Marchand, S.; Barbe, J.C.; Moine, V.; de Revel, G. Influence of lactic acid bacteria strains on ester concentrations in red wines: Specific impact on branched hydroxylated compounds. Food Chem. 2018, 239, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H.; Costello, P.J.; Remize, F.; Guzzo, J.; Guilloux-Benatier, M. Saccharomyces cerevisiae-Oenococcus oeni interactions in wine: Current knowledge and perspectives. Int. J. Food Microbiol. 2004, 93, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Arnink, K.; Henick-Kling, T. Influence of Saccharomyces cerevisiae and Oenococcus oeni strains on successful malolactic conversion in wine. Am. J. Enol. Vitic. 2005, 56, 228–237. [Google Scholar]
- Muñoz, V.; Beccaria, B.; Abreo, E. Simultaneous and successive inoculations of yeasts and lactic acid bacteria on the fermentation of an unsulfited Tannat grape must. Braz. J. Microbiol. 2014, 45, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Antalick, G.; Perello, M.C.; de Revel, G. Co-inoculation with yeast and LAB under winery conditions: Modification of the aromatic profile of Merlot wines. S. Afr. J. Enol. Vitic. 2013, 34, 223–232. [Google Scholar] [CrossRef]
- Massera, A.; Soria, A.; Catania, C.; Krieger, S.; Combina, M. Simultaneous inoculation of Malbec (Vitis vinifera) musts with yeast and bacteria: Effects on fermentation performance, sensory and sanitary attributes of wines. Food Technol. Biotechnol. 2009, 47, 192–201. [Google Scholar]
- Abrahamse, C.; Bartowsky, E. Timing of malolactic fermentation inoculation in Shiraz grape must and wine: Influence on chemical composition. World J. Microbiol. Biot. 2012, 28, 255–265. [Google Scholar] [CrossRef]
- Du Plessis, H.W.; du Toit, M.; Hoff, J.W.; Hart, R.S.; Ndimba, B.K.; Jolly, N.P. Characterisation of non-Saccharomyces yeasts using different methodologies and evaluation of their compatibility with malolactic fermentation. S. Afr. J. Enol. Vitic. 2017, 38, 46–63. [Google Scholar] [CrossRef]
- Minnaar, P.P.; Ntushelo, N.; Ngqumba, Z.; Van Breda, V.; Jolly, N.P. Effect of Torulaspora delbrueckii yeast on the anthocyanin and flavanol concentrations of Cabernet franc and Pinotage wines. S. Afr. J. Enol. Vitic. 2015, 36, 50–58. [Google Scholar] [CrossRef]
- Anonymous. Methods of Analyses for Wine Laboratories. Compiled by the South African Wine Laboratories Association. South African Society for Viticulture and Oenology, P.O. Box 2092, Dennesig, Stellenbosch 7601, South Africa. Available online: http://www.sasev.org (accessed on 30 November 2017).
- Louw, L.; Roux, K.; Tredoux, A.; Tomic, O.; Naes, T.; Nieuwoudt, H.H.; Van Rensburg, P. Characterisation of selected South African cultivar wines using FTMIR spectroscopy, gas chromatography and multivariate data analysis. J. Agric. Food. Chem. 2009, 57, 2623–2632. [Google Scholar] [CrossRef]
- Dehlholm, C. Descriptive Sensory Evaluations: Comparison and Applicability of Novel Rapid Methodologies. Ph.D. Thesis, Department of Food Science, University of Copenhagen, Copenhagen, Denmark, 2012. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of Variance Test for Normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Ott, R.L. An Introduction to Statistical Methods and Data Analysis; Duxbury Press: Belmont, CA, USA, 1998; pp. 807–837. [Google Scholar]
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Lema, C.; García-Jares, C.; Orriols, I.; Angulo, L. Contribution of Saccharomyces and non-Saccharomyces populations to the production of some components of Albariño wine aroma. Am. J. Enol. Vitic. 1996, 47, 206–216. [Google Scholar]
- Fleet, G.H.; Lafon-Lafourcade, S.; Ribéreau-Gayon, P. Evolution of yeasts and lactic acid bacteria during fermentation and storage of Bordeaux wines. Appl. Environ. Microbiol. 1984, 48, 1034–1038. [Google Scholar] [PubMed]
- López, I.; López, R.; Santamaría, P.; Torres, C.; Ruiz-Larrea, F. Performance of malolactic fermentation by inoculation of selected Lactobacillus plantarum and Oenococcus oeni strains isolated from Rioja red wines. VITIS—J. Grapevine Res. 2008, 47, 123–129. [Google Scholar]
