Simultaneous Determination of Sugars, Carboxylates, Alcohols and Aldehydes from Fermentations by High Performance Liquid Chromatography
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. HPLC Set up
3. Results and Discussions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pereira da Costa, M.; Conte-Junior, C.A. Chromatographic methods for the determination of carbohydrates and organic acids in foods of animal origin. Compr. Rev. Food Sci. Saf. 2015, 14, 586–600. [Google Scholar] [CrossRef]
- Ball, S.; Lloyd, L. Agilent Hi-Plex Columns for Carbohydrates, Alcohols, and Acids. Available online: http://www.agilent.com/cs/library/applications/5990--8264EN.pdf (accessed on 7 March 2016).
- Agblevor, F.A.; Hames, B.R.; Schell, D.; Chum, H.L. Analysis of biomass sugars using a novel hplc method. Appl. Biochem. Biotechnol. 2007, 136, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Womersley, C.; Drinkwater, L.; Crowe, J.H. Separation of tricarboxylic acid cycle acids and other related organic acids in insect haemolymph by high-performance liquid chromatography. J Chromatogr. A 1985, 318, 112–116. [Google Scholar] [CrossRef]
- van Hees, P.A.W.; Dahlén, J.; Lundström, U.S.; Borén, H.; Allard, B. Determination of low molecular weight organic acids in soil solution by hplc. Talanta 1999, 48, 173–179. [Google Scholar] [CrossRef]
