1. Introduction
Sluggish or stuck alcoholic fermentation results in wines containing ≥2 g/L residual sugar and lower than expected ethanol concentrations [
1]. While a number of causative factors have been implicated [
2], rectifying strategies are rather limited [
1,
3]. Most frequently, problem wines are either (a) incrementally added to vigorous fermentations or (b) racked, supplemented with nutrients, and re-inoculated with a different strain of
Saccharomyces cerevisiae [
1,
4]. Even using these procedures, success can be limited due to inhibitory amounts of ethanol or other unidentified factors [
4]. Of additional concern is that
Saccharomyces prefers glucose over fructose, the latter therefore remaining in higher relative amounts in sluggish fermentations [
5,
6,
7].
As an alternative method to remove residual sugar from sluggish fermentations, Santos
et al. [
8] and others suggested inoculation with non-
Saccharomyces yeasts such as
Zygosaccharomyces bailii. This species can be fructophilic and grows under conditions of ≥18%
v/
v ethanol, characteristics that would be advantageous to restarting fermentation [
8,
9,
10]. In support, Santos
et al. demonstrated that
Z. bailii metabolized 25 g/L fructose within six days in a medium that simulated a stuck fermentation [
8]. However,
Z. bailii is traditionally associated with spoilage,
i.e., formation of gas, sediment, haze, and/or off-flavors or odors such as excessive volatile acidity in bottled wines [
2,
11]. In contrast, some reports have indicated that selected strains may, in fact, improve the texture and body of resultant wines [
12,
13,
14]. To date, vinification conditions encountered by the wine industry including ethanol concentrations >14% or fructose >25g/L have not been examined.
Recently, two strains of Z. bailii were isolated from a large, commercial winery without a history of known spoilage issues. As these strains did not cause obvious quality problems, their abilities to metabolize residual sugar under various conditions and in partially-fermented red wines were compared to that of a commercial strain of Z. bailli (W3) as well as S. cerevisiae EC1118, the latter commonly used to restart fermentation.
2. Materials and Methods
2.1. Yeasts
Zygosaccharomyces bailii B2 and B6 were isolated from two barrel samples of Cabernet Sauvignon wines obtained from a commercial winery located in California. The wines were not identified by the winery as exhibiting off-sensory characteristics typically associated with
Z. bailii contamination. The strains were sequenced (28S ribosomal RNA) and identified by MIDI Laboratories (
www.midilabs.com).
Z. bailii “fructoferm W3” and
S. cerevisiae EC1118 and D254 were received from Lallemand Inc. (Montréal, Canada). All yeasts were maintained using yeast mold (YM; pH 4.5) media (Difco, Sparks, MD, USA).
2.2. Starter Cultures
Yeast inoculums for the Cabernet Sauvignon wines were prepared by transferring a single colony into 10 mL YM broth. After incubation at 27 °C until late exponential phase (optical density measured at 600 nm), cultures were transferred to a 100 mL YM broth containing 5% v/v ethanol as a means to acclimate cells to wine conditions. Cells were grown to late exponential phase, harvested by centrifugation at 2000× g for 20 min, and washed twice with 0.2 M Na2HPO4 (pH 7.0) buffer before inoculation into wines at 108 cfu/mL.
For the Syrah wines, single colonies were inoculated into 10 mL YM broth followed by incubation for 48 h at 22 °C. Cultures were then transferred into 200 mL and to 2000 mL YM broth after 48 h with continuous shaking. Once populations of 107 to 108 cfu/mL were achieved as determined by hemocytometer (Sigma, St. Louis, MO, USA), cells were harvested by centrifugation at 3000× g for 10 min and washed twice with 0.2 M Na2HPO4 prior to inoculation.