- González-Arenzana, L.; López, R.; Santamaría, P.; Tenorio, C.; López-Alfaro, I. Dynamics of indigenous lactic acid bacteria populations in wine fermentations from La Rioja (Spain) during three vintages. Microb. Ecol. 2012, 63, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Farías, M.E. Improvement of wine organoleptic characteristics by non-Saccharomyces yeasts. Curr. Res. Technol. Ed. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 908–919. [Google Scholar]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its Importance to Wine Aroma—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 77–129. [Google Scholar]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Silva, R.D.; Chaves, S.R.; Sousa, M.J.; Côrte-Real, M. The impact of acetate metabolism on yeast fermentative performance and wine quality: Reduction of volatile acidity of grape musts and wines. Appl. Microbiol. Biotechnol. 2011, 89, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, L.M.; Merín, M.G.; Morata, V.I.; Farías, M.E. Characterization of wines produced by mixed culture of autochthonous yeasts and Oenococcus oeni from the northwest region of Argentina. J. Ind. Microbiol. Biotechnol. 2011, 38, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.; Miot-Sertier, C.; Marullo, P.; Hernández-Orte, P.; Lagarrigue, L.; Lonvaud-Funel, A.; Bely, M. Genetic characterization and phenotypic variability in Torulaspora delbrueckii species: Potential applications in the wine industry. Int. J. Food Microbiol. 2009, 134, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Vejarano, R.; Bañuelos, M.A.; Morata, A.; Tesfaye, W.; Uthurry, C.; Villa, A.; Cintora, I.; Suárez-Lepe, J.A. Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT Food Sci. Technol. 2014, 59, 915–922. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G.; Comi, G.; Zironi, R. Higher alcohol and acetic acid production by apiculate wine yeasts. J. Appl. Bact. 1992, 73, 126–130. [Google Scholar] [CrossRef]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The oenological potential of Hanseniaspora uvarum in simultaneous and sequential co-fermentation with Saccharomyces cerevisiae for industrial wine production. Front. Microbiol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Palomero, F.; Gálvez, L.; Morata, A.; Calderón, F.; Palmero, D.; Suárez-Lepe, J.A. Quality and composition of red wine fermented with Schizosaccharomyces pombe as sole fermentative yeast, and in mixed and sequential fermentations with Saccharomyces cerevisiae. Food Technol. Biotechnol. 2014, 52, 376–382. [Google Scholar]
- Tristezza, M.; di Feo, L.; Tufariello, M.; Grieco, F.; Capozzi, V.; Spano, G.; Mita, G. Simultaneous inoculation of yeasts and lactic acid bacteria: Effects on fermentation dynamics and chemical composition of Negroamaro wine. LWT Food Sci. Technol. 2016, 66, 406–412. [Google Scholar] [CrossRef]
- Izquierdo-Cañas, P.M.; Mena-Morales, A.; García-Romero, E. Malolactic fermentation before or during wine aging in barrels. LWT Food Sci. Technol. 2016, 66, 468–474. [Google Scholar] [CrossRef]
- Izquierdo-Cañas, P.M.; Romero, E.G.; Pérez-Martín, F.; Seseña, S.; Palop, M.L. Sequential inoculation versus co-inoculation in Cabernet Franc wine fermentation. Food Sci. Technol. Int. 2015, 21, 203–212. [Google Scholar] [CrossRef]
- Guth, H. Quantification and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food. Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterisation of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G. Flavor chemistry and odor thresholds. In Flavor Chemistry: 30 Years of Progress; Teranishi, R., Wick, E., Hornstein, I., Eds.; Kluwer Academic: New York, NY, USA, 1999; pp. 353–365. [Google Scholar]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparitive study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 589–590. [Google Scholar] [CrossRef]
