A Review of Process-Design Challenges for Industrial Fermentation of Butanol from Crude Glycerol by Non-Biphasic Clostridium pasteurianum
Abstract
:1. Introduction
2. Characterization of Biodiesel-Derived Crude Glycerol
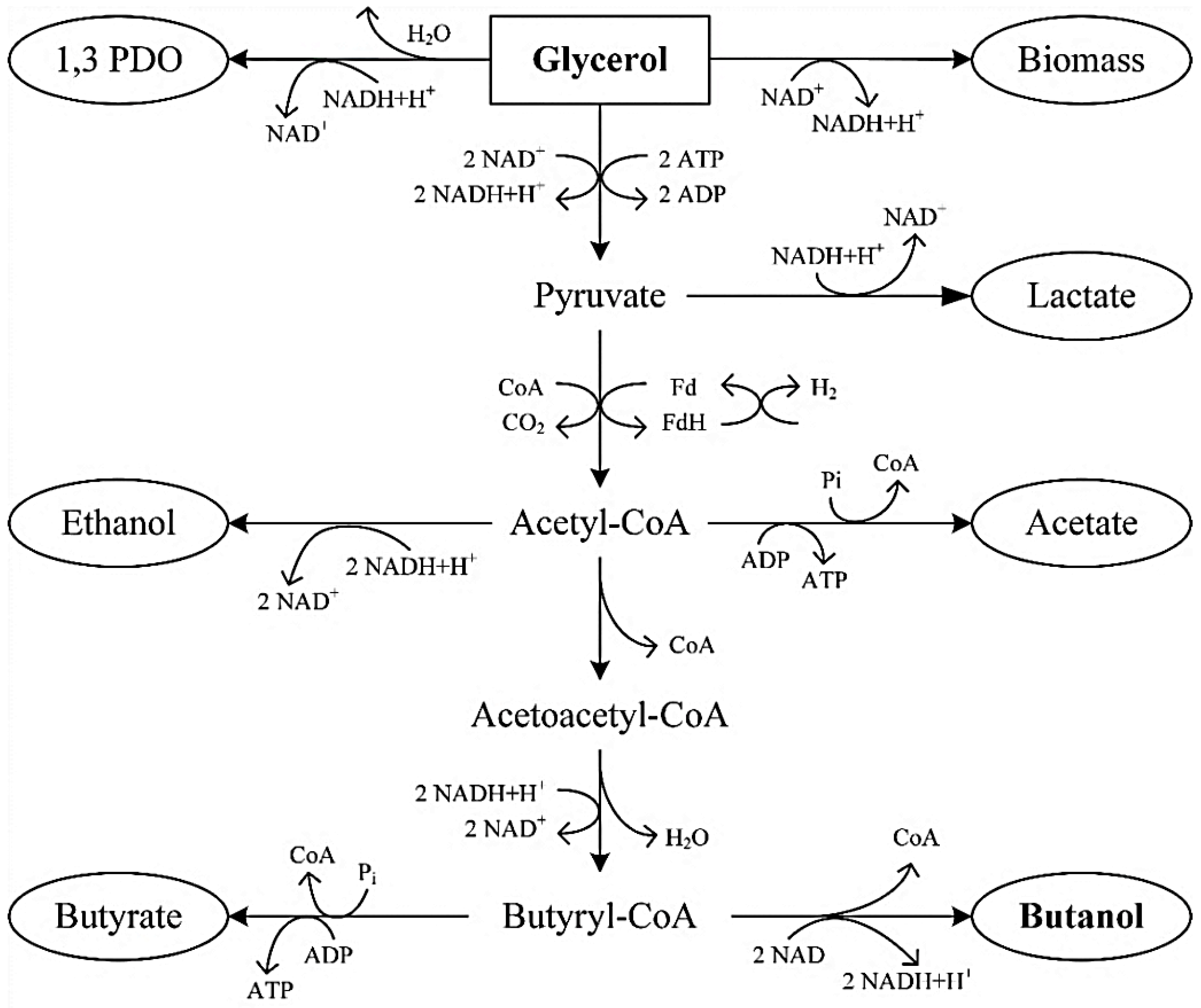
3. Microbial Metabolism of Glycerol
4. Biodiesel-Derived Crude Glycerol Pretreatment
5. Media Composition and Fermentation Condition
6. Metabolic Engineering and Mutagenesis
7. Advanced Fermentative Technologies for High Productivity
7.1. High Cell Density
7.2. Continuous Bioreactors for High Productivity
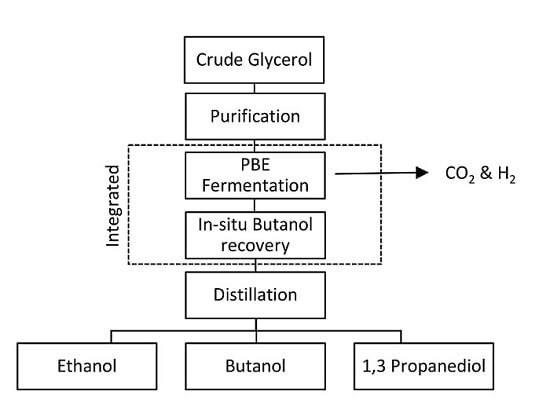
8. One Stage In-Situ Butanol Recovery Technologies
8.1. Integrated PBE Fermentation with In-Situ Butanol Recovery
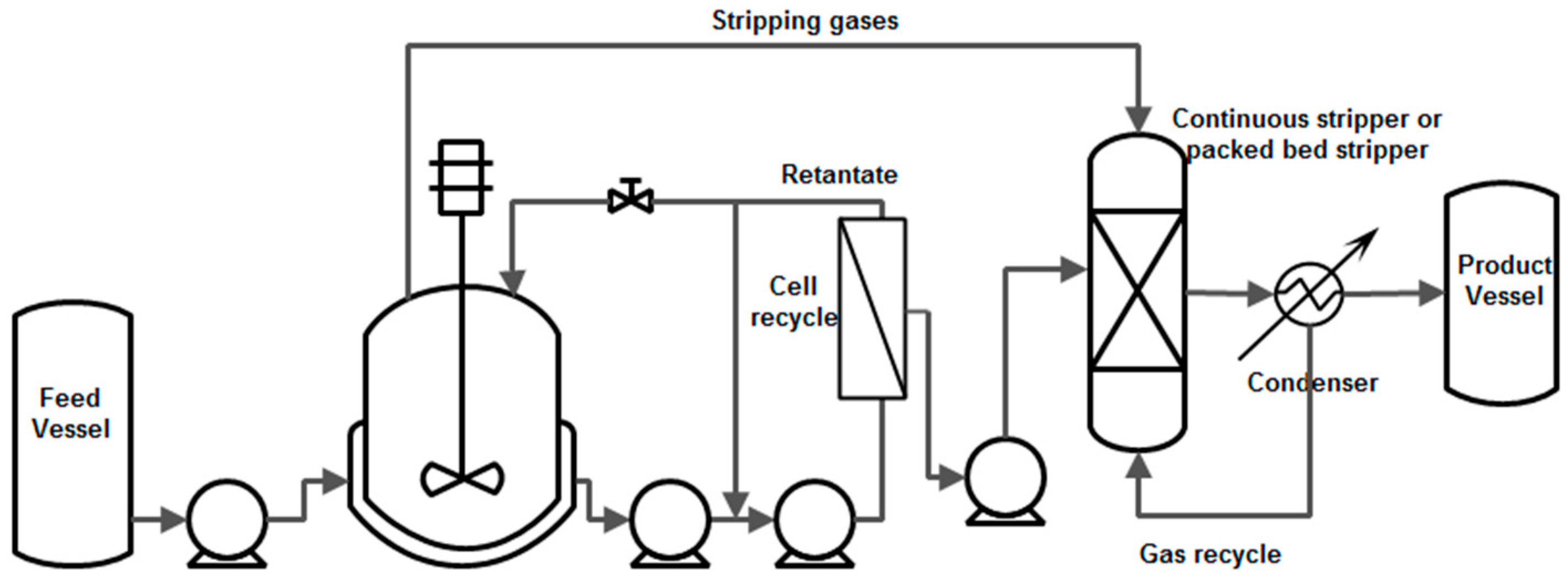
8.1.1. Gas Stripping
8.1.2. Liquid-Liquid Extraction
8.2. Integrated Continuous ABE Fermentation with In-Situ Butanol Recovery
8.2.1. Gas Stripping
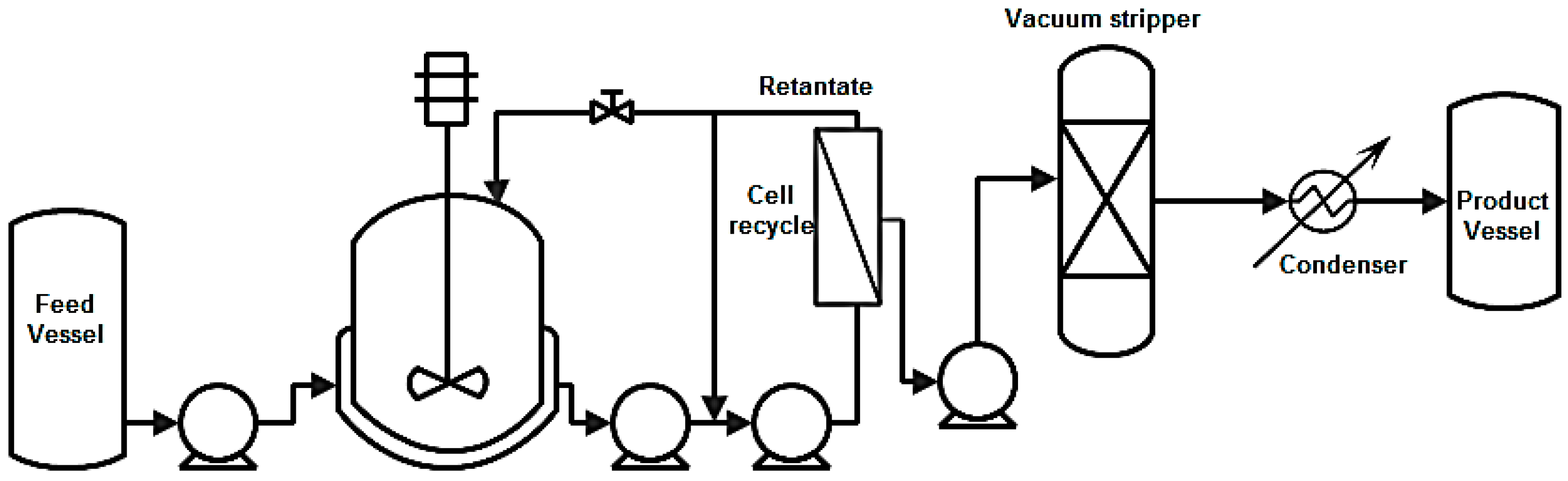
8.2.2. Vacuum Stripping
8.2.3. Pervaporation
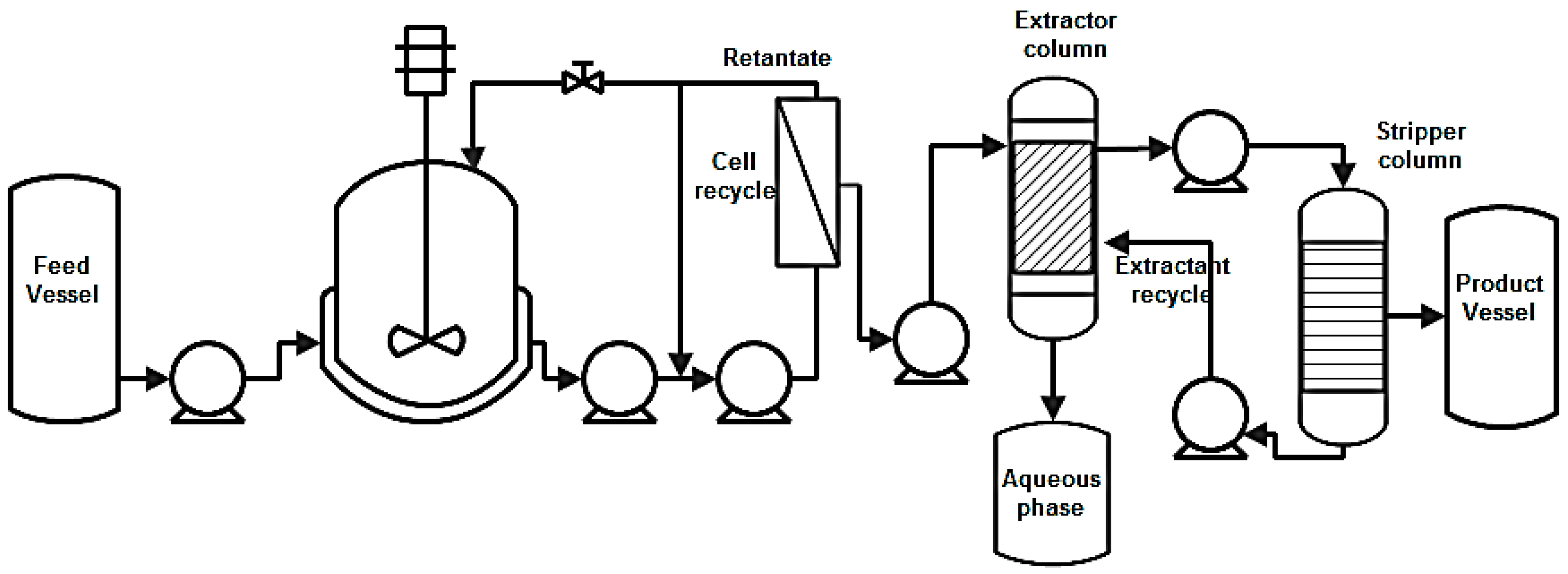
8.3. Liquid-Liquid Extraction (LLE)
8.4. Perstraction
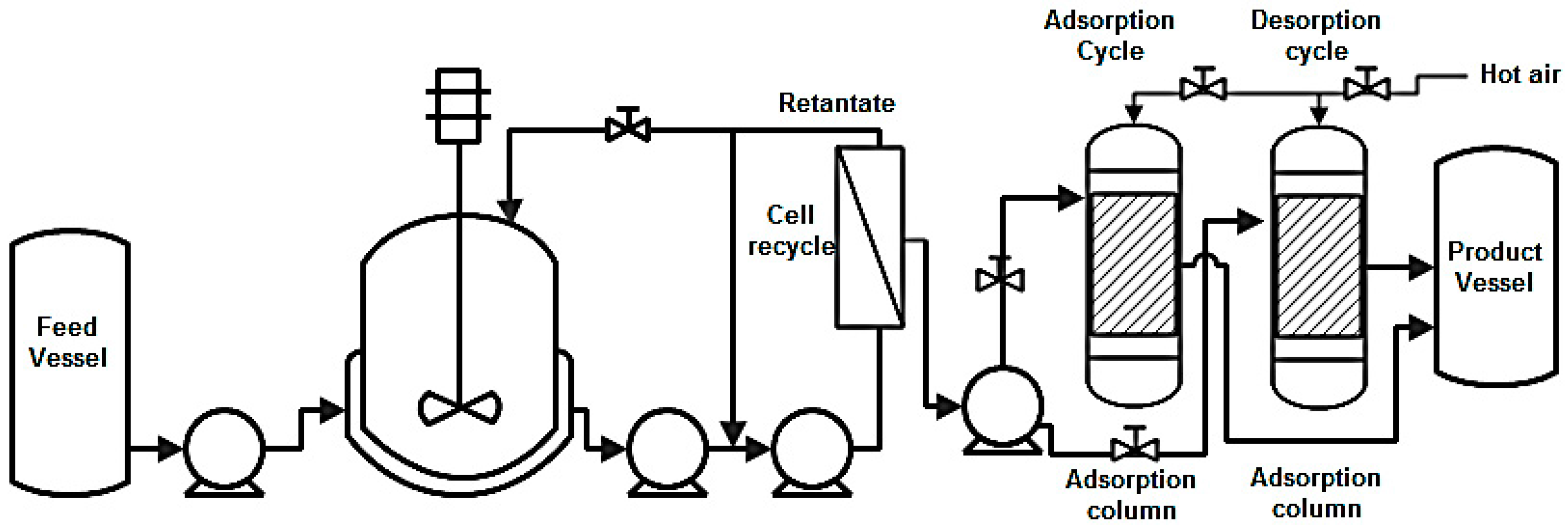
8.5. Adsorption
8.6. Transferring In-Situ Recovery Techniques from ABE Fermentation to PBE Fermentation
9. Hybrid In-Situ Butanol Recovery Processes
9.1. Two-Stage Gas Stripping
9.2. Gas Stripping-Pervaporation
9.3. Gas Stripping—Gas Permeation
9.4. Extraction-Gas Stripping
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malaviya, A.; Jang, Y.S.; Lee, S.Y. Continuous butanol production with reduced byproducts formation from glycerol by a hyper producing mutant of Clostridium pasteurianum. Appl. Microbiol. Biotechnol. 2012, 93, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-S.; Malaviya, A.; Cho, C.; Lee, J.; Lee, S.Y. Butanol production from renewable biomass by clostridia. Bioresour. Technol. 2012, 123, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Survase, S.A.; Ojamo, H.; Granström, T. Biobutanol: The outlook of an academic and industrialist. RSC Adv. 2013, 3. [Google Scholar] [CrossRef]
- Mariano, A.P.; Dias, M.O.S.; Junqueira, T.L.; Cunha, M.P.; Bonomi, A.; Maciel Filho, R. Butanol production in a first-generation Brazilian sugarcane biorefinery: Technical aspects and economics of greenfield projects. Bioresour. Technol. 2013, 135, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Sarchami, T.; Rehmann, L. Optimizing acid hydrolysis of jerusalem artichoke-derived inulin for fermentative butanol production. BioEnergy Res. 2014. [Google Scholar] [CrossRef]
- Lee, S.-M.; Cho, M.O.; Park, C.H.; Chung, Y.C.; Kim, J.H.; Sang, B.I.; Um, Y. Continuous butanol production using suspended and immobilized Clostridium beijerinckii NCIMB 8052 with supplementary butyrate. Energy Fuels 2008, 22, 3459–3464. [Google Scholar]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by Clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Cann, A.F.; Connor, M.R.; Shen, C.R.; Smith, K.M.; Brynildsen, M.P.; Chou, K.J.; Hanai, T.; Liao, J.C. Metabolic engineering of Escherichia coli for 1-butanol production. Metab. Eng. 2008, 10, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Sun, Z. Recent progress on industrial fermentative production of acetone-butanol-ethanol by Clostridium acetobutylicum in China. Appl. Microbiol. Biotechnol. 2009, 83, 415–423. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Päkkilä, J.; Ojamo, H.; Muurinen, E.; Keiski, R.L. Challenges in biobutanol production: How to improve the efficiency? Renew. Sustain. Energy Rev. 2011, 15, 964–980. [Google Scholar] [CrossRef]
- Green, E.M. Fermentative production of butanol-the industrial perspective. Curr. Opin. Biotechnol. 2011, 22, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Ezeji, T.C.; Ebener, J.; Dien, B.S.; Cotta, M.A.; Blaschek, H.P. Butanol production by Clostridium beijerinckii. Part I: Use of acid and enzyme hydrolyzed corn fiber. Bioresour. Technol. 2008, 99, 5915–5922. [Google Scholar] [CrossRef] [PubMed]
- Sabra, W.; Groeger, C.; Sharma, P.N.; Zeng, A.P. Improved n-butanol production by a non-acetone producing Clostridium pasteurianum DSMZ 525 in mixed substrate fermentation. Appl. Microbiol. Biotechnol. 2014, 98, 4267–4276. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Dabrock, B.; Bahl, H.; Gottschalk, G. Parameters affecting solvent production by Clostridium pasteurianum. Appl. Environ. Microbiol. 1992, 58, 1233–1239. [Google Scholar] [PubMed]