2.3. Cabernet Sauvignon Wines
A 2009 commercially-produced Cabernet Sauvignon wine (pH 3.92, 13.4% v/v alcohol, 0.70 g/L volatile acidity) was initially filtered through 1 μm nominal pads (Gusmer Enterprises, Fresno, CA, USA). After filtration, total SO2 was removed using 3% v/v hydrogen peroxide, pH was adjusted to 3.7 using 500 g/L tartaric acid, and 0.1 g/L yeast extract was added. The wine was then sterile-filtered through 0.2 μm Nylon® 10 inch cartridge membranes placed in sanitary filter housings (Pall, Port Washington, NY, USA) before addition of 0.1 g/L microcrystalline cellulose (Sigmacell Type 20, Sigma-Aldrich, St. Louis, MO, USA). Wines were adjusted to contain 40 or 60 g/L fructose and 13%, 15%, or 17% v/v alcohol by addition of sterile-filtered solutions of fructose in wine with added ethanol. Completed wines were transferred into sterile dilution bottles (100 mL) and incubated at 18 °C.
2.4. Syrah Wines
Syrah grapes (25.9°Brix, pH 3.4) were obtained in 2013 from a vineyard located at the Irrigated Agricultural and Extension Center (Washington State University, Prosser, WA, USA). Grapes were crushed/destemmed and 45 mg/L total SO2 was added as K2S2O5. The must (56 L) was placed into a 100 L stainless steel closed fermentation tank and, after 24 h, 0.4 g/L Fermaid-O (Lallemand Inc.) was added while the sugar concentration was adjusted to 29°Brix using sucrose. An active dry form of S. cerevisiae, strain D254, was rehydrated and inoculated according to the manufacturer’s instructions. The fermentation was punched down twice daily and when 0.5°Brix was reached, free-run (non-pressed) wine was racked into a sanitized 100 L tank. After 26 days, the fermentation was estimated to contain 20 to 25 g/L residual sugar and was considered stuck. Portions of the stuck wine were transferred into sterile 1000 mL glass bottles (Wheaton, Wheaton Science Products, Millville, NJ) for inoculation with S. cerevisiae EC1118 or Z. bailii W3 (1 × 107 cfu/mL each or a mixed culture of Z. bailii B2 and B6 (5 × 106 cfu/mL) before storage at 16.6° or 22.3 °C.
2.5. Analyses
Culturabilities were determined by plating on WL agar [
