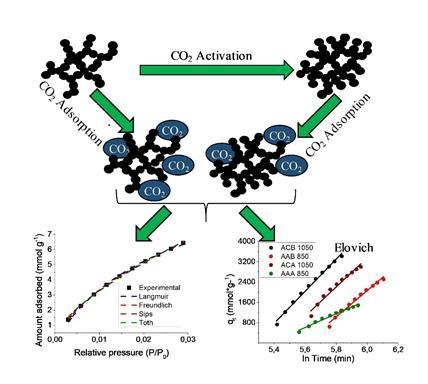
Kinetic and Equilibrium Study of the Adsorption of CO2 in Ultramicropores of Resorcinol-Formaldehyde Aerogels Obtained in Acidic and Basic Medium
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Carbonization
2.3. Activation with CO2
2.4. Characterization
2.4.1. Physisorption of N2 at −196 °C and CO2 at 0 °C
2.4.2. Raman Spectroscopy
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Infrared Spectroscopy
2.5. Kinetic Study of the CO2 Adsorption Process at 0 °C and Atmospheric Pressure
2.5.1. Pseudo First Order and Pseudo Second Order Models
2.5.2. Modified Pseudo First Order
2.5.3. Elovich Model
2.5.4. Intra-Particle Diffusion Model
2.6. Adsorption Studies
2.6.1. Langmuir Model
2.6.2. Freundlich Model
2.6.3. Sips Model
2.6.4. Toth Model
3. Results and Discussion
3.1. Physisorption
3.1.1. Physisorption of N2 to −196 °C
3.1.2. Physisorption of CO2 at 0 °C
3.2. Raman Spectroscopy
3.3. Scanning Electron Microscopy (SEM)
3.4. Infrared Spectroscopy
3.5. Adsorption of CO2 at 0 °C
3.5.1. Kinetic Study of the CO2 Adsorption Process at 0 °C
3.5.2. Study of the CO2 Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anas, M.; Gönel, A.G.; Bozbag, S.E.; Erkey, C. Thermodynamics of adsorption of carbon dioxide on various aerogels. J. CO2 Util. 2017, 21, 82–88. [Google Scholar] [CrossRef]
- Jedli, H.; Brahmi, J.; Hedfi, H.; Mbarek, M.; Bouzgarrou, S.; Slimi, K. Adsorption kinetics and thermodynamics properties of supercritical CO2 on activated clay. J. Pet. Sci. Eng. 2018, 166, 476–481. [Google Scholar] [CrossRef]
- Kumar, S.; Wani, M.Y.; Koh, J.; Gil, J.M.; Sobral, A.J.F.N. Carbon dioxide adsorption and cycloaddition reaction of epoxides using chitosan-graphene oxide nanocomposite as a catalyst. J. Environ. Sci. 2018, 69, 77–84. [Google Scholar]
- Yaumi, A.L.; Bakar, M.Z.A.; Hameed, B.H. Melamine-nitrogenated mesoporous activated carbon derived from rice husk for carbon dioxide adsorption in fixed-bed. Energy 2018, 155, 46–55. [Google Scholar] [CrossRef]
- Kouetcha, D.N.; Ramézani, H.; Mathieu-Cohaut, N.; Bhatia, S.K. Carbon dioxide adsorption through carbon adsorbent structures: Effect of the porosity size, chemical potential and temperature. Comput. Mater. Sci. 2018, 151, 255–272. [Google Scholar] [CrossRef]
- Lillia, S.; Bonalumi, D.; Grande, C.; Manzolini, G. A comprehensive modeling of the hybrid temperature electric swing adsorption process for CO2 capture. Int. J. Greenh. Gas Control 2018, 74, 155–173. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Raganati, F.; Alfe, M.; Gargiulo, V.; Chirone, R.; Ammendola, P. Isotherms and thermodynamics of CO2 adsorption on a novel carbon-magnetite composite sorbent. Chem. Eng. Res. Des. 2018, 134, 540–552. [Google Scholar] [CrossRef]
- Zeleňák, V.; Skřínska, M.; Zukal, A.; Čejka, J. Carbon dioxide adsorption over amine modified silica: Effect of amine basicity and entropy factor on isosteric heats of adsorption. Chem. Eng. J. 2018, 348, 327–337. [Google Scholar] [CrossRef]
- Zukal, A.; Shamzhy, M.; Kubů, M.; Čejka, J. The effect of pore size dimensions in isoreticular zeolites on carbon dioxide adsorption heats. J. CO2 Util. 2018, 24, 157–163. [Google Scholar] [CrossRef]