- Rehman, A.; Wijesekara, S.; Nomura, N.; Sato, S.; Matsumura, M. Pre-treatment and utilization of raw glycerol from sunflower oil biodiesel for growth and 1,3-propanediol production by Clostridium butyricum. J. Chem. Technol. Biotechnol. 2008, 82, 1072–1080. [Google Scholar]
- Quispe, C.A.; Coronado, C.J.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Johnson, D.; Taconi, K. The Glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007, 26, 404–409. [Google Scholar] [CrossRef]
- Khanna, S.; Goyal, A.; Moholkar, V.S. Production of n-butanol from biodiesel derived crude glycerol using Clostridium pasteurianum immobilized on Amberlite. Fuel 2013, 112, 557–561. [Google Scholar] [CrossRef]
- Gallardo, R.; Alves, M.; Rodrigues, L.R. Modulation of crude glycerol fermentation by Clostridium pasteurianum DSM 525 towards the production of butanol. Biomass Bioenergy 2014, 71, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Taconi, K.A.; Venkataramanan, K.P.; Johnson, D.T. Growth and solvent production by Clostridium pasteyrianum ATCC 6013 utilizing biodiesel-derived crude glycerol as the sole carbon source. Environ. Prog. Sustain. Energy 2009, 28, 100–110. [Google Scholar] [CrossRef]
- Venkataramanan, K.P.; Kurniawan, Y.; Boatman, J.J.; Haynes, C.H.; Taconi, K.A.; Martin, L.; Bothun, G.D.; Scholz, C. Homeoviscous response of Clostridium pasteurianum to butanol toxicity during glycerol fermentation. J. Biotechnol. 2014, 179, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Yoshida, T.; Noguchi, T.; Sonomoto, K. Recent advances and future prospects for increased butanol production by acetone-butanol-ethanol fermentation. Eng. Life Sci. 2013, 13, 432–445. [Google Scholar] [CrossRef]
- Branduardi, P.; de Ferra, F.; Longo, V.; Porro, D. Microbial n-butanol production from Clostridia to non-Clostridial hosts. Eng. Life Sci. 2014, 14, 16–26. [Google Scholar] [CrossRef]
- Jensen, T.Ø.; Kvist, T.; Mikkelsen, M.J.; Westermann, P. Production of 1,3-PDO and butanol by a mutant strain of Clostridium pasteurianum with increased tolerance towards crude glycerol. AMB Express 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Shukla, A.K.; Goyal, A.; Moholkar, V.S. Alcoholic biofuels production from biodiesel derived glycerol by Clostridium pasteurianum whole cells immobilized on silica. Waste Biomass Valoriz. 2014, 5, 789–798. [Google Scholar] [CrossRef]
- Zheng, J.; Tashiro, Y.; Yoshida, T.; Gao, M.; Wang, Q.; Sonomoto, K. Continuous butanol fermentation from xylose with high cell density by cell recycling system. Bioresour. Technol. 2013, 129, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Abdehagh, N.; Tezel, F.H.; Thibault, J. Adsorbent screening for biobutanol separation by adsorption: Kinetics, isotherms and competitive effect of other compounds. Adsorption 2013, 19, 1263–1272. [Google Scholar] [CrossRef]
- Wiehn, M.; Staggs, K.; Wang, Y.; Nielsen, D.R. In situ butanol recovery from Clostridium acetobutylicum fermentations by expanded bed adsorption. Biotechnol. Prog. 2014, 30, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Survase, S.A.; Singhal, R.S.; Granström, T. Continuous two stage acetone-butanol-ethanol fermentation with integrated solvent removal using Clostridium acetobutylicum B 5313. Bioresour. Technol. 2012, 106, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Mai, N.L.; Koo, Y.M. Butanol recovery from aqueous solution into ionic liquids by liquid-liquid extraction. Process Biochem. 2010, 45, 1899–1903. [Google Scholar] [CrossRef]
- Hu, S.; Luo, X.; Wan, C.; Li, Y. Characterization of crude glycerol from biodiesel plants. J. Agric. Food Chem. 2012, 60, 5915–5921. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.F.A.; Hernandez, A.A.; Mullan, B.P.B.; Moore, K.; Trezona-Murray, M.; King, R.H.; Pluske, J.R. A chemical analysis of samples of crude glycerol from the production of biodiesel in Australia, and the effects of feeding crude glycerol to growing-finishing pigs on performance, plasma metabolites and meat quality at slaughter. Anim. Prod. Sci. 2009, 49, 154–161. [Google Scholar] [CrossRef]
- Mu, Y.; Teng, H.; Zhang, D.J.; Wang, W.; Xiu, Z.L. Microbial production of 1,3-propanediol by Klebsiella pneumoniae using crude glycerol from biodiesel preparations. Biotechnol. Lett. 2006, 28, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- De Carvalho, P.L.O.; Moreira, I.; Martins, E.N.; Piano, L.M.; Toledo, J.B.; Costa Filho, C.D.L. Crude glycerine in diets for piglets. Rev. Bras. Zootec. 2012, 41, 1654–1661. [Google Scholar] [CrossRef]