2] using an Autoplate 4000 spiral plater (Spiral Biotech, Bethesda, MD, USA). Glucose and fructose concentrations were determined using enzymatic assays obtained from R-Biopharm AG (Darmstadt, Germany) for Cabernet Sauvignon fermentations or Megazyme (Wicklow, Ireland) for Syrah. Volatile acidities were measured using a Cash still (Research and Development Glass, Berkeley, CA, USA) while residual (reducing) sugars were estimated using Clinitest
® [
15]. Progress of the Syrah fermentation (°Brix) was evaluated using a portable density meter (DMA 35, Anton Paar, Graz, Austria).
All treatments were conducted in triplicate and reported as means. Two-way analysis of variance (ANOVA) and Fisher’s LSD were applied for mean separation using XLSTAT software (Addinsoft, New York, NY, USA) for significance (p ≤ 0.05).
3. Results and Discussion
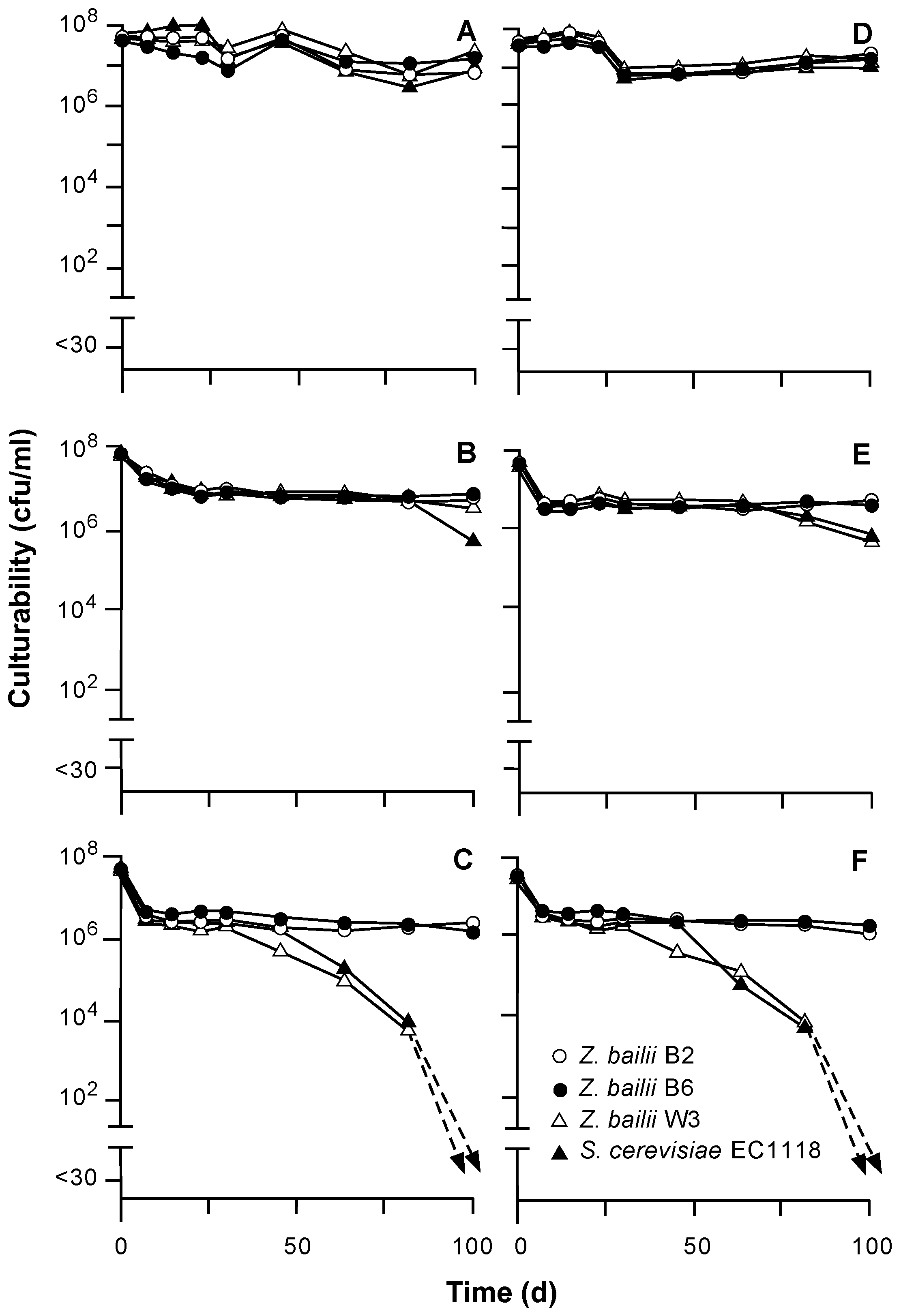
In general, survivabilities of the yeasts in the Cabernet Sauvignon wines depended on alcohol content. For example, populations of yeasts remained close to inoculation levels of approximately 10
8 cfu/mL for 100 days in wines containing 13% alcohol (
Figure 1). In 15% and 17% alcohol, the culturabilities of most yeasts quickly declined by approximately one log but remained between 10
6 and 10
8 cfu/mL for at least 50 days. The exceptions were
Z. bailii W3 and
S. cerevisiae EC1118, which slowly declined in 17%
v/
v alcohol wines to 10
5 cfu/mL by day 65 and <30 cfu/mL by day 100. In general, high inoculum levels (10
8 cfu/mL) have been recommended for stuck fermentations [
2] and were used in previous studies [
4].
Figure 1.
Culturabilities of different yeasts inoculated into Cabernet Sauvignon wines containing 40 (A,B,C) or 60 (D,E,F) g/L fructose and 13% (A,D), 15% (B,E), or 17% (C,F) v/v alcohol.
Figure 1.
Culturabilities of different yeasts inoculated into Cabernet Sauvignon wines containing 40 (A,B,C) or 60 (D,E,F) g/L fructose and 13% (A,D), 15% (B,E), or 17% (C,F) v/v alcohol.
Although
Z. bailii W3 eventually died off, the other strains of
Zygosaccharomyces exhibited extended survival even in 17% alcohol wine, in agreement with other studies [
8,
16]. In contrast, other authors have proposed that
S. cerevisiae can be the more ethanol-tolerant yeast [
17,
18]. The greater ethanol tolerance of some of
Z. bailii strains studied may be related to a diminished degree of unsaturation reported in the cellular membrane fatty acids [
19].