- Kumar, M.; Saini, S.; Gayen, K. Acetone-butanol-ethanol fermentation analysis using only high performance liquid chromatography. Anal. Methods 2014, 6, 774–781. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Castellari, M.; Versari, A.; Spinabelli, U.; Galassi, S.; Amati, A. An improved hplc method for the analysis of organic acids, carbohydrates, and alcohols in grape musts and wines. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 2047–2056. [Google Scholar] [CrossRef]
- López, E.F.; Gómez, E.F. Simultaneous determination of the major organic acids, sugars, glycerol, and ethanol by hplc in grape musts and white wines. J. Chromatogr. Sci. 1996, 34, 254–257. [Google Scholar] [CrossRef]
- Chinnici, F.; Spinabelli, U.; Riponi, C.; Amati, A. Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J. Food Compos. Anal. 2005, 18, 121–130. [Google Scholar] [CrossRef]
- Vertes, A.A. Biomass to Biofuels: Strategies for Global Industries; Wiley: Chichester, UK, 2010; Volume 1. [Google Scholar]
- Wang, P.-M.; Zheng, D.-Q.; Chi, X.-Q.; Li, O.; Qian, C.-D.; Liu, T.-Z.; Zhang, X.-Y.; Du, F.-G.; Sun, P.-Y.; Qu, A.-M.; et al. Relationship of trehalose accumulation with ethanol fermentation in industrial saccharomyces cerevisiae yeast strains. Bioresour. Technol. 