- Bournay, L.; Casanave, D.; Delfort, B.; Hillion, G.; Chodorge, J.A. New heterogeneous process for biodiesel production: A way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal. Today 2005, 106, 190–192. [Google Scholar] [CrossRef]
- Daniel, R.; Stuertz, K.; Gottschalk, G. Biochemical and molecular characterization of the oxidative branch of glycerol utilization by Citrobacter freundii. J. Bacteriol. 1995, 177, 4392–4401. [Google Scholar] [PubMed]
- Seifert, C.; Bowien, S.; Gottschalk, G.; Daniel, R. Identification and expression of the genes and purification and characterization of the gene products involved in reactivation of coenzyme B12-dependent glycerol dehydratase of Citrobacter freundii. Eur. J. Biochem. 2001, 268, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Németh, A.; Kupcsulik, B.; Sevella, B. 1,3-Propanediol oxidoreductase production with Klebsiella pneumoniae DSM2026. World J. Microbiol. Biotechnol. 2003, 19, 659–663. [Google Scholar] [CrossRef]
- Biebl, H.; Zeng, A.P.; Menzel, K.; Deckwer, W.D. Fermentation of glycerol to 1,3-propanediol and 2,3-butanediol by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 1998, 50, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Malaoui, H.; Marczak, R. Separation and characterization of the 1,3-propanediol and glycerol dehydrogenase activities from Clostridium butyricum E5 wild-type and mutant D. J. Appl. Microbiol. 2001, 90, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Colin, T.; Bories, A.; Lavigne, C.; Moulin, G. Effects of acetate and butyrate during glycerol fermentation by Clostridium butyricum. Curr. Microbiol. 2001, 43, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Barbirato, F.; Chedaille, D.; Bories, A. Propionic acid fermentation from glycerol: Comparison with conventional substrates. Appl. Microbiol. Biotechnol. 1997, 47, 441–446. [Google Scholar] [CrossRef]
- Barbirato, F.; Bories, A. Relationship between the physiology of Enterobacter agglomerans CNCM 1210 grown anaerobically on glycerol and the culture conditions. Res. Microbiol. 1997, 148, 475–484. [Google Scholar] [CrossRef]
- Ito, T.; Nakashimada, Y.; Senba, K.; Matsui, T.; Nishio, N. Hydrogen and ethanol production from glycerol-containing wastes discharged after biodiesel manufacturing process. J. Biosci. Bioeng. 2005, 100, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Talarico, T.L.; Axelsson, L.T.; Novotny, J.; Fiuzat, M.; Dobrogosz, W.J. Utilization of glycerol as a hydrogen acceptor by Lactobacillus reuteri: Purification of 1,3-propanediol: NAD+ oxidoreductase. Appl. Environ. Microbiol. 1990, 56, 943–948. [Google Scholar] [PubMed]
- Johnson, E.E.; Rehmann, L. The role of 1,3-propanediol production in fermentation of glycerol by Clostridium pasteurianum. Bioresour. Technol. 2016, 209. [Google Scholar] [CrossRef] [PubMed]
- Biebl, H. Fermentation of glycerol by Clostridium pasteurianum—Batch and continuous culture studies. J. Ind. Microbiol. Biotechnol. 2001, 27, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, K.P.; Boatman, J.J.; Kurniawan, Y.; Taconi, K.A.; Bothun, G.D.; Scholz, C. Impact of impurities in biodiesel-derived crude glycerol on the fermentation by Clostridium pasteurianum ATCC 6013. Appl. Microbiol. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Manosak, R.; Limpattayanate, S.; Hunsom, M. Sequential-refining of crude glycerol derived from waste used-oil methyl ester plant via a combined process of chemical and adsorption. Fuel Process Technol. 2011, 92, 92–99. [Google Scholar] [CrossRef]
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J. Chem. Eng. 2010, 27, 944–949. [Google Scholar] [CrossRef]
- Hájek, M.; Skopal, F. Purification of the glycerol phase after transesterification of vegetable oils. In Proceedings of the 44th International Petroleum Conference, Bratislava, Slovak Republic, 21–22 September 2009; pp. 1–6.
- Sarchami, T.; Johnson, E.; Rehmann, L. Optimization of fermentation condition favoring butanol production from glycerol by Clostridium pasteurianum DSM 525. Bioresour. Technol. 2016, 208, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, K.P. A Study of Anaerobic Fermentation of Biodiesel Derived Crude Glycerol into Butanol Using Clostridium pasteurianum ATCC 6013; The University of Alabama in Huntsville: Huntsville, AL, USA, 2012; p. 218. [Google Scholar]