- Deng, S.; Chen, T.; Zhao, T.; Yao, X.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Role of micropores and nitrogen-containing groups in CO2 adsorption on indole-3-butyric acid potassium derived carbons. Chem. Eng. J. 2016, 286, 98–105. [Google Scholar] [CrossRef]
- Pires, J.; Juźków, J.; Pinto, M.L. Amino acid modified montmorillonite clays as sustainable materials for carbon dioxide adsorption and separation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 105–110. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Wdowin, M.; Tarkowski, R.; Franus, W. Determination of changes in the reservoir and cap rocks of the chabowo anticline caused by CO2-brine-rock interactions. Int. J. Coal Geol. 2014, 130, 79–88. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Hu, X.; Feng, R.; Zhou, M.; Cui, W. Development of zeolitic imidazolate framework-67 functionalized Co-Al LDH for CO2 adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 16–23. [Google Scholar] [CrossRef]
- Álvarez-Gutiérrez, N.; Gil, M.V.; Rubiera, F.; Pevida, C. Kinetics of CO2 adsorption on cherry stone-based carbons in CO2/CH4 separations. Chem. Eng. J. 2017, 307, 249–257. [Google Scholar] [CrossRef]
- Liu, N.; Shen, J.; Liu, D. Activated high specific surface area carbon aerogels for EDLCs. Microporous Mesoporous Mater. 2013, 167, 176–181. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H. The use of low temperature van der waals adsorption isotherms in determining the surface areas of various adsorbents. J. Am. Chem. Soc. 1937, 59, 2682–2689. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H. The use of van der waals adsorption isotherms in determining the surface area of iron synthetic ammonia catalysts. J. Am. Chem. Soc. 1935, 57, 1754–1755. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a theory of the van der waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Dubinin, M.M. Physical adsorption of gases and vapors in micropores. In Progress in Surface and Membrane Science; Cadenhead, D.A., Danielli, J.F., Rosenberg, M.D., Eds.; Elsevier: Moscow, Russia, 1975; Volume 9, pp. 1–70. [Google Scholar]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, L.; Xiong, Y.; Li, J.; Peng, P.A. Nanopore structure characterization for organic-rich shale using the non-local-density functional theory by a combination of N2 and CO2 adsorption. Microporous Mesoporous Mater. 2016, 227, 88–94. [Google Scholar] [CrossRef]
- Inyang, H.I.; Onwawoma, A.; Bae, S. The elovich equation as a predictor of lead and cadmium sorption rates on contaminant barrier minerals. Soil Tillage Res. 2016, 155, 124–132. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Silva, C.M. What’s wrong with lagergreen pseudo first order model for adsorption kinetics? Chem. Eng. J. 2016, 306, 1138–1142. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Dynamic studies and pseudo-second order modeling of copper(II) biosorption onto pine cone powder. Desalination 2010, 251, 112–122. [Google Scholar] [CrossRef]
- Yang, X.; Al-Duri, B. Kinetic modeling of liquid-phase adsorption of reactive dyes on activated carbon. J. Colloid Interface Sci. 2005, 287, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M. UT (University of Tehran) isotherm as a novel and useful adsorption isotherm for investigation of adsorptive removal of pollutants. J. Environ. Chem. Eng. 2017, 5, 1906–1919. [Google Scholar] [CrossRef]