2014, 152, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Nikel, P.I.; Martinez-Garcia, E.; de Lorenzo, V. Biotechnological domestication of pseudomonads using synthetic biology. Nat. Rev. Microbiol. 2014, 12, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Dietmair, S.; Timmins, N.E.; Gray, P.P.; Nielsen, L.K.; Krömer, J.O. Towards quantitative metabolomics of mammalian cells: Development of a metabolite extraction protocol. Anal. Biochem. 2010, 404, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Yu, S.; Bernhardt, P.V.; Rabaey, K.; Virdis, B.; Krömer, J.O. Anoxic metabolism and biochemical production in pseudomonas putida f1 driven by a bioelectrochemical system. Biotechnol. Biofuels 2016, 9, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
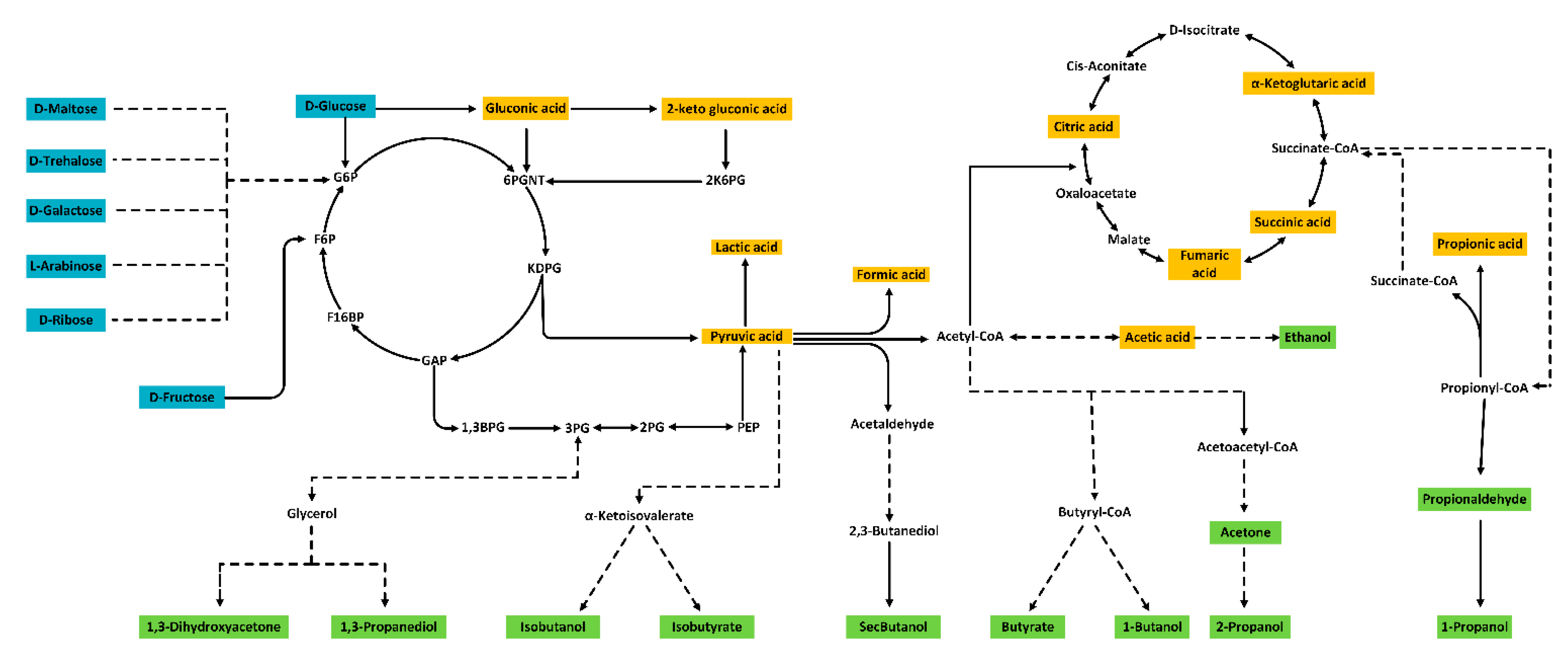
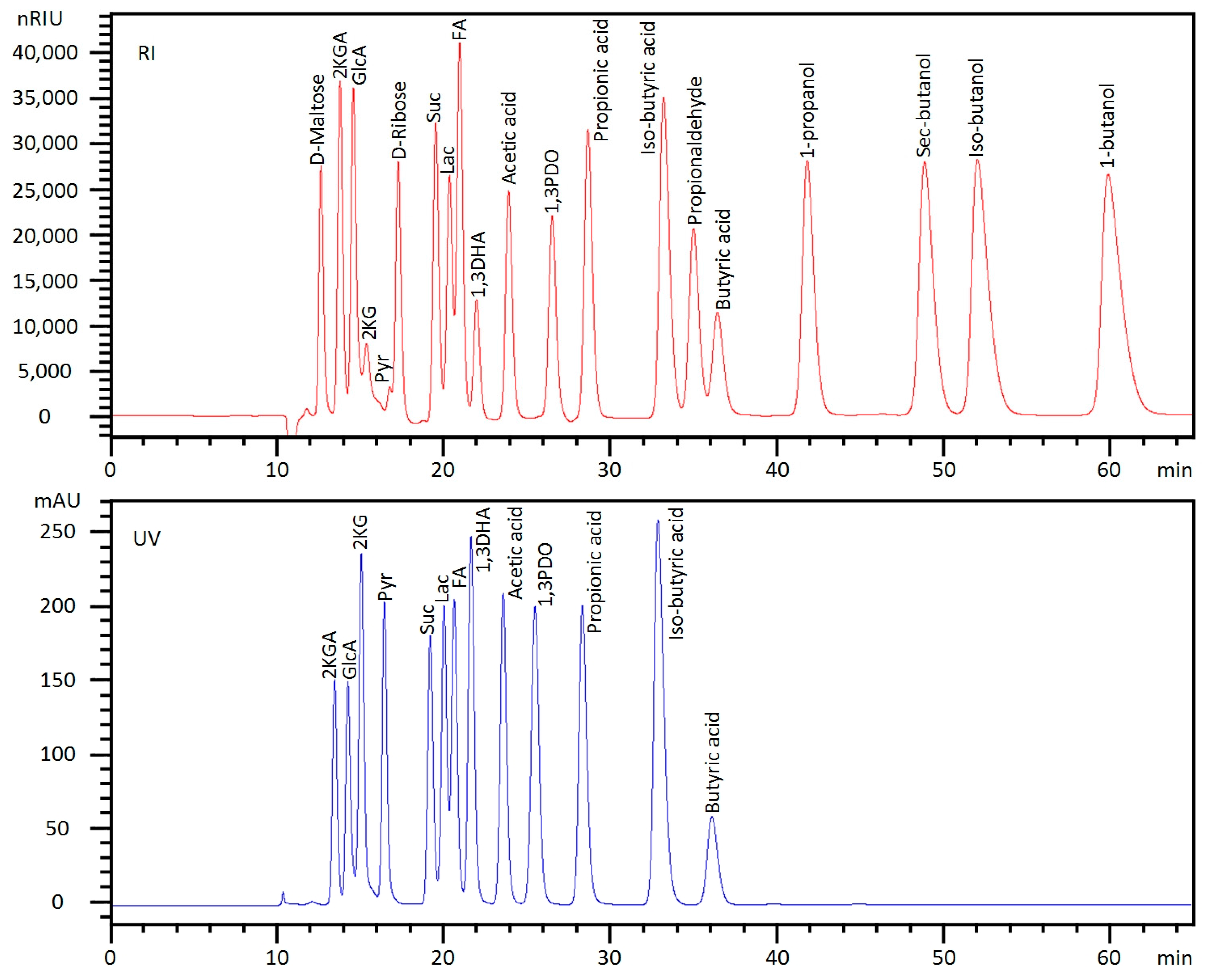
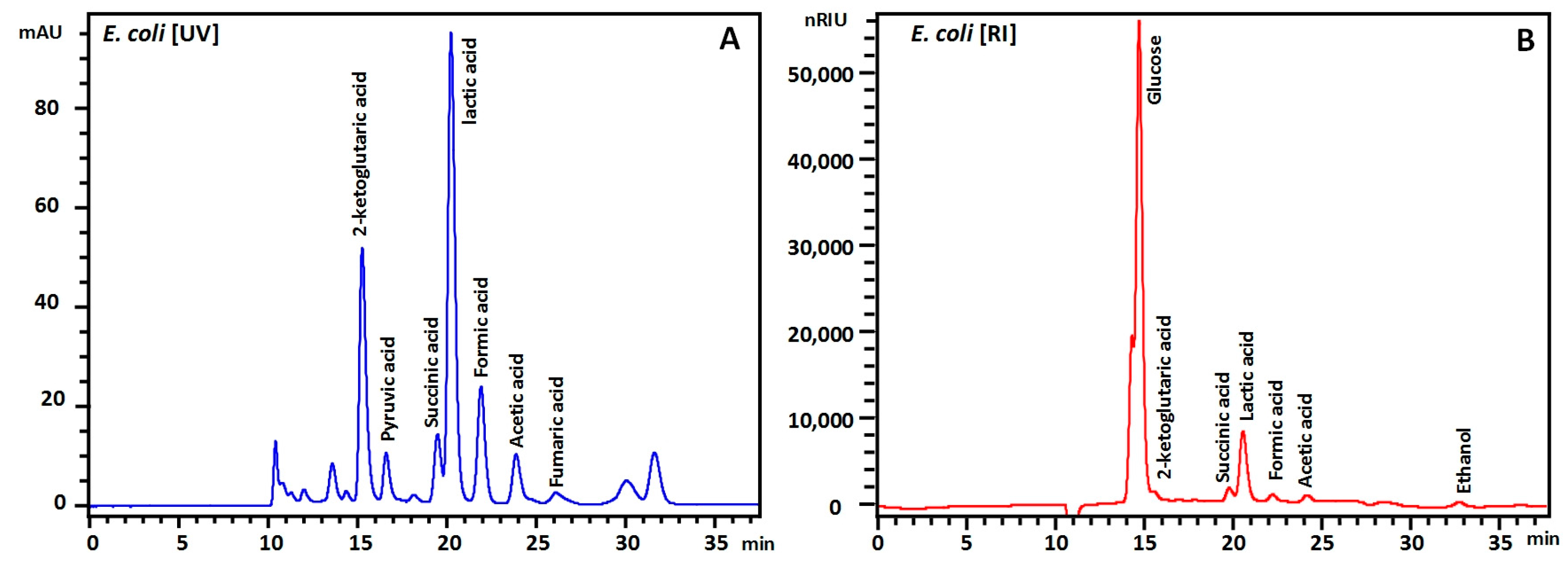
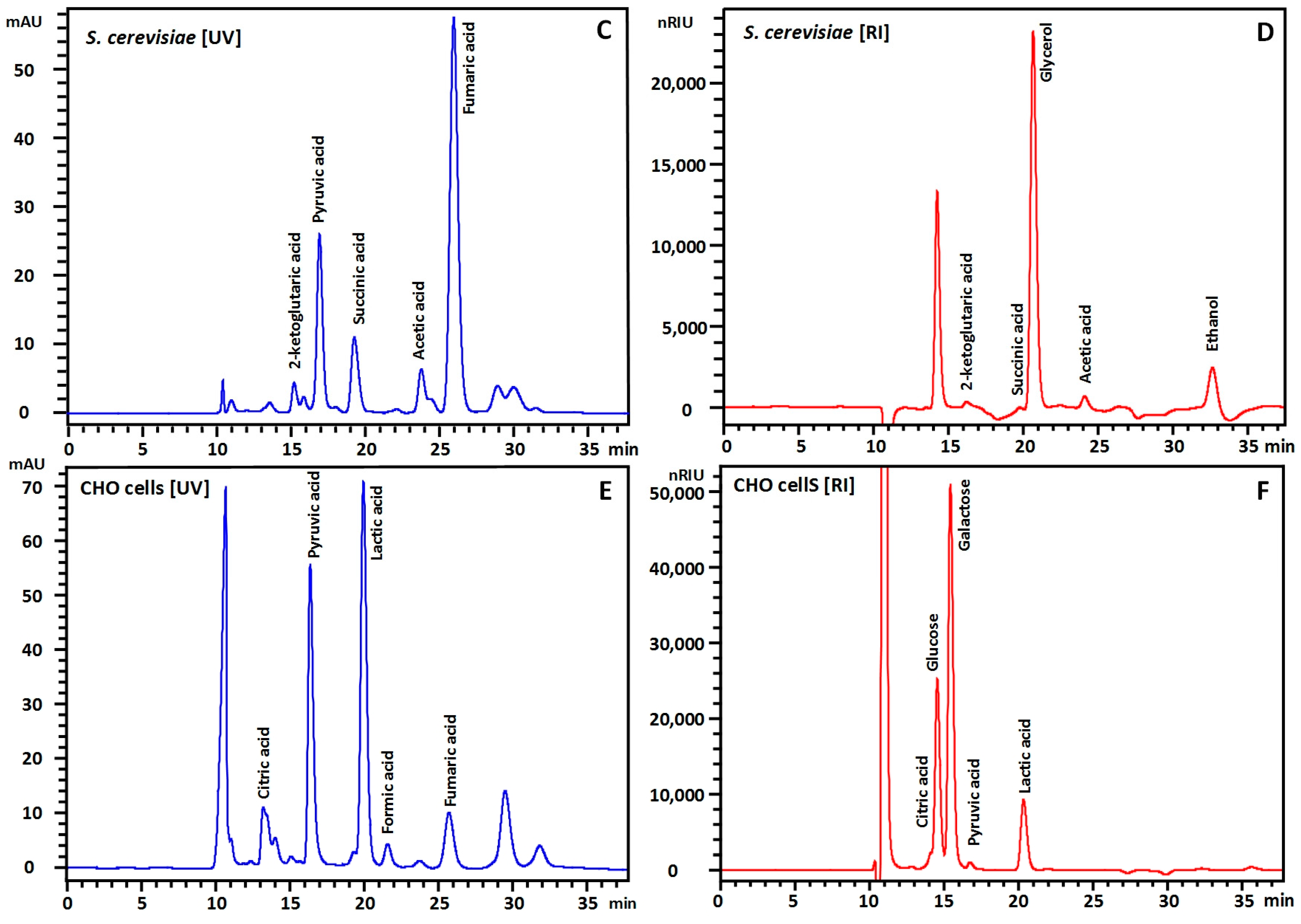
| Peak | Compound | RT (min) | LOQ | S/NLOQ | LOD | S/NLOD | Calibration Curve | Recovery (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UV | RI | mM | nmol | mM | nmol | Detector | Slope | R2 | |||||
| 1 | d-Trehalose | - | 12.53 | 0.098 | 2.94 | 10.9 | 0.012 | 0.37 | 2.8 | RI | 203,384 | 0.99999 | 100.6 |
| d-Maltose | - | 12.56 | 0.117 | 3.52 | 12.4 | 0.029 | 0.88 | 2.9 | RI | 218,036 | 0.99998 | 100.3 | |