- Moon, C.; Hwan, C.; Sang, B.; Um, Y. Optimization of medium compositions favoring butanol and 1,3-propanediol production from glycerol by Clostridium pasteurianum. Bioresour. Technol. 2011, 102, 10561–10568. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Goyal, A.; Moholkar, V.S. Effect of fermentation parameters on bio-alcohols production from glycerol using immobilized Clostridium pasteurianum: An optimization study. Prep. Biochem. Biotechnol. 2013, 43, 828–847. [Google Scholar] [CrossRef] [PubMed]
- Science, R.L.; Illumina, M.; Diego, S.; Gmbh, T. Complete genome sequence of the nitrogen-fixing and solvent-producing Clostridium pasteurianum DSM 525. Genome Announc. 2015, 3, 1997–1998. [Google Scholar]
- Rotta, C.; Poehlein, A.; Schwarz, K.; McClure, P.; Daniel, R.; Minton, N.P. Closed Genome Sequence of Clostridium pasteurianum ATCC 6013. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-N.; Chen, G.; Yao, S.-J. Microbial production of 1,3-propanediol from glycerol by encapsulated Klebsiella pneumoniae. Biochem. Eng. J. 2006, 32, 93–99. [Google Scholar] [CrossRef]
- Jang, Y.-S.; Malaviya, A.; Lee, S.Y. Acetone-butanol-ethanol production with high productivity using Clostridium acetobutylicum BKM19. Biotechnol. Bioeng. 2013, 110, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Gungormusler, M.; Gonen, C.; Azbar, N. Continuous production of 1,3-propanediol using raw glycerol with immobilized Clostridium beijerinckii NRRL B-593 in comparison to suspended culture. Bioprocess Biosyst. Eng. 2011, 34, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Schlieker, M.; Vorlop, K.-D. A novel immobilization method for entrapment: LentiKats®. In Immobilization of Enzymes and Cells; Guisan, J., Ed.; Humana Press: New York, NY, USA, 2006; pp. 333–343. [Google Scholar]
- Survase, S.A.; van Heiningen, A.; Granström, T. Continuous bio-catalytic conversion of sugar mixture to acetone-butanol-ethanol by immobilized Clostridium acetobutylicum DSM 792. Appl. Microbiol. Biotechnol. 2012, 93, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Gallazzi, A.; Branska, B.; Marinelli, F.; Patakova, P. Continuous production of n-butanol by Clostridium pasteurianum DSM 525 using suspended and surface-immobilized cells. J. Biotechnol. 2015, 216, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Westman, J.O.; Ylitervo, P.; Franzén, C.J.; Taherzadeh, M.J. Effects of encapsulation of microorganisms on product formation during microbial fermentations. Appl. Microbiol. Biotechnol. 2012, 96, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Heng, P.W.S.; Chan, L.W. Microencapsulation of Clostridium acetobutylicum ATCC 824 spores in gellan gum microspheres for the production of biobutanol. J. Microencapsul. 2015, 32, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.N.; Kim, N.-J.; Kang, J.; Jeong, C.M.; Fei, Q.; Kim, B.J.; Kwon, S.; Lee, S.Y.; Kim, J. Multi-stage high cell continuous fermentation for high productivity and titer. Bioprocess Biosyst. Eng. 2011, 34, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Kaeding, T.; DaLuz, J.; Kube, J.; Zeng, A.-P. Integrated study of fermentation and downstream processing in a miniplant significantly improved the microbial 1,3-propanediol production from raw glycerol. Bioprocess Biosyst. Eng. 2015, 38, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gayen, K. Biobutanol: The future biofuel. Biomass Convers. 2012. [Google Scholar] [CrossRef]
- Kadic, E.; Heindel., J.T. To Bioreactor Hydrodynamics and Gas-Liquid Mass Transfer an Introduction to Bioreactor Hydrodynamics and Gas-Liquid Mass; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Chang, Z.; Cai, D.; Wang, Y.; Chen, C.; Fu, C.; Wang, G.; Qin, P.; Wang, Z.; Tan, T. Effective multiple stages continuous acetone-butanol-ethanol fermentation by immobilized bioreactors: Making full use of fresh corn stalk. Bioresour. Technol. 2016, 205, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, J.; Patakova, P.; Paulova, L.; Pokorny, T.; Rychtera, M.; Melzoch, K. Butanol production by Clostridium pasteurianum NRRL B-598 in continuous culture compared to batch and fed-batch systems. Fuel Process Technol. 2016, 144, 139–144. [Google Scholar] [CrossRef]