- Tao, Y.; Li, H.; Wang, H.; Zhang, L. Volatile compounds of young Cabernet Sauvignon red wine from Changli County (China). J. Food Comp. Anal. 2008, 21, 689–694. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. Changes in the concentration of yeast-derived volatile compounds of red wine during malolactic fermentation with four commercial starter cultures of Oenococcus oeni. J. Agric. Food Chem. 2005, 53, 10134–10139. [Google Scholar] [CrossRef] [PubMed]
- Tat, L.; Battistutta, F.; Comuzzo, P.; Zironi, R. Ethyl Phenylacetate as the probable responsible of honey-like character in Aglianico del Vulture wine. J. Agric. Food Chem. 2007, 55, 5205–5212. [Google Scholar] [CrossRef] [PubMed]
- Etiévant, P.X. Wine. In Volatile Compounds of Food and Beverages; Maarse, H., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Fazzalari, F.A. Compilation of Odor and Taste Threshold Values Data; ASTM Data Series: West Conshohocken, PA, USA, 1978. [Google Scholar]

| Reference Code | Species Name | Source |
|---|---|---|
| Sc | Saccharomyces cerevisiae | VIN 13, commercial yeast strain from Anchor Wine Yeast, South Africa |
| C7 | Candida zemplinina (synonym: Starmerella bacillaris) | Yeast isolate from ARC Infruitec-Nietvoorbij culture collection |
| H4 | Hanseniaspora uvarum | Yeast isolate from ARC Infruitec-Nietvoorbij culture collection, South Africa |
| L1 | Lachancea thermotolerans | Viniflora® Rhythm™, commercial yeast strain from Chr. Hansen A/S, Denmark |
| L2 | Lachancea thermotolerans | Yeast isolate from ARC Infruitec-Nietvoorbij culture collection |
| M2 | Metschnikowia pulcherrima | Yeast isolate from ARC Infruitec-Nietvoorbij culture collection |
| T3 | Torulaspora delbrueckii | Level2 TD™, commercial yeast strain from Lallemand Inc. |
| T6 | Torulaspora delbrueckii | Yeast isolate from ARC Infruitec-Nietvoorbij culture collection |
| O. oeni | Oenococcus oeni | Commercial malolactic bacteria culture Viniflora® oenos from Chr. Hansen A/S |
| Treatment 2 | Residual Sugar (g/L) | pH | Volatile Acidity (g/L) | Total Acidity (g/L) | Malic Acid (g/L) | Lactic Acid (g/L) | Alcohol (% v/v) | Glycerol (g/L) | Duration of MLF (Days) |
|---|---|---|---|---|---|---|---|---|---|
| Sc | 2.23 ± 0.13 ef 3 | 3.66 ± 0.01 jkl | 0.25 ± 0.01 k | 6.19 ± 0.04 a | 1.26 ± 0.06 c | <0.20 i | 15.99 ± 0.03 abcd | 11.43 ± 0.05 fgh | No MLF |
| Sc + sim MLF | 2.16 ± 0.16 ef | 3.74 ± 0.04 defg | 0.39 ± 0.02 gh | 5.89 ± 0.12 cde | <0.20 f | 1.01 ± 0.04 b | 16.09 ± 0.12 a | 11.84 ± 0.09 ab | 54 |
| Sc + seq MLF | 2.18 ± 0.27 ef | 3.76 ± 0.01 cdef | 0.39 ± 0.02 gh | 5.52 ± 0.03 ij | <0.20 f | 0.86 ± 0.02 d | 16.01 ± 0.07 abc | 11.75 ± 0.09 bc | 53 |
| C7 + Sc | 2.23 ± 0.13 ef | 3.59 ± 0.01 m | 0.33 ± 0.01 i | 6.21 ± 0.03 a | 1.21 ± 0.05 c | <0.20 i | 15.49 ± 0.01 k | 11.08 ± 0.13 k | No MLF |
| C7 + Sc + sim MLF | 2.20 ± 0.25 ef | 3.67 ± 0 ijkl | 0.40 ± 0.01 fg | 6.03 ± 0.13 bc | <0.20 f | 0.95 ± 0.04 c | 15.93 ± 0.04 bcdef | 11.85 ± 0.14 ab | 63 |
| C7 + Sc + seq MLF | 2.32 ± 0.22 bcdef | 3.70 ± 0.01 ghij | 0.47 ± 0.02 c | 5.65 ± 0.04 ghi | <0.20 f | 0.77 ± 0.02 g | 15.54 ± 0.03 k | 11.24 ± 0.04 ij | 40 |
| H4 + Sc | 2.77 ± 0.16 a | 3.76 ± 0.04 cdef | 0.37 ± 0.01 h | 5.69 ± 0.03 gh | 0.77 ± 0.09 e | <0.20 i | 15.94 ± 0.03 bcde | 11.26 ± 0.04 ij | No MLF |
| H4 + Sc + sim MLF | 2.42 ± 0.19 bcde | 3.73 ± 0.04 efgh | 0.42 ± 0.02 ef | 5.76 ± 0.05 efg | <0.20 f | 1.06 ± 0.06 a | 15.82 ± 0.09 fghij | 11.76 ± 0.16 bc | 48 |
| H4 + Sc + seq MLF | 2.78 ± 0.18 a | 3.85 ± 0.01 b | 0.52 ± 0.01 b | 5.19 ± 0.04 m | <0.20 f | 0.81 ± 0.02 efg | 15.96 ± 0.04 bcd | 11.59 ± 0.05 de | 38 |
| L1 + Sc | 2.32 ± 0.26 bcdef | 3.72 ± 0.01 efghi | 0.30 ± 0.02 j | 5.88 ± 0.01 def | 1.12 ± 0.02 d | <0.20 i | 15.77 ± 0.02 ij | 11.33 ± 0.05 hi | No MLF |
| L1 + Sc + sim MLF | 2.60 ± 0.09 ab | 3.76 ± 0.25 def | 0.43 ± 0.01 ef | 5.58 ± 0.06 hij | <0.20 f | 1.00 ± 0.02 b | 15.93 ± 0.08 bcdef | 11.61 ± 0.08 cde | 48 |
| L1 + Sc + seq MLF | 2.22 ± 0.27 ef | 3.80 ± 0.01 bcd | 0.44 ± 0.01 de | 5.35 ± 0.02 kl | <0.20 f | 0.86 ± 0.02 d | 15.80 ± 0.04 hij | 11.71 ± 0.03 bcd | 48 |
| L2 + Sc | 2.55 ± 0.13 abcd | 3.83 ± 0 b | 0.42 ± 0.01 ef | 5.48 ± 0.05 jk | 1.68 ± 0.01 a | <0.20 i | 16.04 ± 0.04 ab | 11.06 ± 0.05 k | No MLF |