| 2 | 2-Ketogluconic acid | 13.34 | 13.70 | 0.158 | 4.73 | 12.3 | 0.005 | 0.16 | 2.9 | UV | 374 | 0.99997 | 102.3 |
| Citric acid | 13.42 | 13.78 | 0.060 | 1.80 | 15.0 | 0.010 | 0.30 | 2.9 | UV | 827 | 0.99999 | 100.0 | |
| 3 | Gluconic acid | 14.13 | 14.49 | 0.159 | 4.77 | 11.4 | 0.005 | 0.16 | 3.2 | UV | 343 | 0.99999 | 100.6 |
| d-Glucose | - | 14.50 | 0.391 | 11.72 | 9.4 | 0.049 | 1.46 | 1.9 | RI | 116,091 | 0.99977 | 101.1 | |
| 4 | 2-Ketoglutaric acid | 14.94 | 15.29 | 0.025 | 0.75 | 10.7 | 0.003 | 0.09 | 2.1 | UV | 706 | 0.99996 | 99.9 |
| d-Galactose | - | 15.38 | 0.300 | 9.00 | 20.1 | 0.075 | 2.24 | 3.1 | RI | 119,106 | 0.99974 | 96.0 | |
| d-Fructose | - | 15.64 | 0.365 | 10.94 | 14.9 | 0.024 | 0.73 | 3.6 | RI | 109,365 | 0.99996 | 101.8 | |
| 5 | Pyruvic acid | 16.33 | 16.68 | 0.020 | 0.59 | 7.2 | 0.005 | 0.15 | 2.3 | UV | 1976 | 0.99992 | 96.5 |
| l-Arabinose | - | 16.68 | 0.365 | 10.95 | 13.8 | 0.091 | 2.74 | 1.8 | RI | a | 0.99999 | 100.0 | |
| 6 | d-Ribose | - | 17.17 | 0.266 | 7.99 | 11.3 | 0.018 | 0.53 | 1.6 | RI | 86,258 | 0.99996 | 102.7 |
| 7 | Succinic acid | 19.01 | 19.43 | 0.196 | 5.87 | 9.4 | 0.024 | 0.73 | 1.8 | UV | 285 | 0.99996 | 99.8 |
| 8 | Lactic acid | 19.90 | 20.27 | 0.235 | 7.06 | 10.9 | 0.029 | 0.88 | 1.7 | UV | 297 | 0.99997 | 99.4 |