- Napoli, F.; Olivieri, G.; Russo, M.E.; Marzocchella, A.; Salatino, P. Butanol production by Clostridium acetobutylicum in a continuous packed bed reactor. J. Ind. Microbiol. Biotechnol. 2010, 37, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y.; Yang, F.; Zhang, C. Continuous acetone-butanol-ethanol production by corn stalk immobilized cells. J. Ind. Microbiol. Biotechnol. 2009, 36, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ai, H.; Zhang, S.; Li, S.; Liang, Z.; Wu, Z.Q.; Yang, S.T.; Wang, J.F. Enhanced butanol production by coculture of Clostridium beijerinckii and Clostridium tyrobutyricum. Bioresour. Technol. 2013, 143, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-L. Acetone-Butanol-Ethanol Fermentation by Engineered Clostridium beijerinckii and Clostridium tyrobutyricum. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2010. [Google Scholar]
- Huang, W.; Ramey, D.; Yang, S. Continuous production of butanol by Clostridium acetobutylicum immobilized in a fibrous bed Bioreactor. Appl. Biochem. Biotechnol. 2004, 113, 887–898. [Google Scholar] [CrossRef]
- Qureshi, N.; Hughes, S.; Maddox, I.S.; Cotta, M.A. Energy-efficient recovery of butanol from model solutions and fermentation broth by adsorption. Bioprocess Biosyst. Eng. 2005, 27, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Butanol fermentation research: Upstream and downstream manipulations. Chem. Rec. 2004, 4, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Abdehagh, N.; Tezel, F.H.; Thibault, J. Separation techniques in butanol production: Challenges and developments. Biomass Bioenergy 2014, 60, 222–246. [Google Scholar] [CrossRef]
- Abdehagh, N.; Gurnani, P.; Tezel, F.H.; Thibault, J. Adsorptive separation and recovery of biobutanol from ABE model solutions. Adsorption 2015, 21, 185–194. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, X.Q.; Liu, C.G.; Chen, L.J.; Bai, F.W. Prospective and development of butanol as an advanced biofuel. Biotechnol. Adv. 2013, 31, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Vane, L.M. Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuels Bioprod. Biorefin. 2008, 2, 553–558. [Google Scholar] [CrossRef]
- Errico, M.; Sanchez-Ramirez, E.; Quiroz-Ramìrez, J.J.; Segovia-Hernandez, J.G.; Rong, B.G. Synthesis and design of new hybrid configurations for biobutanol purification. Comput. Chem. Eng. 2016, 84, 482–492. [Google Scholar] [CrossRef]
- Kraemer, K.; Harwardt, A.; Bronneberg, R.; Marquardt, W. Separation of butanol from acetone-butanol-ethanol fermentation by a hybrid extraction-distillation process. Comput. Chem. Eng. 2011, 35, 949–963. [Google Scholar] [CrossRef]
- Mariano, A.P.; Qureshi, N.; Filho, R.M.; Ezeji, T.C. Bioproduction of butanol in bioreactors: New insights from simultaneous in situ butanol recovery to eliminate product toxicity. Biotechnol. Bioeng. 2011, 108, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Mariano, A.P.; Qureshi, N.; Maciel Filho, R.; Ezeji, T.C. Assessment of in situ butanol recovery by vacuum during acetone butanol ethanol (ABE) fermentation. J. Chem. Technol. Biotechnol. 2012, 87, 334–340. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, J.; Lu, C.; Yang, S.T.; Bai, F.; Tang, I. High-titer n-butanol production by clostridium acetobutylicum JB200 in fed-batch fermentation with intermittent gas stripping. Biotechnol. Bioeng. 2012, 109, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Microbial production of a biofuel (acetone-butanol-ethanol) in a continuous bioreactor: Impact of bleed and simultaneous product removal. Bioprocess Biosyst. Eng. 2013, 36, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhao, J.; Liu, F.; Lu, C.; Yang, S.T.; Bai, F.W. Two-stage in situ gas stripping for enhanced butanol fermentation and energy-saving product recovery. Bioresour. Technol. 2013, 135, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhao, J.; Yang, S.-T.; Wei, D. Fed-batch fermentation for n-butanol production from cassava bagasse hydrolysate in a fibrous bed bioreactor with continuous gas stripping. Bioresour. Technol. 2012, 104, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.Ø.; Kvist, T.; Mikkelsen, M.J.; Westermann, P. Fermentation of crude glycerol from biodiesel production by Clostridium pasteurianum. J. Ind. Microbiol. Biotechnol. 2012, 39, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, M.; Hua, D.; Li, Y.; Xu, H.; Liang, X.; Zhao, Y.; Jin, F.; Chen, L.; Meng, G.; et al. Butanol production of Clostridium pasteurianum SE-5 from transesterification reaction solution using fermentation and extraction coupling system. In Proceedings of the 2013 International IEEE Conference on Materials for Renewable Energy and Environment (ICMREE), Chengdu, China, 19–21 August 2013; Volume 1, pp. 174–178.