- Rill, C.; Kolar, Z.I.; Kickelbick, G.; Wolterbeek, H.T.; Peters, J.A. Kinetics and thermodynamics of adsorption on hydroxyapatite of the [160Tb] terbium complexes of the bone-targeting ligands DOTP and BPPED. Langmuir 2009, 25, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Bai, P.; Guo, X.; Ni, X. Adsorptive separation of acetic acid from dilute aqueous solutions: Adsorption kinetic, isotherms, and thermodynamic studies. J. Chem. Eng. Data 2015, 61, 213–219. [Google Scholar] [CrossRef]
- Uslu, H.; Majumder, S. Adsorption studies of lactic acid by polymeric adsorbent amberlite XAD-7: Equilibrium and kinetics. J. Chem. Eng. Data 2017, 62, 1501–1506. [Google Scholar] [CrossRef]
- Chen, J.; Yang, H.; Ring, Z. Study of intra-particle diffusion effect on hydrodesulphurization of dibenzothiophenic compounds. Catal. Today 2005, 109, 93–98. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Setiadi, T. Adsorption and desorption of copper (II) ions onto garden grass. Bioresour. Technol. 2012, 121, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- McKay, G.; Mesdaghinia, A.; Nasseri, S.; Hadi, M.; Solaimany, A.M. Optimum isotherms of dyes sorption by activated carbon: Fractional theoretical capacity & error analysis. Chem. Eng. J. 2014, 251, 236–247. [Google Scholar]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Barka, N.; Ouzaouit, K.; Abdennouri, M.; Makhfouk, M.E. Dried prickly pear cactus (opuntia ficus indica) cladodes as a low-cost and eco-friendly biosorbent for dyes removal from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2013, 44, 52–60. [Google Scholar] [CrossRef]
- Brdar, M.; Šćiban, M.; Takači, A.; Došenović, T. Comparison of two and three parameters adsorption isotherm for Cr (VI) onto Kraft lignin. Chem. Eng. J. 2012, 183, 108–111. [Google Scholar] [CrossRef]
- Deniz, F.; Ersanli, E.T. Simultaneous bioremoval of two unsafe dyes from aqueous solution using a novel green composite biosorbent. Microchem. J. 2016, 128, 312–319. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Adsorption of congo red by chitosan hydrogel beads impregnated with carbon nanotubes. Bioresour. Technol. 2010, 101, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Kumar, T.; Ojha, K.; Mandal, A. Adsorption of surfactants on sand surface in enhanced oil recovery: Isotherms, kinetics and thermodynamic studies. Appl. Surf. Sci. 2013, 284, 87–99. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Anu, N.; Nandagopal, M.S.G.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Hall, P.J. The control of porosity at nano scale in resorcinol formaldehyde carbon aerogels. J. Mater. Sci. 2009, 44, 2705–2713. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Fei, B.; Ma, J.; Liu, X.; Yang, S.; Tian, G.; Jiang, Z. Porous nanoplatelets wrapped carbon aerogels by pyrolysis of regenerated bamboo cellulose aerogels as supercapacitor electrodes. Carbohydr. Polym. 2018, 180, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Arenillas, A.; Calvo, E.; Bermúdez, J.; Menéndez, J. Carbonisation of resorcinol-formaldehyde organic xerogels: Effect of temperature, particle size and heating rate on the porosity of carbon xerogels. J. Anal. Appl. Pyrolysis 2013, 100, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Hu, H.; Chen, G. Zeolite with tunable intracrystal mesoporosity synthesized with carbon aerogel as a secondary template. Microporous Mesoporous Mater. 2008, 113, 481–489. [Google Scholar] [CrossRef]