| L2 + Sc + sim MLF | 2.18 ± 0.30 ef | 3.62 ± 0.13 lm | 0.39 ± 0.05 gh | 6.04 ± 0.03 b | <0.20 f | 0.82 ± 0.07 def | 15.89 ± 0.02 defgh | 11.62 ± 0.26 cde | 68 |
| L2 + Sc + seq MLF | 2.59 ± 0.18 abc | 3.92 ± 0.02 a | 0.56 ± 0.01 a | 4.95 ± 0.03 n | <0.20 f | 0.70 ± 0.04 h | 16.08 ± 0.02 a | 11.37 ± 0.06 ghi | 40 |
| M2 + Sc | 2.20 ± 0.28 ef | 3.67 ± 0.02 hijk | 0.31 ± 0.10 ij | 6.01 ± 0.02 bcd | 1.21 ± 0.03 c | <0.20 i | 15.81 ± 0.13 ghij | 11.32 ± 0.03 hi | No MLF |
| M2 + Sc + sim MLF | 2.08 ± 0.22 f | 3.74 ± 0.04 defg | 0.43 ± 0.02 de | 5.76 ± 0.12 efg | <0.20 f | 0.96 ± 0.012 bc | 15.81 ± 0.09 ghij | 11.71 ± 0.02 bcd | 52 |
| M2 + Sc + seq MLF | 2.28 ± 0.19 cdef | 3.77 ± 0.02 cde | 0.48 ± 0.01 c | 5.44 ± 0.03 jk | <0.20 f | 0.78 ± 0.03 fg | 15.92 ± 0.02 cdefg | 11.57 ± 0.04 def | 48 |
| T3 + Sc | 2.45 ± 0.20 bcde | 3.63 ± 0.01 klm | 0.31 ± 0.01 ij | 6.08 ± 0.07 ab | 1.25 ± 0.08 c | <0.20 i | 15.80 ± 0.15 hij | 10.98 ± 0.11 k | No MLF |
| T3 + Sc + sim MLF | 2.26 ± 0.11 def | 3.74 ± 0.02 defg | 0.41 ± 0.01 fg | 5.76 ± 0.10 efg | <0.20 f | 0.99 ± 0.04 bc | 15.91 ± 0.01 cdefgh | 11.98 ± 0.07 a | 51 |
| T3 + Sc + seq MLF | 2.29 ± 0.21 bcdef | 3.73 ± 0.01 efg | 0.46 ± 0.01 cd | 5.48 ± 0.03 jk | <0.20 f | 0.85 ± 0.04 de | 15.88 ± 0.02 defghi | 11.25 ± 0.78 ij | 48 |
| T6 + Sc | 2.45 ± 0.06 bcde | 3.72 ± 0.02 fghi | 0.40 ± 0.01 fg | 5.74 ± 0.05 fg | 1.59 ± 0.04 b | <0.20 i | 15.84 ± 0.14 efghij | 11.14 ± 0.04 jk | No MLF |
| T6 + Sc + sim MLF | 2.22 ± 0.10 ef | 3.67 ± 0.01 ijkl | 0.41 ± 0.02 fg | 6.00 ± 0.06 bcd | <0.20 f | 0.95 ± 0.04 c | 15.75 ± 0.09 j | 11.75 ± 0.09 bc | 53 |
| T6 + Sc + seq MLF | 2.58 ± 0.16 abcd | 3.82 ± 0.01 bc | 0.54 ± 0.01 a | 5.27 ± 0.02 lm | <0.20 f | 0.65 ± 0.02 h | 15.94 ± 0.02 bcde | 11.49 ± 0.11 efg | 40 |
| Treatment 1 | Ethyl Acetate | OAV | Ethyl Butanoate | OAV | Isoamyl Acetate | OAV | Ethyl Lactate | OAV | Ethyl-3-hydroxy Butanoate | OAV | Diethyl Succinate | OAV | Ethyl Hexanoate | OAV | Ethyl Octanoate | OAV |
| Sc | 40.20 p 2 | 3.3 | 0.49 kl | 1.2 | 1.33 ijk | 8.3 | 1.59 l | 0.1 | 1.68 efg | 1.7 | 2.103 f | 1.8 | 0.77 ij | 9.6 | 0.33 f | 0.6 |
| Sc + sim MLF | 52.98 m | 4.4 | 0.49 kl | 1.2 | 1.05 n | 6.6 | 9.22 b | 0.7 | 1.68 ef | 1.7 | 2.466 c | 2.1 | 0.76 j | 9.4 | 0.25 i | 0.4 |
| Sc + seq MLF | 55.77 l | 4.6 | 0.48 l | 1.2 | 1.48 def | 9.3 | 6.48 g | 0.5 | 1.60 j | 1.6 | 2.076 f | 1.7 | 0.77 ij | 9.6 | 0.33 f | 0.6 |
| C7 + Sc | 58.55 jk | 4.9 | 0.53 efgh | 1.3 | 1.06 n | 6.7 | 1.62 l | 0.1 | 1.76 bc | 1.8 | 1.941 h | 1.6 | 0.80 fg | 10.0 | 0.32 f | 0.6 |
| C7 + Sc + sim MLF | 62.08 ghi | 5.2 | 0.53 efgh | 1.3 | 1.07 n | 6.7 | 8.81 c | 0.6 | 1.75 cd | 1.8 | 2.581 b | 2.2 | 0.80 fg | 10.0 | 0.28 gh | 0.5 |
| C7 + Sc + seq MLF | 76.02 b | 6.3 | 0.55 ab | 1.4 | 1.20 lm | 7.5 | 6.77 ef | 0.5 | 1.79 ab | 1.8 | 2.002 g | 1.7 | 0.83 cd | 10.4 | 0.37 e | 0.6 |
| H4 + Sc | 65.72 f | 5.5 | 0.52 efgh | 1.3 | 1.47 defg | 9.2 | 2.08 k | 0.1 | 1.67 fgh | 1.7 | 1.544 m | 1.3 | 0.81 ef | 10.1 | 0.37 e | 0.6 |
| H4 + Sc + sim MLF | 64.15 fg | 5.3 | 0.50 jk | 1.3 | 1.23 klm | 7.7 | 7.54 d | 0.5 | 1.70 e | 1.7 | 2.486 c | 2.1 | 0.78 hi | 9.8 | 0.27 h | 0.5 |
| H4 + Sc + seq MLF | 73.35 c | 6.1 | 0.54 bcd | 1.4 | 1.60 abc | 10.0 | 4.80 j | 0.3 | 1.64 hi | 1.6 | 1.586 m | 1.3 | 0.84 c | 10.5 | 0.41 c | 0.7 |
| L1 + Sc | 45.83 o | 3.8 | 0.53 cdef | 1.3 | 1.44 efgh | 9.0 | 1.53 l | 0.1 | 1.59 j | 1.6 | 1.828 k | 1.5 | 0.80 efg | 10.0 | 0.36 e | 0.6 |
| L1 + Sc + sim MLF | 63.54 fgh | 5.3 | 0.52 ghi | 1.3 | 1.39 fghi | 8.7 | 7.46 d | 0.5 | 1.67 fgh | 1.7 | 2.459 c | 2.0 | 0.79 gh | 9.9 | 0.29 gh | 0.5 |
| L1 + Sc + seq MLF | 60.39 ij | 5.0 | 0.53 cde | 1.3 | 1.53 cde | 9.5 | 5.63 hi | 0.4 | 1.64 i | 1.6 | 1.854 jk | 1.5 | 0.81 efg | 10.1 | 0.38 de | 0.7 |
| L2 + Sc | 69.35 e | 5.8 | 0.55 ab | 1.4 | 1.64 ab | 10.2 | 2.01 k | 0.1 | 1.74 cd | 1.7 | 1.710 l | 1.4 | 0.88 a | 11.0 | 0.43 b | 0.7 |
| L2 + Sc + sim MLF | 72.04 cd | 6.0 | 0.52 fghi | 1.3 | 0.95 o | 5.9 | 9.64 a | 0.7 | 1.80 a | 1.8 | 2.595 b | 2.2 | 0.81 ef | 10.1 | 0.31 f | 0.5 |
| L2 + Sc + seq MLF | 81.31 a | 6.8 | 0.56 a | 1.4 | 1.67 a | 10.5 | 5.03 j | 0.4 | 1.74 cd | 1.7 | 1.714 l | 1.4 | 0.88 a | 11.1 | 0.46 a | 0.8 |