- Qureshi, N.; Maddox, I.S. Integration of continuous production and recovery of solvents from whey permeate: Use of immobilized cells of Clostridium acetobutylicum in a flutilized bed reactor coupled with gas stripping. Bioprocess Eng. 1990, 6, 63–69. [Google Scholar] [CrossRef]
- Groot, W.J.; van der Lans, R.G.J.M.; Luyben, K.C.A. Batch and continuous butanol fermentations with free cells: Integration with product recovery by gas-stripping. Appl. Microbiol. Biotechnol. 1989, 32, 305–308. [Google Scholar] [CrossRef]
- Ennis, B.M.; Marshall, C.T.; Maddox, I.S.; Paterson, A.H.J. Continuous product recovery by in-situ gas stripping/condensation during solvent production from whey permeate using Clostridium acetobutylicum. Biotechnol. Lett. 1986, 8, 725–730. [Google Scholar] [CrossRef]
- Yao, P.; Xiao, Z.; Chen, C.; Li, W.; Deng, Q. Cell growth behaviors of Clostridium acetobutylicum in a pervaporation membrane bioreactor for butanol fermentation. Biotechnol. Appl. Biochem. 2016, 63, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, X.; Qi, B.; Luo, J.; Zhuang, X.; Su, Y.; Wan, Y. Continuous acetone-butanol-ethanol (ABE) fermentation with in situ solvent recovery by silicalite-1 filled PDMS/PAN composite membrane. Energy Fuels 2014, 28, 555–562. [Google Scholar] [CrossRef]
- Van Hecke, W.; Hofmann, T.; de Wever, H. Pervaporative recovery of ABE during continuous cultivation: Enhancement of performance. Bioresour. Technol. 2013, 129, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Takehara, S.; Kataoka, H. Continuous butanol/isopropanol fermentation in down-flow column reactor coupled with pervaporation using supported liquid membrane. Biotechnol. Bioeng. 1992, 39, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Survase, S.A.; Ojamo, H.; Granström, T. The two stage immobilized column reactor with an integrated solvent recovery module for enhanced ABE production. Bioresour. Technol. 2013, 140, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Maddox, I.S. Continuous production of acetone-butanol-ethanol using immobilized cells of Clostridium acetobutylicum and integration with product removal by liquid-liquid extraction. J. Ferment. Bioeng. 1995, 80, 185–189. [Google Scholar] [CrossRef]
- Qureshi, N.; Maddox, I.S.; Friedlt, A. Application of continuous substrate feeding to the ABE fermentation: Relief of product inhibition using extraction, perstraction, stripping, and pervaporation. Biotechnol. Prog. 1992, 8, 382–390. [Google Scholar] [CrossRef]
- Yang, X.; Tsaot, G.T. Enhanced acetone-butanol fermentation using repeated fed-batch operation coupled with cell recycle by membrane and simultaneous removal of inhibitory products by adsorption. Biotechnol. Bioeng. 1995, 47, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Izák, P.; Schwarz, K.; Ruth, W.; Bahl, H.; Kragl, U. Increased productivity of Clostridium acetobutylicum fermentation of acetone, butanol, and ethanol by pervaporation through supported ionic liquid membrane. Appl. Microbiol. Biotechnol. 2008, 78, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, W.; Vandezande, P.; Dubreuil, M.; Uyttebroek, M.; Beckers, H.; de Wever, H. Biobutanol production from C5/C6 carbohydrates integrated with pervaporation: Experimental results and conceptual plant design. J. Ind. Microbiol. Biotechnol. 2016, 43, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, A.; Kujawski, J.; Bryjak, M.; Kujawski, W. ABE fermentation products recovery methods—A review. Renew. Sustain. Energy Rev. 2015, 48, 648–661. [Google Scholar] [CrossRef]
- Xue, C.; Du, G.Q.; Sun, J.X.; Chen, L.J.; Gao, S.S.; Yu, M.L.; Yang, S.T.; Bai, F.W. Characterization of gas stripping and its integration with acetone-butanol-ethanol fermentation for high-efficient butanol production and recovery. Biochem. Eng. J. 2014, 83, 55–61. [Google Scholar] [CrossRef]
- Cai, D.; Chen, H.; Chen, C.; Hu, S.; Wang, Y.; Chang, Z.; Miao, Q.; Qin, P.; Wang, Z.; Wang, J.; Tan, T. Gas stripping-pervaporation hybrid process for energy-saving product recovery from acetone-butanol-ethanol (ABE) fermentation broth. Chem. Eng. J. 2016, 287, 1–10. [Google Scholar] [CrossRef]
- Xue, C.; Liu, F.; Xu, M.; Zhao, J.; Chen, L.; Ren, J.; Bai, F.; Yang, S.T. A novel in situ gas stripping-pervaporation process integrated with acetone-butanol-ethanol fermentation for hyper n-butanol production. Biotechnol. Bioeng. 2016, 113, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Vane, L.M.; Alvarez, F.R. Hybrid vapor stripping-vapor permeation process for recovery and dehydration of 1-butanol and acetone/butanol/ethanol from dilute aqueous solutions. Part 1. Process Simulations. J. Chem. Technol. Biotechnol. 2013, 88, 1436–1447. [Google Scholar] [CrossRef]
- Lu, K.M.; Li, S.Y. An integrated in situ extraction-gas stripping process for Acetone-Butanol-Ethanol (ABE) fermentation. J. Taiwan Inst. Chem. Eng. 2014, 45, 2106–2110. [Google Scholar] [CrossRef]
- Anand, P.; Saxena, R.K. A comparative study of solvent-assisted pretreatment of biodiesel derived crude glycerol on growth and 1,3-propanediol production from Citrobacter freundii. New Biotechnol. 2012, 29, 199–205. [Google Scholar] [CrossRef] [PubMed]



| Compound | Crude Glycerol wt % | Concentrated Glycerol Phase | Organic Phase | ||
|---|---|---|---|---|---|
| Molar Ratio of KOH: Esters | Molar Ratio of KOH: Esters | ||||
| 1:1 | 1.2:1 | 1:1 | 1.2:1 | ||
| Glycerol | 55.5 ± 3.9 | 84.7 | 85.1 | Nt | Nt |
| Soap | 18.6 ± 2.8 | Nt | Nt | Nt | Nt |
| Salts | 1.7 ± 0.28 | 2.39 | 2.87 | Nt | Nt |
| Water | 13.3 ± 1.37 | 12.1 | 11.1 | Nt | Nt |
| Methanol | 2.9 ± 1.48 | 0.46 | 0.37 | Nt | Nt |
| Esters | 8.1 ± 1.65 | Nt | Nt | 4.8 | 0 |
| FFAs | Nt | Nt | Nt | 95 | 99.5 |
| Others | Nt | 0.35 | 0.56 | 0.2 | 0.5 |
| Strains | Crude Glycerol Pretreatment/(Fermentation Time) | Culture Condition | Max. Butanol Yield a g·g−1 (mol·mol−1) | Overall Butanol Productivity g·L−1·h−1 | Reference | ||
|---|---|---|---|---|---|---|---|
| Pure Glycerol | Crude Glycerol | Pure Glycerol | Crude Glycerol | ||||
| C. pasteurianum (wild type; DSM 525) | Filtration (35 h) | Batch, Free cells, Vol ~ 5 L | 0.28 (0.35) | 0.27 (0.34) | 0.41 | 0.35 | [55] |