Similar to the trends observed for culturability, utilization of fructose depended not only on species and strain but also on the amount of ethanol present (
Figure 2). With the exception of
Z. bailli B6, all strains consumed 40 g/L fructose to dryness in wines containing 13% ethanol. Despite an initial decline in culturability,
Z. bailii W3 and
S. cerevisiae EC1118 metabolized >90% of the 40 g/L or 33% of the 60 g/L fructose present in the 15% alcohol wines. However, none of the wines inoculated with
Zygosaccharomyces and containing 15% or 17% alcohol reached dryness (<2 g/L). Of these wines, upon completion of the experiment the lowest fructose concentration (3 g/L) was found in wines inoculated with strain W3, which initially contained 15% alcohol and 40 g/L fructose. Few differences in sugar utilization were noted between wines containing 17% alcohol, where metabolic activities ceased at approximately 50% of the original amount of fructose present.
Figure 2.
Declines in fructose concentrations of Cabernet Sauvignon wines containing 40 (A,B,C) or 60 (D,E,F) g/L fructose and 13% (A,D), 15% (B,E), or 17% (C,F) v/v alcohol inoculated with different yeasts.
Figure 2.
Declines in fructose concentrations of Cabernet Sauvignon wines containing 40 (A,B,C) or 60 (D,E,F) g/L fructose and 13% (A,D), 15% (B,E), or 17% (C,F) v/v alcohol inoculated with different yeasts.
While hexose transport in most yeasts is a function of carrier-mediated facilitated diffusion and active proton symporters [
6,
20], the fructophilic nature of
Z. bailii may be due to an added specific high-capacity transport system [
9,
21]. However, Santos
et al. suggested that the fructose transport system in
Z. bailii could be more sensitive to ethanol than other metabolic pathways [
8]. Thus, while
Z. bailii may survive in high ethanol environments, it may be less able to efficiently utilize fructose as the relative ethanol concentration becomes greater, in agreement with the results of the present study.
Differences were noted regarding the synthesis of volatile acidity (VA) calculated as acetic acid. As illustrated in
Table 1, 15% alcohol wines inoculated with
S. cerevisiae contained less VA than those inoculated with
Z. bailii. While VA values of wines with
Saccharomyces were 0.71 to 0.77 g/L, those inoculated with
Zygosaccharomyces ranged between 0.80 g/L up to 1.0 g/L.
Zygosaccharomyces are well known to produce VA but synthesis of ethyl acetate, sometimes produced along with acetic acid by other non-
Saccharomyces yeasts but with a lower sensory threshhold, is generally limited [
14].
While many non-
Saccharomyces yeasts are believed to possess lower ethanol tolerances compared to
Saccharomyces, responses are also a function of temperature [
22]. For example, Heard and Fleet reported that in mixed culture fermentation at 10 °C,
Kloeckera apiculata or
Candida stellata achieved populations of 10
7 cfu/mL and completed fermentation rather than
S. cerevisiae [
23]. Similarly, Erten observed that
K. apiculata survived longer when fermentations were maintained at <15 °C than those conducted above 20 °C [
24], in agreement with previous findings [
25]. Consequently, temperatures lower than the 18 °C used in the first wine experiment might also extend the alcohol tolerance range of
Zygosaccharomyces yet allow utilization of fructose from a stuck wine.
Table 1.
Volatile acidities (g/L) of Cabernet Sauvignon wines containing 15% or 17% v/v alcohol and 40 or 60 g/L fructose as measured 100 days after inoculation.
Table 1.