- Sierra, I.; Iriarte-Velasco, U.; Gamero, M.; Aguayo, A.T. Upgrading of sewage sludge by demineralization and physical activation with CO2: Application for methylene blue and phenol removal. Microporous Mesoporous Mater. 2017, 250, 88–99. [Google Scholar] [CrossRef]
- Kong, Y.; Zhong, Y.; Shen, X.; Cui, S.; Fan, M. Effect of silica sources on nanostructures of resorcinol-formaldehyde/silica and carbon/silicon carbide composite aerogels. Microporous Mesoporous Mater. 2014, 197, 77–82. [Google Scholar] [CrossRef]
- Shoaib, M.; Al-Swaidan, H.M. Optimization and characterization of sliced activated carbon prepared from date palm tree fronds by physical activation. Biomass Bioenergy 2015, 73, 124–134. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Rodríguez-Reinoso, F. Chemical versus physical activation of coconut shell: A comparative study. Microporous Mesoporous Mater. 2012, 152, 163–171. [Google Scholar] [CrossRef]
- Zafra, M.; Lavela, P.; Rasines, G.; Macías, C.; Tirado, J. Effect of the resorcinol/catalyst ratio in the capacitive performance of carbon xerogels with potential use in sodium chloride removal from saline water. J. Solid State Electrochem. 2014, 18, 2847–2856. [Google Scholar] [CrossRef]
- Mahani, A.A.; Motahari, S.; Nayyeri, V. Synthesis, characterization and dielectric properties of one-step pyrolyzed/activated resorcinol-formaldehyde based carbon aerogels for electromagnetic interference shielding applications. Mater. Chem. Phys. 2018, 213, 492–501. [Google Scholar] [CrossRef]
- Laskowski, J.; Milow, B.; Ratke, L. Aerogel-aerogel composites for normal temperature range thermal insulations. J. Non-Cryst. Solids 2016, 441, 42–48. [Google Scholar] [CrossRef]
- Kong, Y.; Zhong, Y.; Shen, X.; Cui, S.; Yang, M.; Teng, K.; Zhang, J. Facile synthesis of resorcinol-formaldehyde/silica composite aerogels and their transformation to monolithic carbon/silica and carbon/silicon carbide composite aerogels. J. Non-Cryst. Solids 2012, 358, 3150–3155. [Google Scholar] [CrossRef]
- Laskowski, J.; Milow, B.; Ratke, L. Subcritically dried resorcinol-formaldehyde aerogels from a base-acid catalyzed synthesis route. Microporous Mesoporous Mater. 2014, 197, 308–315. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Du, A.; Zhu, X.; Shen, J.; Wu, G.; Zhang, Z.; Zhou, B. One-pot synthesis, characterization and properties of acid-catalyzed resorcinol/formaldehyde cross-linked silica aerogels and their conversion to hierarchical porous carbon monoliths. J. Sol-Gel Sci. Technol. 2012, 62, 294–303. [Google Scholar] [CrossRef]
- Kinnertová, E.; Slovák, V. Influence of catalyst amount on properties of resorcinol-formaldehyde xerogels. Thermochim. Acta 2018, 660, 37–43. [Google Scholar] [CrossRef]
- Schwan, M.; Naikade, M.; Raabe, D.; Ratke, L. From hard to rubber-like: Mechanical properties of resorcinol-formaldehyde aerogels. J. Mater. Sci. 2015, 50, 5482–5493. [Google Scholar] [CrossRef]