| Glycerol | - | 20.45 | 0.156 | 4.68 | 44.1 | 0.078 | 2.34 | 3.0 | RI | 199,288 | 0.99999 | 98.9 | |
| 9 | 1,3-Dihydroxyacetone | 20.51 | 20.88 | 0.363 | 10.90 | 9.2 | 0.060 | 1.8 | 2.6 | UV | 192 | 0.9995 | 105.6 |
| 11 | Formic acid | 21.53 | 21.89 | 0.469 | 14.08 | 12.3 | 0.059 | 1.76 | 2.0 | UV | 186 | 0.99999 | 99.8 |
| 11 | Acetic acid | 23.46 | 23.83 | 0.361 | 10.84 | 9.2 | 0.045 | 1.36 | 1.7 | UV | 147 | 0.99999 | 100.2 |
| 12 | Fumaric acid | 25.33 | - | 0.006 | 0.19 | 32.6 | 0.001 | 0.02 | 4.2 | UV | 44,143 | 0.99967 | 95.8 |
| 13 | 1,3-Propanediol | - | 26.48 | 0.811 | 24.33 | 7.3 | 0.203 | 6.08 | 3.1 | RI | 37,638 | 0.99986 | 98.9 |
| 14 | Propionic acid | 28.22 | 28.59 | 0.276 | 8.27 | 6.5 | 0.034 | 1.03 | 1.8 | UV | 177 | 0.99998 | 100.2 |
| 15 | Ethanol | - | 32.45 | 9.609 | 288.26 | 10.8 | 0.601 | 18.02 | 2.0 | RI | 12,451 | 0.99994 | 99.7 |
| iso-Butyric acid | 32.76 | 33.13 | 0.394 | 11.82 | 17.8 | 0.049 | 1.48 | 2.9 | UV | 274 | 0.99987 | 99.5 | |
| 16 | Propionaldehyde | - | 34.94 | 1.634 | 49.03 | 9.8 | 0.204 | 6.13 | 1.8 | RI | 21,298 | 0.99982 | 97.5 |
| Acetone | - | 35.16 | 1.568 | 47.05 | 9.7 | 0.399 | 11.97 | 2.3 | RI | 16,063 | 0.99999 | 101.0 | |
| 17 | 2-Propanol | - | 35.96 | 1.616 | 48.48 | 10.1 | 0.404 | 12.12 | 1.7 | RI | 23,012 | 0.99998 | 101.2 |
| Butyric acid | 36.02 | 36.38 | 0.450 | 13.51 | 12.9 | 0.074 | 2.23 | 2.3 | UV | 212 | 0.99998 | 102.3 | |