| M2 + Sc | 48.61 n | 4.1 | 0.54 bc | 1.4 | 1.52 cde | 9.5 | 1.39 l | 0.1 | 1.65 ghi | 1.7 | 1.870 ijk | 1.6 | 0.83 c | 10.4 | 0.40 cd | 0.7 |
| M2 + Sc + sim MLF | 57.62 kl | 4.8 | 0.51 ij | 1.3 | 1.21 lm | 7.6 | 6.69 fg | 0.5 | 1.69 ef | 1.7 | 2.283 e | 1.9 | 0.77 ij | 9.6 | 0.27 gh | 0.5 |
| M2 + Sc + seq MLF | 63.71 fgh | 5.3 | 0.53 cdefg | 1.3 | 1.44 defgh | 9.0 | 5.69 hi | 0.4 | 1.67 fgh | 1.7 | 1.852 jk | 1.5 | 0.82 de | 10.2 | 0.39 cd | 0.7 |
| T3 + Sc | 51.47 m | 4.3 | 0.54 bcd | 1.3 | 1.32 ijk | 8.3 | 1.50 l | 0.1 | 1.73 d | 1.7 | 1.885 ij | 1.6 | 0.83 cd | 10.3 | 0.37 e | 0.6 |
| T3 + Sc + sim MLF | 60.75 ij | 5.1 | 0.53 cdefg | 1.3 | 1.36 hij | 8.5 | 6.99 e | 0.5 | 1.66 fghi | 1.7 | 2.353 d | 2.0 | 0.79 gh | 9.9 | 0.29 g | 0.5 |
| T3 + Sc + seq MLF | 63.41 fgh | 5.3 | 0.54 bcd | 1.4 | 1.29 jkl | 8.0 | 5.88 h | 0.4 | 1.73 d | 1.7 | 1.912 hi | 1.6 | 0.84 c | 10.5 | 0.40 cd | 0.7 |
| T6 + Sc | 70.27 de | 5.9 | 0.55 ab | 1.4 | 1.38 ghij | 8.6 | 2.10 k | 0.1 | 1.67 fgh | 1.7 | 1.581 m | 1.3 | 0.86 b | 10.7 | 0.40 cd | 0.7 |
| T6 + Sc + sim MLF | 61.55 hi | 5.1 | 0.52 hi | 1.3 | 1.18 m | 7.4 | 8.65 c | 0.6 | 1.75 cd | 1.8 | 2.717 a | 2.3 | 0.80 fgh | 10.0 | 0.28 gh | 0.5 |
| T6 + Sc + seq MLF | 83.26 a | 6.9 | 0.56 a | 1.4 | 1.55 bcd | 9.7 | 5.59 i | 0.4 | 1.68 efg | 1.7 | 1.595 m | 1.3 | 0.88 a | 11.0 | 0.46 a | 0.8 |
| Treatment | Ethyl Decanoate | OAV | Ethyl Phenyl Acetate | OAV | 2-Phenyl ethyl Acetate | OAV | Methanol | OAV | Propanol | OAV | Butanol | OAV | Isobutanol | OAV | Pentanol | OAV |
| Sc | 0.097 ij | 0.2 | 0.61 c | 8.4 | 1.18 jk | 0.7 | 156.39 de | 0.3 | 47.65 fg | 0.2 | 3.34 a | 0.02 | 42.39 hi | 1.1 | 0.710 j | 0.01 |
| Sc + sim MLF | 0.123 bc | 0.2 | 0.64 b | 8.7 | 1.15 k | 0.6 | 184.84 abc | 0.4 | 53.65 bc | 0.2 | 2.62 e | 0.02 | 47.77 cd | 1.2 | 0.734 b | 0.01 |
| Sc + seq MLF | 0.096 j | 0.2 | 0.67 a | 9.2 | 1.18 jk | 0.7 | 146.68 gh | 0.3 | 43.27 ij | 0.1 | 3.15 b | 0.02 | 38.50 k | 1.0 | 0.706 kl | 0.01 |
| C7 + Sc | 0.098 hij | 0.2 | 0.44 n | 6.1 | 1.31 efg | 0.7 | 178.68 c | 0.4 | 42.25 j | 0.1 | 2.07 m | 0.01 | 52.69 b | 1.3 | 0.723 gh | 0.01 |
| C7 + Sc + sim MLF | 0.132 a | 0.3 | 0.49 kl | 6.8 | 1.27 hi | 0.7 | 184.83 abc | 0.4 | 51.70 cd | 0.2 | 2.50 fg | 0.02 | 51.95 b | 1.3 | 0.737 ab | 0.01 |
| C7 + Sc + seq MLF | 0.125 abc | 0.2 | 0.48 klm | 6.6 | 1.38 d | 0.8 | 177.87 c | 0.4 | 42.76 ij | 0.1 | 2.15 l | 0.01 | 54.62 a | 1.4 | 0.733 cd | 0.01 |
| H4 + Sc | 0.101 ghij | 0.2 | 0.51 ij | 7.0 | 1.27 hi | 0.7 | 179.10 c | 0.4 | 49.41 ef | 0.2 | 2.36 i | 0.02 | 38.02 k | 1.0 | 0.719 h | 0.01 |
| H4 + Sc + sim MLF | 0.121 bcd | 0.2 | 0.59 d | 8.0 | 1.19 j | 0.7 | 182.98 bc | 0.4 | 43.93 hij | 0.1 | 2.35 ij | 0.02 | 49.38 c | 1.2 | 0.733 cd | 0.01 |
| H4 + Sc + seq MLF | 0.111 ef | 0.2 | 0.55 fg | 7.6 | 1.37 d | 0.8 | 161.97 d | 0.3 | 44.95 hi | 0.1 | 2.26 k | 0.02 | 35.61 l | 0.9 | 0.723 gh | 0.01 |
| L1 + Sc | 0.102 ghij | 0.2 | 0.50 kl | 6.8 | 1.28 ghi | 0.7 | 152.48 efg | 0.3 | 54.69 ab | 0.2 | 2.98 c | 0.02 | 34.72 l | 0.9 | 0.715 i | 0.01 |
| L1 + Sc + sim MLF | 0.096 j | 0.2 | 0.57 de | 7.8 | 1.28 fghi | 0.7 | 190.70 a | 0.4 | 49.37 ef | 0.2 | 2.24 k | 0.01 | 52.49 b | 1.3 | 0.727 ef | 0.01 |
| L1 + Sc + seq MLF | 0.105 fghi | 0.2 | 0.56 ef | 7.7 | 1.29 fgh | 0.7 | 156.00 def | 0.3 | 56.13 a | 0.2 | 3.03 c | 0.02 | 35.65 l | 0.9 | 0.721 h | 0.01 |
| L2 + Sc | 0.113 def | 0.2 | 0.57 de | 7.8 | 1.47 b | 0.8 | 183.45 abc | 0.4 | 42.93 ij | 0.1 | 2.51 fg | 0.02 | 44.82 efg | 1.1 | 0.726 efg | 0.01 |
| L2 + Sc + sim MLF | 0.117 cde | 0.2 | 0.48 lm | 6.6 | 1.29 fgh | 0.7 | 190.62 a | 0.4 | 50.31 de | 0.2 | 2.70 d | 0.02 | 56.45 a | 1.4 | 0.739 a | 0.01 |
| L2 + Sc + seq MLF | 0.122 bcd | 0.2 | 0.67 a | 9.2 | 1.80 a | 1.0 | 186.66 ab | 0.4 | 43.49 ij | 0.1 | 2.51 fg | 0.02 | 45.19 ef | 1.1 | 0.732 cd | 0.01 |
| M2 + Sc | 0.107 fg | 0.2 | 0.50 jk | 6.8 | 1.34 de | 0.7 | 143.03 h | 0.3 | 38.41 k | 0.1 | 2.52 fg | 0.02 | 39.81 jk | 1.0 | 0.704 kl | 0.01 |
| M2 + Sc + sim MLF | 0.126 ab | 0.3 | 0.57 ef | 7.8 | 1.21 j | 0.7 | 183.91 abc | 0.4 | 43.72 ij | 0.1 | 2.40 hi | 0.02 | 49.32 c | 1.2 | 0.720 h | 0.01 |