| C. pasteurianum (wild type; ATCC 116) | None (120 h) | Batch, Free cells, Vol < 1 L | 0.18 (0.22) | 0.13 (0.16) | <0.10 | <0.10 | [27] |
| C. pasteurianum (wild type; ATCC 116) | None (120 h) | Batch, Immobilized cells, Vol < 1 L | 0.36 (0.45) | 0.23 (0.29) | <0.10 | <0.10 | [27] |
| C. pasteurianum (wild type; ATCC 6013) | None (14–24 days) | Batch, Free cells, Vol < 1 L | 0.26 (0.32) | 0.21 (0.26) | <0.1 | <0.02 | [56] |
| C. pasteurianum (wild type; ATCC 6013) | Acid precipitation (4 days) | Batch, Free cells, Vol < 1 L | 0.26 (0.32) | 0.28 (0.35) | <0.1 | <0.1 | [56] |
| C. pasteurianum (wild type; ATCC 6103) | None (25 days) | Batch, Free cells, Vol < 1 L | 0.31 (0.39) | 0.30 (0.37) | 0.04 | <0.02 b,c | [22] |
| Strains | Culture Condition | Initial Glycerol Titer g·L−1 | Inoculum Age h | Initial Cell Density g·L−1DCW | pH | Temperature °C | Agitation Rate rpm | Max. Butanol Titer g·L−1 | Max. Butanol Yield a g·g−1 (mol·mol−1) | Overall Butanol Productivity g·L−1·h−1 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. pasteurianum (wild type; DSM 525) | Batch, Free cells, Vol < 1 L | Pure Non-Sig. 50 | Sig. 16 | Sig. 0.4 | Sig 7.0 | Sig. 30 | Not-studied | 12.3 | 0.28 (0.35) | 0.41 | [55] |
| C. pasteurianum (wild type; ATCC 6013) | Batch, Immobolized cells, Vol < 1 L | Pure Non-Sig. 25 | Not-studied | Not-stdied | Sig 7.0 | Non-Sig. 30 | Non-Sig. 200 | 7.7 | 0.21 (0.26) | 0.04 | [58] |
| C. pasteurianum (wild type; ATCC 6013) | Batch, Immobolized cells, Vol < 1 L | Crude Sig. 25 | Not-studied | Not-stdied | Sig 7.0 | Non-Sig. 30 | Non-Sig. 200 | 6.8 | 0.17 (0.21) | 0.035 | [58] |
| C. pasteurianum (wild type; ATCC6103) | Batch, Free cells, Iron limitation, Vol < 1 L | Pure Not-studied 86 a | Sig. 18 | Sig 0.42 | Sig 5.5–6.0 | Not-studied 37 | Not-studied | 10.0 | 0.25 (0.31) | 0.27 | [1] |
| Strains | Process Parameters | Glycerol Consumed g·L−1 | Max. Bioreactor Butanol Titer g·L−1 | Max. Butanol Yield a g·g−1 (mol·mol−1) | Overall Butanol Productivity g L−1 h−1 | Reference |
|---|---|---|---|---|---|---|
| C. pasteurianum (mutant MNO6; DSMZ 525) | Fed Batch, Free cells, in-situ butanol removal, Vol < 1 L | Crude 100–122 | 12.6 | 0.20 (0.25) | 1.80 c,d | [26] |
| C. pasteurianum (mutant MBEL_GLY2; ATCC 6103) | Batch, Free cells, Vol < 1 L | Pure 86.0 | 13.7 | 0.30 (0.37) | 0.31 | [1] |
| C. pasteurianum (mutant MBEL_GLY2; ATCC 6103) | Batch, Free cells, Vol < 1 L, Optimized medium | Pure 79.3 | 17.3 | 0.30 (0.37) | 0.33 | [1] |
| C. pasteurianum (mutant MBEL_GLY2; ATCC 6103) High initial cell concentration | Batch, Free cells, Vol < 1 L, Optimized medium | Pure 82.0 | 17.8 | 0.30 (0.37) | 0.43 | [1] |
| C. pasteurianum (mutant MBEL_GLY2; ATCC 6103) High Cells/Cell Recycle | Continuous, Free cells, (D = 0.9 h−1) Vol < 1 L, Optimized medium | Pure 35 b | 8.6 | 0.25 (0.31) b | 7.8 | [1] |
| C. pasteurianum (spontaneous asporogenous mutant; DSM 525) | Continuous, Free cells, D = 0.05 h−1 V ~ 1 L | Pure 30.85 | 7.45 | 0.24 (0.30) | 0.372 | [16] |
| Strains | Culture Condition | Carbon Source | Max. Butanol Yield a g·g−1 (mol·mol−1) | Butanol Productivity g·L−1·h−1 | Reference | ||
|---|---|---|---|---|---|---|---|
| Cell Immobilization | Free Cells | Cell Immobilization | Free cells | Cell Immobilization | |||
| C. pasteurianum (wild type; DSM 525) | Continuous (D = 0.44 h−1 for immobilized cells and D = 0.01 h−1 for free cells) Vol ~ 400 mL | Pure glycerol | 0.4 (0.50) | 0.33 (0.41) | 0.1 | 4.2 | [66] |
| C. pasteurianum (wild type; MTCC 116) | Batch Vol < 1 L | Pure glycerol | 0.18 (0.22) | 0.36 (0.45) | <0.10 | <0.10 | [27] |
| C. pasteurianum (wild type; MTCC 116) | Batch Vol < 1 L | Crude glycerol | 0.13 (0.16) | 0.23 (0.29) | <0.10 | <0.10 | [27] |
| C. pasteurianum (wild type; MTCC 116) | Batch Vol < 1 L | Crude glycerol | Nt | 0.35 (0.43) | Nt | <0.10 | [20] |
| C. pasteurianum (wild type; MTCC 6013) | Batch Vol < 1 L | Pure glycerol | Nt | 0.21 (0.26) | Nt | 0.04 | [58] |
| C. pasteurianum (wild type; MTCC 6013) | Batch Vol < 1 L | Crude glycerol | Nt | 0.17 (0.21) | Nt | 0.035 | [58] |
| Cell Recycling | Free cells | Cell Recycling | Free cells | Cell Recycling | |||
| C. pasteurianum (mutant MBEL_GLY2; ATCC 6103) | Continuous (D = 0.9 h−1), Vol < 1 L Optimized medium | Pure glycerol | 0.3 (0.37) b | 0.25 (0.31) | 0.43 b | 7.8 | [1] |
| Bioreactor Type | Fermentation Mode | Cell Configuration/Support | Bacteria | Dilution Rate h−1 | Hours of Operation h | Substrate | Max Prod (g·L−1·h−1) | Reference |
|---|---|---|---|---|---|---|---|---|
| CSTR with cell recycle | ||||||||
| 3-stage CSTR (600 mL) | Continuous single pass | Immobilized on corn stover | C. acetobutylicum ABE 1201 | 0.04 overall | ~400 | Corn stover juice | 0.45 | [73] |
| Single stage CSTR (400 mL) | Continuous single pass | Cell recycle with ultrafiltration | C. saccharoper-butylacetonicum N1-4 ATCC 13564 (DCW = 18.0 g/L) | 0.78 | ~100 | xylose | 3.32 | [28] |
| Single stage CSTR (400 mL) | Continuous single pass | Cell recycle with ultrafiltration | C. pasteurianum ATCC 6013 | 0.9 | ~50 | glycerol | 7.8 | [1] |
| Packed Bed Bioreactor | ||||||||
| Single stage PBB (200 mL) | Continuous single pass | Immobilized on corn cob residue | C.pasteurianum NRRL B-598 | 0.12 | ~700 | glucose | 0.48 | [74] |
| Single stage PBB (180 mL) | Continuous single pass | Immobilized on corn stover pieces (1 cm3) | C. pasteurianum DSM 525 | 0.44 | ~300 | glycerol | 4.2 | [66] |
| Single stage PBB (250 mL) | Continuous single pass | Tygon ring carriers (ID = 3.2 mm) | C. acetobutylicum DSM 792 (DCW = 74 g/L) | 0.97 | ~750 | lactose | 4.4 | [75] |
| Single stage PBB (100 mL) | Continuous single pass | Immobilized on corn stover (5–8 mm) | C.beijerinckii ATCC 55025 on corn stock | 1.00 | ~480 | glucose | 5.06 | [76] |
| Fibrous Bed Bioreactor | ||||||||
| Two-stage FBB (2 L) | Continuous single pass | Immobilized on spiral wound fibrous material | Co-culture C. tyrobutyricum ATCC 25755 C. beijerinckii ATCC 55025 | 0.144 | ~100 | cassava starch | 0.96 | [77] |
| Single stage FBB (150 mL) | Continuous single pass | Immobilized on spiral wound fibrous cotton sheets | C. beijerinckii ATCC 55025 DCW = 100 g/L, 70% viable | 1.88 | ~350 | glucose/butyric acid | 17.29 | [78] |