- Li, W.-C.; Lu, A.-H.; Guo, S.-C. Characterization of the microstructures of organic and carbon aerogels based upon mixed cresol-formaldehyde. Carbon 2001, 39, 1989–1994. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Time-efficient acid-catalyzed synthesis of resorcinol-formaldehyde aerogels. Chem. Mater. 2007, 19, 6138–6144. [Google Scholar] [CrossRef]
- Pekala, R. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Yan, L.-G.; Qin, L.-L.; Yu, H.-Q.; Li, S.; Shan, R.-R.; Du, B. Adsorption of acid dyes from aqueous solution by CTMAB modified bentonite: Kinetic and isotherm modeling. J. Mol. Liq. 2015, 211, 1074–1081. [Google Scholar] [CrossRef]
- Liu, B.; Ge, N.; Peng, B.; Pan, S. Kinetic and isotherm studies on the adsorption of tenuazonic acid from fruit juice using inactivated LAB. J. Food Eng. 2017, 224, 45–52. [Google Scholar] [CrossRef]









| Sample | SBET (m2 g−1) |
|---|---|
| ACB 1050 | 1927 |
| AAB 850 | 1491 |
| ACA 1050 | 893 |
| AAA 850 | 629 |
| Sample | Vn (cm3 g−1) |
|---|---|
| ACB 1050 | 0.698 |
| AAB 850 | 0.357 |
| ACA 1050 | 0.451 |
| AAA 850 | 0.230 |
| Kinetic Model | Parameter | Samples | |||
|---|---|---|---|---|---|
| ACB 1050 | AAB 850 | ACA 1050 | AAA 850 | ||
| Pseudo first order | K1 | 0.0233 | 0.0212 | 0.0283 | 0.02395 |
| R2 | 0.945 | 0.952 | 0.947 | 0.941 | |
| Pseudo Second order | K2 | 0.0000866 | 0.0000903 | 0.0001.21 | 0.000230 |
| R2 | 0.6521 | 0.6615 | 0.6734 | 0.689 | |
| Modified Pseudo first order | Km | 0.0164 | 0.0154 | 0.0221 | 0.0181 |
| R2 | 0.869 | 0.8872 | 0.895 | 0.8771 | |
| Elovich | α | 0.0618 | 0.0368 | 0.0490 | 0.0239 |
| β | 0.0838 | 0.102 | 0.0921 | 0.197 | |
| R2 | 0.991 | 0.982 | 0.969 | 0.985 | |
| Intra-particle diffusion | kdi1 | 2.04 | 1.82 | 2.37 | 0.888 |
| R2 | 0.998 | 0.996 | 0.995 | 0.999 | |
| kdi2 | 1.27 | 0.876 | 0.971 | 0.487 | |
| R2 | 0.995 | 0.992 | 0.992 | 0.994 | |
| Models | Parameters | Sample | |||
|---|---|---|---|---|---|
| ACB 1050 | AAB 850 | ACA 1050 | AAA 850 | ||
| Langmuir | qL | 12.01 | 7.70 | 7.29 | 4.04 |
| KL | 38.48 | 52.07 | 107.84 | 72.17 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | |
| Freundlich | KF | 62.88 | 36.95 | 26.50 | 17.41 |
| N | 1.56 | 1.73 | 2.32 | 1.95 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | |
| Sips | qS | 36.92 | 5.62 | 10.75 | 6.27 |
| KS | 18.98 | 6.57 | 10.19 | 2.77 | |
| ms | 0.99 | 0.58 | 0.66 | 0.51 | |
| R2 | 0.99 | 0.99 | 1.00 | 0.99 | |
| Toth | qT | 0.020 | 0.023 | 0.108 | 0.043 |
| KT | 4.15 | 2.96 | 1.50 | 2.44 | |
| mT | −0.19 | −0.18 | −0.16 | −0.19 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarín Romero, J.R.; Moreno-Piraján, J.C.; Giraldo Gutierrez, L. Kinetic and Equilibrium Study of the Adsorption of CO2 in Ultramicropores of Resorcinol-Formaldehyde Aerogels Obtained in Acidic and Basic Medium. C 2018, 4, 52. https://doi.org/10.3390/c4040052
Guarín Romero JR, Moreno-Piraján JC, Giraldo Gutierrez L. Kinetic and Equilibrium Study of the Adsorption of CO2 in Ultramicropores of Resorcinol-Formaldehyde Aerogels Obtained in Acidic and Basic Medium. C. 2018; 4(4):52. https://doi.org/10.3390/c4040052
Chicago/Turabian StyleGuarín Romero, Jhonatan R., Juan Carlos Moreno-Piraján, and Liliana Giraldo Gutierrez. 2018. "Kinetic and Equilibrium Study of the Adsorption of CO2 in Ultramicropores of Resorcinol-Formaldehyde Aerogels Obtained in Acidic and Basic Medium" C 4, no. 4: 52. https://doi.org/10.3390/c4040052