| M2 + Sc + seq MLF | 0.105 fghi | 0.2 | 0.54 gh | 7.4 | 1.32 ef | 0.7 | 148.78 fgh | 0.3 | 39.55 k | 0.1 | 2.56 ef | 0.02 | 40.80 ij | 1.0 | 0.706 kl | 0.01 |
| T3 + Sc | 0.106 fgh | 0.2 | 0.47 m | 6.5 | 1.35 e | 0.7 | 149.18 efgh | 0.3 | 35.70 l | 0.1 | 2.22 lk | 0.01 | 42.94 gh | 1.1 | 0.703 l | 0.01 |
| T3 + Sc + sim MLF | 0.100 ghij | 0.2 | 0.53 gh | 7.3 | 1.25 i | 0.7 | 178.55 c | 0.4 | 49.52 def | 0.2 | 2.46 gh | 0.02 | 46.60 de | 1.2 | 0.729 de | 0.01 |
| T3 + Sc + seq MLF | 0.103 ghij | 0.2 | 0.54 gh | 7.4 | 1.38 d | 0.8 | 150.39 efgh | 0.3 | 35.75 l | 0.1 | 2.28 jk | 0.02 | 43.44 fgh | 1.1 | 0.707 jk | 0.01 |
| T6 + Sc | 0.107 fg | 0.2 | 0.47 m | 6.4 | 1.43 c | 0.8 | 179.67 bc | 0.4 | 46.10 gh | 0.2 | 2.35 ij | 0.02 | 38.90 jk | 1.0 | 0.721 h | 0.01 |
| T6 + Sc + sim MLF | 0.126 abc | 0.3 | 0.53 hi | 7.2 | 1.28 fghi | 0.7 | 179.36 bc | 0.4 | 54.32 ab | 0.2 | 2.38 hi | 0.02 | 52.08 b | 1.3 | 0.740 a | 0.01 |
| T6 + Sc + seq MLF | 0.120 bcd | 0.2 | 0.51 ij | 7.1 | 1.47 b | 0.8 | 181.11 bc | 0.4 | 46.09 gh | 0.2 | 2.40 hi | 0.02 | 39.14 jk | 1.0 | 0.725 fg | 0.01 |
| Treatment | Isoamyl Alcohol | OAV | 3-Ethoxy-1-propanol | OAV | 3-Methyl-1-pentanol | OAV | Hexanol | OAV | 2-Phenyl Ethanol | OAV | Acetoin | OAV | Acetic Acid | OAV | ||
| Sc | 338.70 ef | 5.6 | 2.43 jk | 24.3 | 0.65 d | 0.6 | 37.73 kl | 4.7 | 79.45 bc | 5.7 | 5.26 kl | 0.04 | 180.02 p | 0.9 | ||
| Sc + sim MLF | 370.68 c | 6.2 | 2.71 h | 27.1 | 0.69 a | 0.7 | 45.05 bc | 5.6 | 75.78 e | 5.4 | 13.95 de | 0.09 | 269.45 l | 1.3 | ||
| Sc + seq MLF | 335.27 f | 5.6 | 2.41 k | 24.1 | 0.65 d | 0.7 | 36.92 klm | 4.6 | 77.00 cde | 5.5 | 12.59 efg | 0.08 | 278.61 kl | 1.4 | ||
| C7 + Sc | 302.06 h | 5.0 | 3.86 b | 38.6 | 0.59 i | 0.6 | 39.42 hi | 4.9 | 57.37 j | 4.1 | 4.59 l | 0.03 | 272.79l | 1.4 | ||
| C7 + Sc + sim MLF | 381.58 b | 6.4 | 2.51 ij | 25.1 | 0.67 b | 0.7 | 44.20 bcde | 5.5 | 80.14 b | 5.7 | 13.57 efg | 0.09 | 298.04 ij | 1.5 | ||
| C7 + Sc + seq MLF | 319.82 g | 5.3 | 4.06 a | 40.6 | 0.60 h | 0.6 | 42.16 g | 5.3 | 59.73 ij | 4.3 | 13.64 def | 0.09 | 385.22 c | 1.9 | ||
| H4 + Sc | 228.40 m | 3.8 | 3.23 f | 32.3 | 0.56 k | 0.6 | 40.21 h | 5.0 | 39.14 l | 2.8 | 5.44 kl | 0.04 | 306.70 ghi | 1.5 | ||
| H4 + Sc + sim MLF | 366.74 cd | 6.1 | 2.44 jk | 24.4 | 0.67 b | 0.7 | 45.58 ab | 5.7 | 76.33 de | 5.5 | 8.65 ij | 0.06 | 316.47 gh | 1.6 | ||
| H4 + Sc + seq MLF | 228.74 m | 3.8 | 3.38 e | 33.8 | 0.56 jk | 0.6 | 43.17 efg | 5.4 | 39.77 l | 2.8 | 15.90 b | 0.11 | 384.48 c | 1.9 | ||
| L1 + Sc | 285.62 j | 4.8 | 2.56 i | 25.6 | 0.62 e | 0.6 | 37.94 jk | 4.7 | 63.25 gh | 4.5 | 5.45 kl | 0.04 | 201.73 o | 1.0 | ||
| L1 + Sc + sim MLF | 366.73 cd | 6.1 | 2.79 h | 27.9 | 0.62 e | 0.6 | 44.76 bcd | 5.6 | 74.72 e | 5.3 | 15.54 bc | 0.10 | 318.76 fg | 1.6 | ||
| L1 + Sc + seq MLF | 290.64 ij | 4.8 | 2.57 i | 25.7 | 0.62 e | 0.6 | 38.13 ijk | 4.8 | 64.89 g | 4.6 | 12.25 gh | 0.08 | 313.81 gh | 1.6 | ||
| L2 + Sc | 260.06 k | 4.3 | 3.34 e | 33.4 | 0.57 j | 0.6 | 43.55 defg | 5.4 | 44.24 k | 3.2 | 6.02 k | 0.04 | 350.01 d | 1.8 | ||
| L2 + Sc + sim MLF | 400.33 a | 6.7 | 2.57 i | 25.7 | 0.65 d | 0.7 | 45.52 ab | 5.7 | 87.48 a | 6.2 | 11.78 h | 0.08 | 305.54 hi | 1.5 | ||
| L2 + Sc + seq MLF | 259.57 k | 4.3 | 3.53 d | 35.3 | 0.57 j | 0.6 | 44.27 bcde | 5.5 | 43.81 k | 3.1 | 12.53 fgh | 0.08 | 468.89 a | 2.3 | ||
| M2 + Sc | 290.00 ij | 4.8 | 3.56 d | 35.6 | 0.61 ef | 0.6 | 36.44 lm | 4.6 | 62.50 gh | 4.5 | 4.19 l | 0.03 | 219.49 n | 1.1 | ||
| M2 + Sc + sim MLF | 346.22 e | 5.8 | 3.58 cd | 35.8 | 0.65 d | 0.7 | 43.28 efg | 5.4 | 69.30 f | 4.9 | 9.79 i | 0.07 | 290.89 jk | 1.5 | ||
| M2 + Sc + seq MLF | 290.23 ij | 4.8 | 3.61 cd | 36.1 | 0.61 fg | 0.6 | 36.24 m | 4.5 | 61.54 hi | 4.4 | 7.94 j | 0.05 | 334.87 e | 1.7 | ||
| T3 + Sc | 294.95 hi | 4.9 | 3.54 d | 35.4 | 0.60 gh | 0.6 | 38.02 ijk | 4.8 | 57.63 j | 4.1 | 4.28 l | 0.03 | 242.29 m | 1.2 | ||
| T3 + Sc + sim MLF | 358.92 d | 6.0 | 2.56 i | 25.6 | 0.66 c | 0.7 | 42.66 fg | 5.3 | 78.99 bcd | 5.6 | 12.16 h | 0.08 | 298.90 ij | 1.5 | ||