Volatile acidities (g/L) of Cabernet Sauvignon wines containing 15% or 17% v/v alcohol and 40 or 60 g/L fructose as measured 100 days after inoculation.
| Treatment | Yeast Inoculated |
|---|
| Ethanol (% v/v) | Fructose (g/L) | Z. bailii ZB2 | Z. bailii ZB6 | Z. bailii W3 | S. cerevisiae EC1118 |
|---|
| 15% | 40 | 0.98 a | 0.94 ab | 0.86 abc | 0.77 c |
| 60 | 1.00 a | 0.89 ab | 0.94 abc | 0.79 bc |
| 17% | 40 | 0.82 a | 0.92 a | 0.80 a | 0.71 a |
| 60 | 0.82 a | 0.80 a | nd | nd |
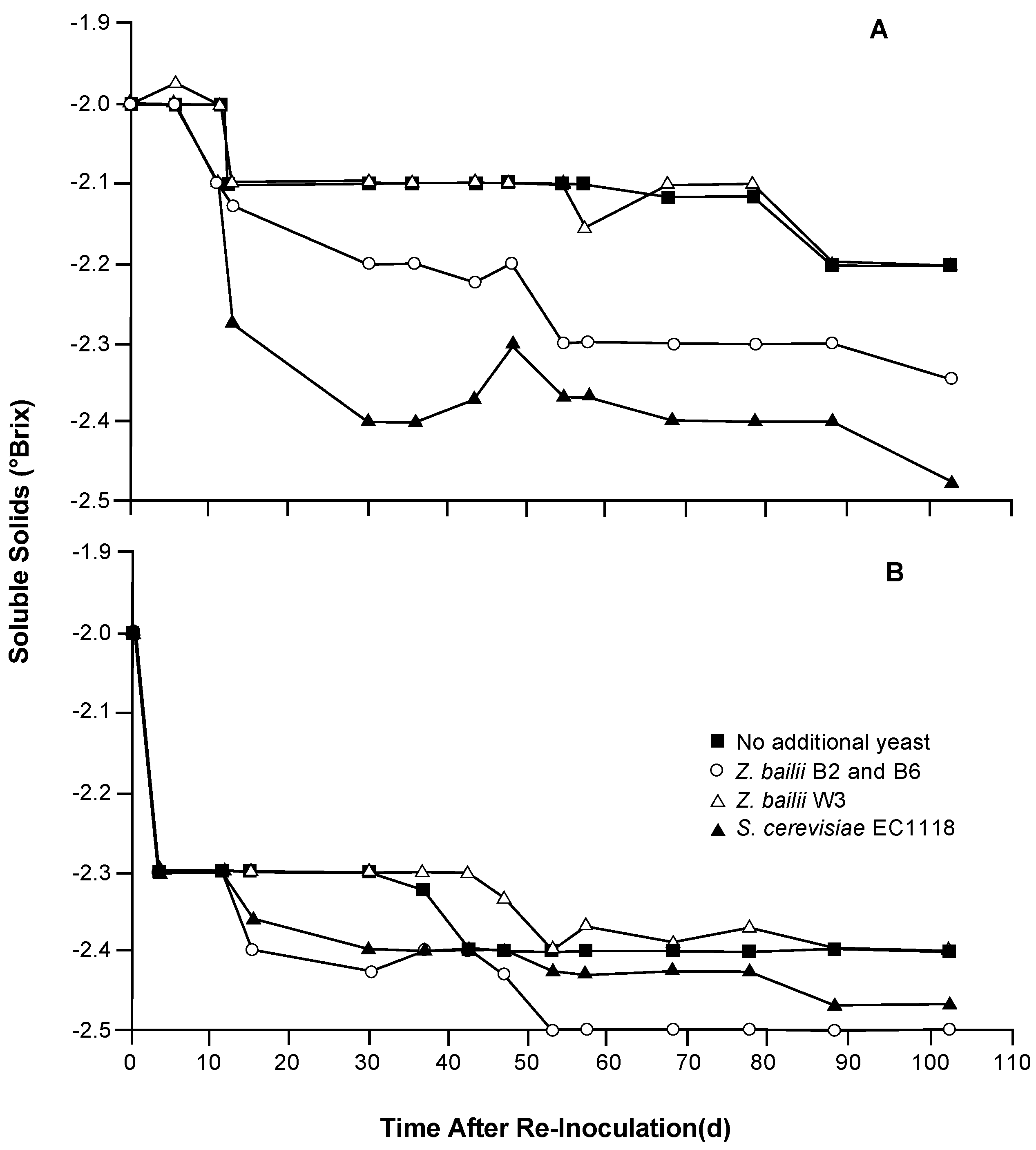
Thus, a sluggish fermentation was induced in a Syrah wine by adding a high amount of sugar (29°Brix) before initial fermentation by S. cerevisiae. As the fermentation became sluggish at approximately −2°Brix, portions were transferred into smaller volumes prior to addition of Z. bailii B2 and B6 (equal populations), Z. bailii W3, or S. cerevisiae EC1118 as means to remove the residual sugar.