| Single stage FBB (200 mL) | Continuous single pass | Immobilized on spiral wound fibrous sheets | C. acetobutylicum ATCC 55025 | 0.90 | ~1100 | glucose/butyric acid | 4.6 | [79] |
| Advantages | Disadvantages | |
|---|---|---|
|
|
|
|
|
|
| Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Gas stripping |
|
|
|
| Vacuum stripping |
|
|
|
| Pervaporation |
|
|
|
| Liquid-liquid extraction |
|
|
|
| Perstraction |
|
|
|
| Adsorption |
|
|
|
| Bacteria | Fermentation Mode | Substrate | Max Butanol Yield g·g−1 | Overall Butanol Productivity g·L−1·h−1 | Hours of Operation h | Comment | Reference |
|---|---|---|---|---|---|---|---|
| Gas Stripping | |||||||
| C. pasteurianum (mutant MNO6; DSMZ 525) | Fed Batch, Single-stage, Free cells, Vol < 1 L | Crude glycerol | 0.20 | 1.8 (1.2) | ~96–120 | H2 and CO2, Stripping temperature 37 °C, Condensation temperature 0 °C | [26] |
| C. pasteurianum (wild type; DSMZ 525) | Fed Batch, Single-stage, Free cells, Vol < 1 L | Crude glycerol | 0.225 | 1.3 (1.2) | ~96–120 | H2 and CO2, Stripping temperature 37 °C, Condensation temperature 0 °C | [94] |
| Liquid-liquid extraction | |||||||
| C. pasteurianum SE-5 | Batch, Single-stage, Free cells, Vol = 1 L | Crude glycerol | 0.30 (0.29) | 0.34 (0.27) | ~72 | Biodiesel was used as extractant | [95] |
| Bacteria | Fermentation Mode | Substrate | ABE Yield g·g−1 | ABE Productivity g·L−1·h−1 | Hours of Operation h | Comment | Reference |
|---|---|---|---|---|---|---|---|
| Gas stripping | |||||||
| C. beijerinckii BA101 | Continuous, Single-stage, Free cells | glucose | 0.41 (0.39) | 0.92 (0.29) | ~504 | H2 & CO2, Stripping temperature 35 °C, Condensation temperature 1 °C | [91] |
| C. acetobutylicum P262 | Continuous, Single-stage, Immobilized cells in a fluidized bed reactor | Whey permeate. | 0.4 (0.33) | 5.1 (1.66) | ~380 | N2, Stripping temperature 65–67 °C, Condensation temperature 3–4 °C | [96] |
| Clostridium sp. DSM 2152 | Continuous, Single-stage, Free cells | Glucose | 0.34 (0.37) | 0.18 (0.17) | ~300 | N2, 10 L·L−1 min, Stripping temperature 30 °C, Condensation temperature −5 to −40 °C | [97] |
| C.acetobutylicum P262 | Continuous, Single-stage, Free cells | Whey permeate | 0.35 (0.32) | 0.62 (0.15) | ~52 | N2, 2.0 L·min−1, Stripping temperature 34 °C, Condensation temperature 4 °C | [98] |
| Vacuum stripping | |||||||
| C. beijerinckii 8052 | Batch a, 7 L fermentation volume, Free cells | Glucose | 0.29 | 0.43 | ~44 | Continuous vacuum | [89] |
| C. beijerinckii P260 | Batch a, 14 L Bioreactor (7 L fermentation volume), Free cells | Glucose | 0.22 | 0.28 | ~48 | Continuous vacuum | [88] |
| C. beijerinckii P260 | Batch a, 14 L Bioreactor (7 L fermentation volume), Free cells | Glucose | 0.26 | 0.34 | ~63 | Intermitten vacuum, 1.5 h vacuum sessions were separated by 4 h time periods | [88] |
| Pervaporation | |||||||
| C. acetbutylicum (CICC 8012) | Continuous, Single-stage, Free cells | glucose | 0.24 | 0.23 b | ~192 | PDMS (800 cm2) | [99] |
| C. acetobutylicum DP 217 | Continuous, Single-stage | glucose | 0.37 | 0.97 | ~268 | PDMS (240 cm2), αbutanol = 31.6 | [100] |
| C. acetobutylicum ATCC 824 | Continuous 2 stage, Free cells | glucose | 0.28 | 0.88 | ~475 | PDMS (180–270 cm2 ), αbutanol = 17.67–19.81 | [101] |
| C. isopropylicum | Continuous, Single-stage, Immobilized cells | molasses | 0.29 b | Nt | ~370 | Liquid (1500 cm2), Butanol flux of 3.3 g·m−2·h−1, αbutanol = 66 | [102] |
| Bacteria | Fermentation Mode | Substrate | ABE Yield g·g−1 | ABE Productivity g·L−1·h−1 | Hours of Operation h | Comment | Reference |
|---|---|---|---|---|---|---|---|
| Liquid-liquid extraction | |||||||
| C. acetobutylicum DSM 792 | Continuous, 2 stage immobilized column reactor, Free cells, D = 1.0 h−1 | Sugar mixture | 0.38 (0.33) | 10.85 (12.14) | ~1152 | oleyl alcohol and decanol (4:1) | [103] |
| C. acetobutylicum B5313 | Continuous, two stage, Free cells, chemostat system, D = 0.05 h−1 | glucose | 0.35 (0.25) | 2.5 (2.12) | ~720 | oleyl alcohol and decanol (4:1) | [31] |
| C. acetobutylicum P262 | Continuous, Single-stage, Immobilized cells | Whey permeate | 0.23 (0.36) 0.39 (0.36) 0.36 (0.35) | 1.5 (3.5) 1.9 (3.6) 1.9 (3.0) | Nt | Dibutyl phthalate Benzyl benzoate Oleyl alcohol | [104] |
| C. acetobutylicum P262 | Continuous, Single-stage, Free cells | Whey permeate | 0.35 (0.32) | 0.14 (0.07) | ~170 | Oleyl alcohol | [105] |
| Perstraction | |||||||
| C.acetobutylicum P262 | Continuous, Single-stage, Free cells, 1 L culture | Whey permeate | 0.37 (0.32) | 0.24 (0.07) | ~290 | Oleyl alcohol, Silicone membrane | [105] |
| Adsorption | |||||||
| C. acetobutylicum ATCC 824 | Fed-batch a, Free cells, 1 L culture, expanded bed adsorption | Glucose | 0.28 (0.17) | 0.72 (0.63) | ~38.5 | hydrophobic polymer resin Dowex Optipore L-493 | [30] |
| C.acetobutylicum | Repeated Fed-batch a, Free cells, Cell recycle | Glucose | 0.32 (30.9) | 1.69 (0.4) | ~250 | Polyvinylpyridine | [106] |
| C.acetobutylicum | Fed-batch a, Free cells | Glucose | 0.32 (30.9) | 1.33 (0.4) | ~250 | Polyvinylpyridine | [106] |
| Technology | Green | Energy Demand | Efficiency |
|---|---|---|---|
| Gas stripping | Yes | High | High |
| Vacuum stripping | Yes | Low | High |
| Pervaporation | Yes | Low | High |
| Liquid-liquid extraction | No | Low | Low |
| Perstraction | No | Low | High |
| Adsorption | Yes | Low | High |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarchami, T.; Munch, G.; Johnson, E.; Kießlich, S.; Rehmann, L. A Review of Process-Design Challenges for Industrial Fermentation of Butanol from Crude Glycerol by Non-Biphasic Clostridium pasteurianum. Fermentation 2016, 2, 13. https://doi.org/10.3390/fermentation2020013
Sarchami T, Munch G, Johnson E, Kießlich S, Rehmann L. A Review of Process-Design Challenges for Industrial Fermentation of Butanol from Crude Glycerol by Non-Biphasic Clostridium pasteurianum. Fermentation. 2016; 2(2):13. https://doi.org/10.3390/fermentation2020013
Chicago/Turabian StyleSarchami, Tahereh, Garret Munch, Erin Johnson, Sascha Kießlich, and Lars Rehmann. 2016. "A Review of Process-Design Challenges for Industrial Fermentation of Butanol from Crude Glycerol by Non-Biphasic Clostridium pasteurianum" Fermentation 2, no. 2: 13. https://doi.org/10.3390/fermentation2020013