| T3 + Sc + seq MLF | 297.42 hi | 5.0 | 3.67 c | 36.7 | 0.61 fg | 0.6 | 39.18 hij | 4.9 | 57.92 j | 4.1 | 8.40 ij | 0.06 | 338.07 de | 1.7 | ||
| T6 + Sc | 235.74 m | 3.9 | 3.23 f | 32.3 | 0.56 jk | 0.6 | 43.08 efg | 5.4 | 37.94 l | 2.7 | 6.49 k | 0.04 | 331.14 ef | 1.7 | ||
| T6 + Sc + sim MLF | 401.63 a | 6.7 | 2.91 g | 29.1 | 0.70 a | 0.7 | 46.61 a | 5.8 | 86.07 a | 6.1 | 14.30 cd | 0.10 | 294.87 ij | 1.5 | ||
| T6 + Sc + seq MLF | 237.91 l | 4.0 | 3.36 e | 33.6 | 0.56 jk | 0.6 | 43.86 cdef | 5.5 | 38.32 l | 2.7 | 17.89 a | 0.12 | 432.88 b | 2.2 | ||
| Treatment | Propionic Acid | OAV | Butyric Acid | OAV | Isobutyric Acid | OAV | Valeric Acid | OAV | Isovaleric Acid | OAV | Hexanoic Acid | OAV | Octanoic Acid | OAV | Decanoic Acid | OAV |
| Sc | 3.89 bcde | 0.2 | 1.13 ghi | 0.5 | 1.524 g | 0.05 | 0.417 ab | 1.4 | 9.07 i | 6.0 | 0.64 b | 0.2 | 1.57 j | 0.2 | 1.09 cd | 0.2 |
| Sc + sim MLF | 3.92 bcd | 0.2 | 1.10 ij | 0.5 | 1.759 e | 0.06 | 0.416 b | 1.4 | 18.67 g | 12.4 | 0.52 j | 0.2 | 1.29 m | 0.1 | 1.04 fgh | 0.2 |
| Sc + seq MLF | 3.91 bcd | 0.2 | 1.09 j | 0.5 | 1.443 h | 0.05 | 0.423 a | 1.4 | 18.80 g | 12.5 | 0.64 ab | 0.2 | 1.57 j | 0.2 | 1.06 efg | 0.2 |
| C7 + Sc | 3.52 hi | 0.2 | 1.21 bc | 0.6 | 1.930 c | 0.06 | 0.39 kl | 1.3 | 9.24 i | 6.2 | 0.55 hi | 0.2 | 1.64 i | 0.2 | 1.08 d | 0.2 |
| C7 + Sc + sim MLF | 4.05 ab | 0.2 | 1.21 c | 0.6 | 2.168 a | 0.07 | 0.398 ghij | 1.3 | 22.58 b | 15.1 | 0.55 i | 0.2 | 1.43 kl | 0.1 | 1.06 ef | 0.2 |
| C7 + Sc + seq MLF | 3.49 ij | 0.2 | 1.25 ab | 0.6 | 1.972 c | 0.07 | 0.395 ijkl | 1.3 | 22.82 b | 15.2 | 0.55 i | 0.2 | 1.77 efg | 0.2 | 1.09 cd | 0.2 |
| H4 + Sc | 4.18 a | 0.2 | 1.15 fgh | 0.5 | 1.283 k | 0.04 | 0.404 efg | 1.3 | 8.78 i | 5.9 | 0.57 fgh | 0.2 | 1.67 hi | 0.2 | 1.06 efg | 0.2 |
| H4 + Sc + sim MLF | 3.66 efghi | 0.2 | 1.12 hij | 0.5 | 1.818d | 0.06 | 0.405ef | 1.4 | 18.94 g | 12.6 | 0.58 efg | 0.2 | 1.37 l | 0.1 | 1.03 h | 0.2 |
| H4 + Sc + seq MLF | 3.76 defgh | 0.2 | 1.15 fgh | 0.5 | 1.197 l | 0.04 | 0.406 def | 1.4 | 20.91 ef | 13.9 | 0.59 de | 0.2 | 1.86 bcd | 0.2 | 1.08 d | 0.2 |
| L1 + Sc | 3.54 ghi | 0.2 | 1.17 def | 0.5 | 1.333 ijk | 0.04 | 0.406 def | 1.4 | 10.19 h | 6.8 | 0.66 ab | 0.2 | 1.73 gh | 0.2 | 1.08 de | 0.2 |
| L1 + Sc + sim MLF | 4.09 ab | 0.2 | 1.16 fg | 0.5 | 2.039 b | 0.07 | 0.412 bcd | 1.4 | 20.31 f | 13.5 | 0.59 de | 0.2 | 1.45 k | 0.1 | 1.06 ef | 0.2 |
| L1 + Sc + seq MLF | 3.77 cdefg | 0.2 | 1.20 cde | 0.5 | 1.282 k | 0.04 | 0.416 b | 1.4 | 24.19 a | 16.1 | 0.65 ab | 0.2 | 1.78 efg | 0.2 | 1.08 d | 0.2 |
| L2 + Sc | 3.63 fghi | 0.2 | 1.25 a | 0.6 | 1.525 g | 0.05 | 0.410 cde | 1.4 | 9.07 i | 6.0 | 0.61 c | 0.2 | 1.92 b | 0.2 | 1.10 bc | 0.2 |
| L2 + Sc + sim MLF | 4.07 ab | 0.2 | 1.26 a | 0.6 | 1.940 c | 0.06 | 0.405 ef | 1.3 | 22.59 b | 15.1 | 0.52 j | 0.2 | 1.44 k | 0.1 | 1.10 bcd | 0.2 |
| L2 + Sc + seq MLF | 4.08 ab | 0.2 | 1.26 a | 0.6 | 1.453 h | 0.05 | 0.415 bc | 1.4 | 21.43 de | 14.3 | 0.58 def | 0.2 | 2.00 a | 0.2 | 1.09 cd | 0.2 |
| M2 + Sc | 2.95 l | 0.1 | 1.15 fgh | 0.5 | 1.378 i | 0.05 | 0.394 jkl | 1.3 | 9.09 i | 6.1 | 0.66 a | 0.2 | 1.74 g | 0.2 | 1.10 bcd | 0.2 |
| M2 + Sc + sim MLF | 3.55 ghi | 0.2 | 1.09 j | 0.5 | 1.676 f | 0.06 | 0.398 ghij | 1.3 | 20.78 ef | 13.9 | 0.56 ghi | 0.2 | 1.39 kl | 0.1 | 1.03 h | 0.2 |
| M2 + Sc + seq MLF | 3.24 jk | 0.2 | 1.15 g | 0.5 | 1.302 k | 0.04 | 0.396 hijk | 1.3 | 22.33 bcd | 14.9 | 0.62c | 0.2 | 1.80 def | 0.2 | 1.08 d | 0.2 |
| T3 + Sc | 3.47 ij | 0.2 | 1.21 cd | 0.5 | 1.482 gh | 0.05 | 0.390 l | 1.3 | 9.21 i | 6.1 | 0.60 cd | 0.2 | 1.75 fg | 0.2 | 1.09 cd | 0.2 |
| T3 + Sc + sim MLF | 3.85 cdef | 0.2 | 1.17 ef | 0.5 | 1.734 e | 0.06 | 0.407 def | 1.4 | 21.34 e | 14.2 | 0.59 de | 0.2 | 1.44 k | 0.1 | 1.04 gh | 0.2 |
| T3 + Sc + seq MLF | 3.03 kl | 0.2 | 1.21 c | 0.5 | 1.440 h | 0.05 | 0.397 hij | 1.3 | 22.81 b | 15.2 | 0.58 def | 0.2 | 1.88 bc | 0.2 | 1.11 ab | 0.2 |
| T6 + Sc | 4.01 abc | 0.2 | 1.17 def | 0.5 | 1.366 ij | 0.05 | 0.396 ijkl | 1.3 | 9.26 i | 6.2 | 0.56 fghi | 0.2 | 1.84 cde | 0.2 | 1.08 cd | 0.2 |