Overall, slight decreases in total soluble solids (≈0.5°Brix) were noted up to day 100, with or without additional yeast inoculation (
Figure 3). Here, those inoculated with
S. cerevisiae EC1118 or a mixture of two strains of
Z. bailii (B2 and B6) yielded lower °Brix values compared to uninoculated wines or those with
Z. bailii W3. In agreement, the only wines to reach dryness (<2 g/L) were those inoculated with
S. cerevisiae EC1118 or
Z. bailii B2/B6. While glucose concentrations in all wines ranged between 0.07 and 0.19 g/L after 100 days, residual fructose levels were all higher (
Table 2). For a given yeast, lower concentrations of fructose were noted in the wines held at 16.6 °C than at 22.3 °C, indicating a higher level of fructose utilization at the lower temperature. Wines without additional yeast or with
Z. bailii W3 contained residual concentrations of fructose >2 g/L at either incubation temperature.
Based on these results, commercial application of these strains of
Z. bailii to rectify stuck fermentation appears to be equivalent compared to using
S. cerevisiae EC1118. For instance, Cabernet Sauvignon wines with 13% alcohol and ≤60 g/L fructose or with 15% and ≤40 g/L achieved dryness with a high inoculation of
S. cerevisiae EC1118. Furthermore, Syrah wines inoculated with EC1118 had equal or lower concentrations of fructose compared to
Z. bailii B2/B6. In fact, this particular strain of
S. cerevisiae has been specifically recommended to restart stuck fermentation [
26] and demonstrated particularly effective ethanol production in high sugar ferments [
27]. However, given the variability between species and strains of
Zygosaccharomyces [
14], evaluation of additional isolates may result in finding those more effective at utilizing residual fructose from stuck wine fermentation, after which a quantitative assesment of potential sensory affects will be necessary.
Figure 3.
Declines in soluble solids (°Brix) of a partially-fermented Syrah wine maintained at 16.6° (A) or 22.3 °C (B) without additional yeast (■) or with inoculation of Z. bailii ZB2 and ZB6 (○), Z. bailii W3(△), or S. cerevisiae EC1118 (▲).
Figure 3.
Declines in soluble solids (°Brix) of a partially-fermented Syrah wine maintained at 16.6° (A) or 22.3 °C (B) without additional yeast (■) or with inoculation of Z. bailii ZB2 and ZB6 (○), Z. bailii W3(△), or S. cerevisiae EC1118 (▲).
Table 2.
Concentrations of residual glucose or fructose in partially-fermented Syrah wines incubated at 16.6 or 22.3 °C without or with inoculation of Z. bailii B2 and B6 (equal populations of each), Z. bailii W3, or S. cerevisiae EC1118.
Table 2.
Concentrations of residual glucose or fructose in partially-fermented Syrah wines incubated at 16.6 or 22.3 °C without or with inoculation of Z. bailii B2 and B6 (equal populations of each), Z. bailii W3, or S. cerevisiae EC1118.
| Treatment | Glucose (g/L) | Fructose (g/L) |
|---|
| Temperature (°C) | Yeast (Strain) |
|---|
| 16.6 | None | 0.19 a | 3.93 a |
| Z. bailii B2 and B6 | 0.07 e | 0.81 e |
| Z. bailii W3 | 0.10 d | 2.52 c |
| S. cerevisiae EC1118 | 0.04 e | 0.33 e |
| 22.3 | None | 0.18 ab | 3.25 b |
| Z. bailii B2 and B6 | 0.12 cd | 1.44 d |
| Z. bailii W3 | 0.15 bc | 2.98 bc |
| S. cerevisiae EC1118 | 0.11 d | 1.84 d |