| 18 | 1-Propanol | - | 41.38 | 1.566 | 46.97 | 15.5 | 0.196 | 5.87 | 2.0 | RI | 10,988 | 0.99966 | 100.7 |
| 19 | sec-Butanol | - | 48.87 | 1.558 | 46.73 | 9.8 | 0.195 | 5.84 | 1.5 | RI | 33,209 | 0.99999 | 99.5 |
| 20 | iso-Butanol | - | 52.07 | 2.286 | 68.58 | 13.9 | 0.377 | 11.3 | 2.3 | RI | 35,461 | 0.99999 | 101.1 |
| 21 | 1-Butanol | - | 59.97 | 1.565 | 46.96 | 11.9 | 0.391 | 11.74 | 2.6 | RI | 34,911 | 0.99999 | 99.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, B.; Plan, M.R.; Hodson, M.P.; Krömer, J.O. Simultaneous Determination of Sugars, Carboxylates, Alcohols and Aldehydes from Fermentations by High Performance Liquid Chromatography. Fermentation 2016, 2, 6. https://doi.org/10.3390/fermentation2010006
Lai B, Plan MR, Hodson MP, Krömer JO. Simultaneous Determination of Sugars, Carboxylates, Alcohols and Aldehydes from Fermentations by High Performance Liquid Chromatography. Fermentation. 2016; 2(1):6. https://doi.org/10.3390/fermentation2010006
Chicago/Turabian StyleLai, Bin, Manuel R. Plan, Mark P. Hodson, and Jens O. Krömer. 2016. "Simultaneous Determination of Sugars, Carboxylates, Alcohols and Aldehydes from Fermentations by High Performance Liquid Chromatography" Fermentation 2, no. 1: 6. https://doi.org/10.3390/fermentation2010006