| T6 + Sc + sim MLF | 3.43 ij | 0.2 | 1.14 fgh | 0.5 | 2.058 b | 0.07 | 0.401 fghi | 1.3 | 22.40 bc | 14.9 | 0.56 fghi | 0.2 | 1.45 k | 0.1 | 1.04 h | 0.2 |
| T6 + Sc + seq MLF | 3.95 abcd | 0.2 | 1.20 cde | 0.5 | 1.321 jk | 0.04 | 0.403 fgh | 1.3 | 21.65 cde | 14.4 | 0.57 fgh | 0.2 | 2.04 a | 0.2 | 1.13 a | 0.2 |
| Compounds | OTH Values (mg/L) | Aroma/Flavor Descriptors |
|---|---|---|
| Esters | ||
| Ethyl acetate | 12 [55] | Fruit, nail polish [41,56] |
| Ethyl butanoate (butyrate) | 0.4 [57] | Strawberry [57], apple [56], fruity [21] |
| Isoamyl acetate | 0.16 [57] | Banana, pear [16,41] |
| Ethyl lactate | 14 [58] | Butter, cream, fruit [56] |
| Ethyl-3-hydroxy butanoate | 1 [55] | Fruity, grape [55], strawberry [59] |
| Diethyl succinate | 1.2 [57] | Fruity, melon [57], berry [56] |
| Ethyl hexanoate (ethyl caproate) | 0.08 [57] | Apple [56], fruity, anise [53], strawberry [58] |
| Ethyl octanoate (ethyl caprylate) | 0.58 [57] | Fruit [56], pear, pineapple [41] |
| Ethyl decanoate (ethyl caprate) | 0.5 [57] | Floral [41,56], grape, soap [16,56] |
| Ethyl phenylacetate | 0.073 [60] | Honey-like [60] |
| 2-Phenylethyl acetate | 0.25 [52] | Flowery, fruity, rose [16,41] |
| Alcohols | ||
| Methanol | 500 [57] | Alcohol [57] |
| N-Propanol | 306 [57] | Alcohol, ripe fruit [57], pungent, harsh [16,56] |
| N-Butanol | 150 [57] | Fusel, spirituous [16,56] |
| Isobutanol | 40 [52] | Wine, solvent, fusel [16] |
| Pentanol | 64 [61] | Fusel, alcoholic, fermented, pungent, bready, yeasty [11] |
| Isoamyl alcohol | 60 [57] | Herbaceous [59], whiskey, malt, burnt [56] |
| 3-Ethoxy-1-propanol | 0.1 [55] | Fruity [57] |
| 3-Methyl-1-pentanol | 1 [55] | Green, pungent, solvent [55] |
| Hexanol | 8 [52] | Herbaceous [55], grass [16,53], resin [53] |
| 2-Phenylethanol | 14 [62] | Floral, rose [16,41], honey, spice, lilac [56] |
| Ketones | ||
| Acetoin | 150 [57] | Buttery, cream [57] |
| Acids | ||
| Acetic acid | 200 [52] | Vinegar [41,62] |
| Propionic acid | 0.42 [41] | Pungent, rancid [41,56], sweat [56] |
| N-Butyric acid | 2.2 [55] | Cheese [53], pungent [41] |
| Isobutyric acid | 30 [55] | Rancid, butter, cheese [56], pungent [41] |
| N-Valeric acid | 3 [63] | unpleasant [41] |
| Isovaleric acid | 1.5 [55] | Cheese [41,52], rancid, sweaty [41] |
| Hexanoic acid | 3 [55] | Sweat [41,56], sour, vinegar, cheese, rancid, fatty, pungent [41] |
| Octanoic acid | 10 [55] | Sweat, cheese [56], oily, fatty, rancid, soapy, sweet, faint fruity, butter [41] |
| Decanoic acid | 6 [57] | Rancid, fat [41,56], unpleasant, citrus, phenolic [41] |
| Descriptor | Treatments | ||
|---|---|---|---|
| Yeast | MLF Strategy | Yeast × MLF Strategy | |
| Berry | 0.0036 | 0.0053 | 0.1643 |
| Fruity | 0.1696 | 0.0857 | 0.4701 |
| Fresh vegetative | 0.0989 | 0.8366 | 0.9774 |
| Cooked vegetative | 0.6539 | 0.1068 | 0.9403 |
| Spicy | 0.1848 | 0.5088 | 0.2219 |
| Floral | 0.3241 | 0.6223 | 0.8284 |
| Acid balance | 0.2679 | 0.0447 | 0.5892 |
| Body | 0.4319 | 0.2718 | 0.1424 |
| Astringency | 0.1749 | 0.0271 | 0.2493 |
| Bitterness | 0.1547 | 0.3787 | 0.6995 |
| Overall quality | 0.2355 | 0.8938 | 0.2737 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du Plessis, H.; Du Toit, M.; Nieuwoudt, H.; Van der Rijst, M.; Kidd, M.; Jolly, N. Effect of Saccharomyces, Non-Saccharomyces Yeasts and Malolactic Fermentation Strategies on Fermentation Kinetics and Flavor of Shiraz Wines. Fermentation 2017, 3, 64. https://doi.org/10.3390/fermentation3040064
Du Plessis H, Du Toit M, Nieuwoudt H, Van der Rijst M, Kidd M, Jolly N. Effect of Saccharomyces, Non-Saccharomyces Yeasts and Malolactic Fermentation Strategies on Fermentation Kinetics and Flavor of Shiraz Wines. Fermentation. 2017; 3(4):64. https://doi.org/10.3390/fermentation3040064
Chicago/Turabian StyleDu Plessis, Heinrich, Maret Du Toit, Hélène Nieuwoudt, Marieta Van der Rijst, Martin Kidd, and Neil Jolly. 2017. "Effect of Saccharomyces, Non-Saccharomyces Yeasts and Malolactic Fermentation Strategies on Fermentation Kinetics and Flavor of Shiraz Wines" Fermentation 3, no. 4: 64. https://doi.org/10.3390/fermentation3040064






