Operando DRIFTS-MS Study of WGS and rWGS Reaction on Biochar-Based Pt Catalysts: The Promotional Effect of Na
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
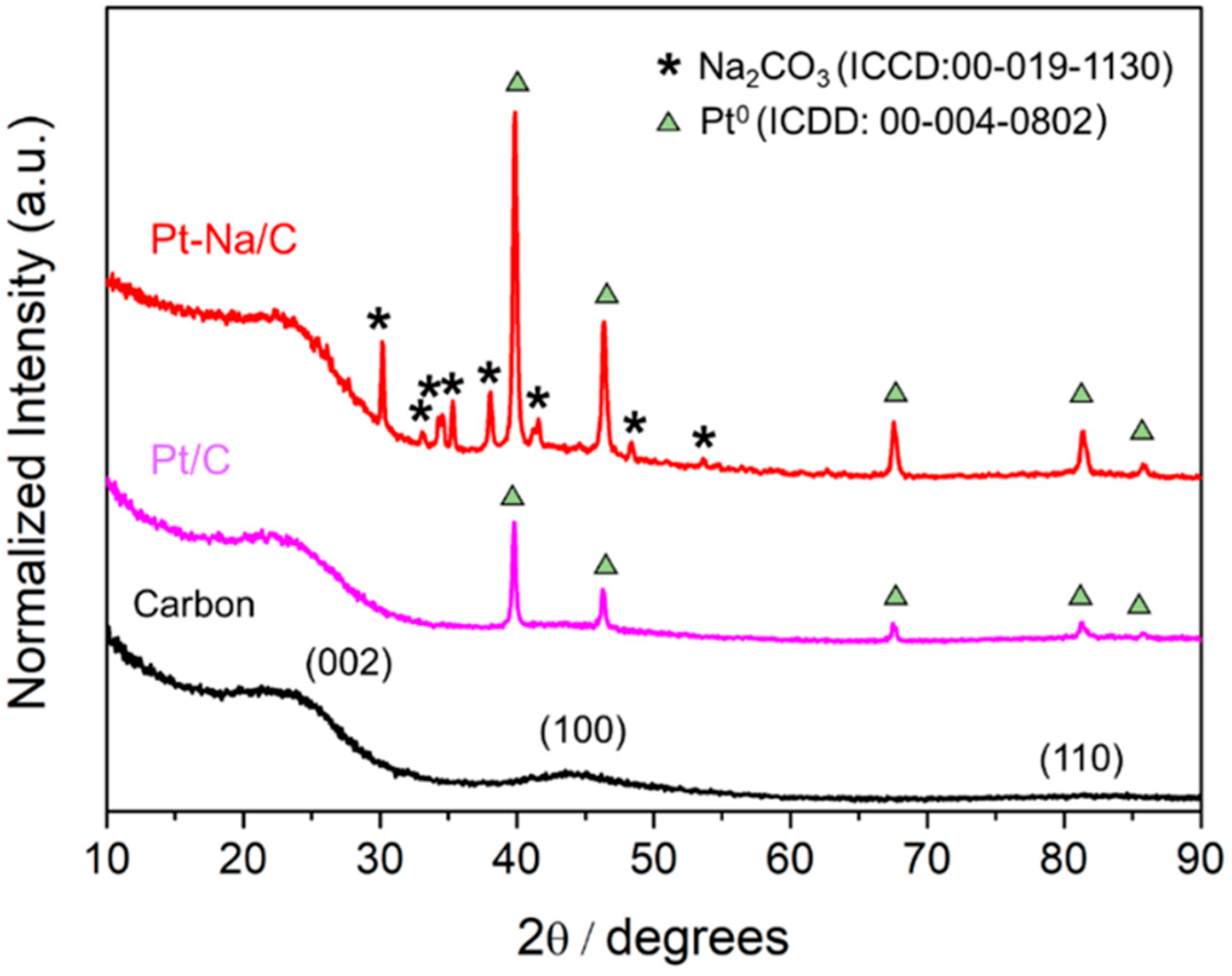
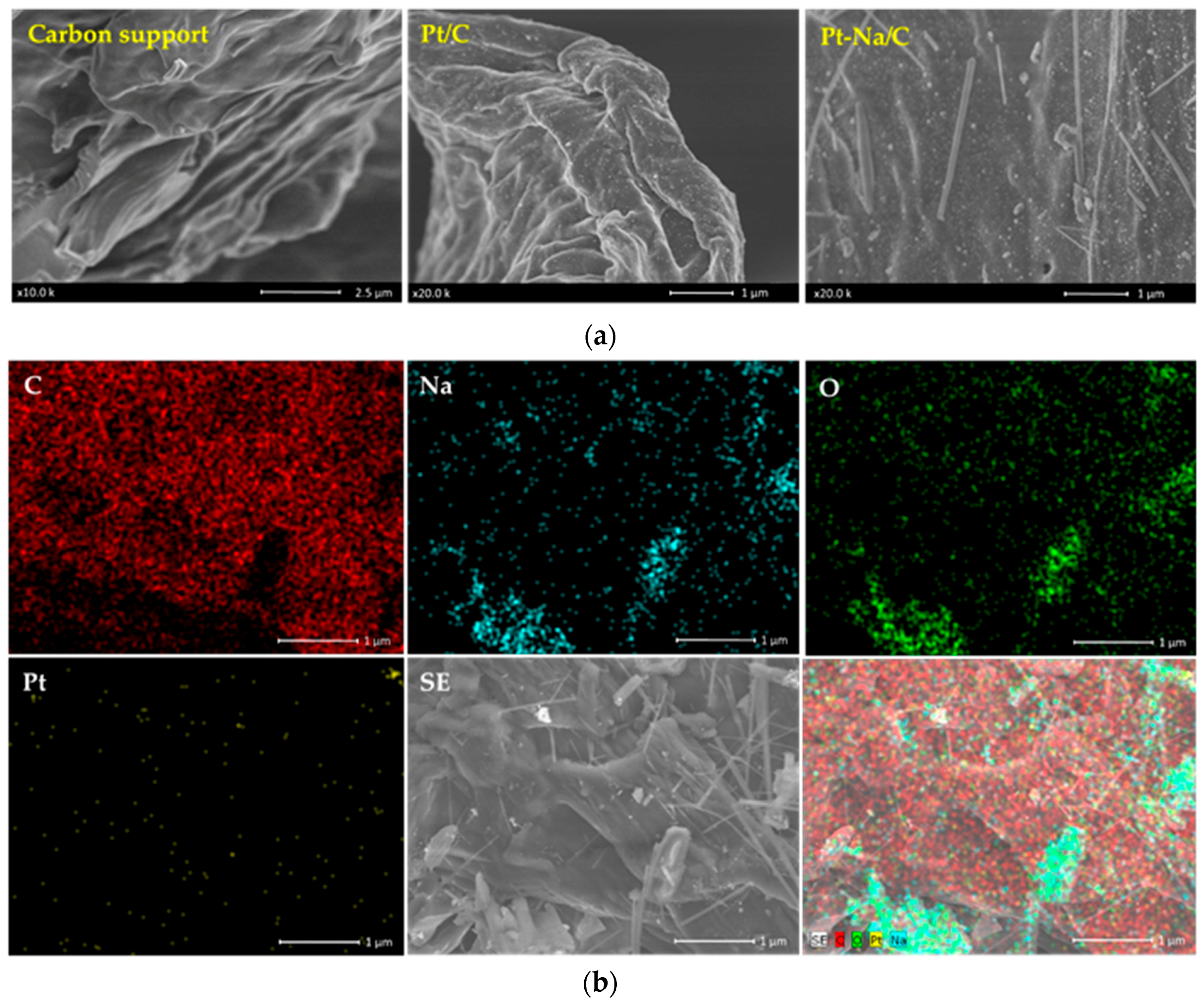
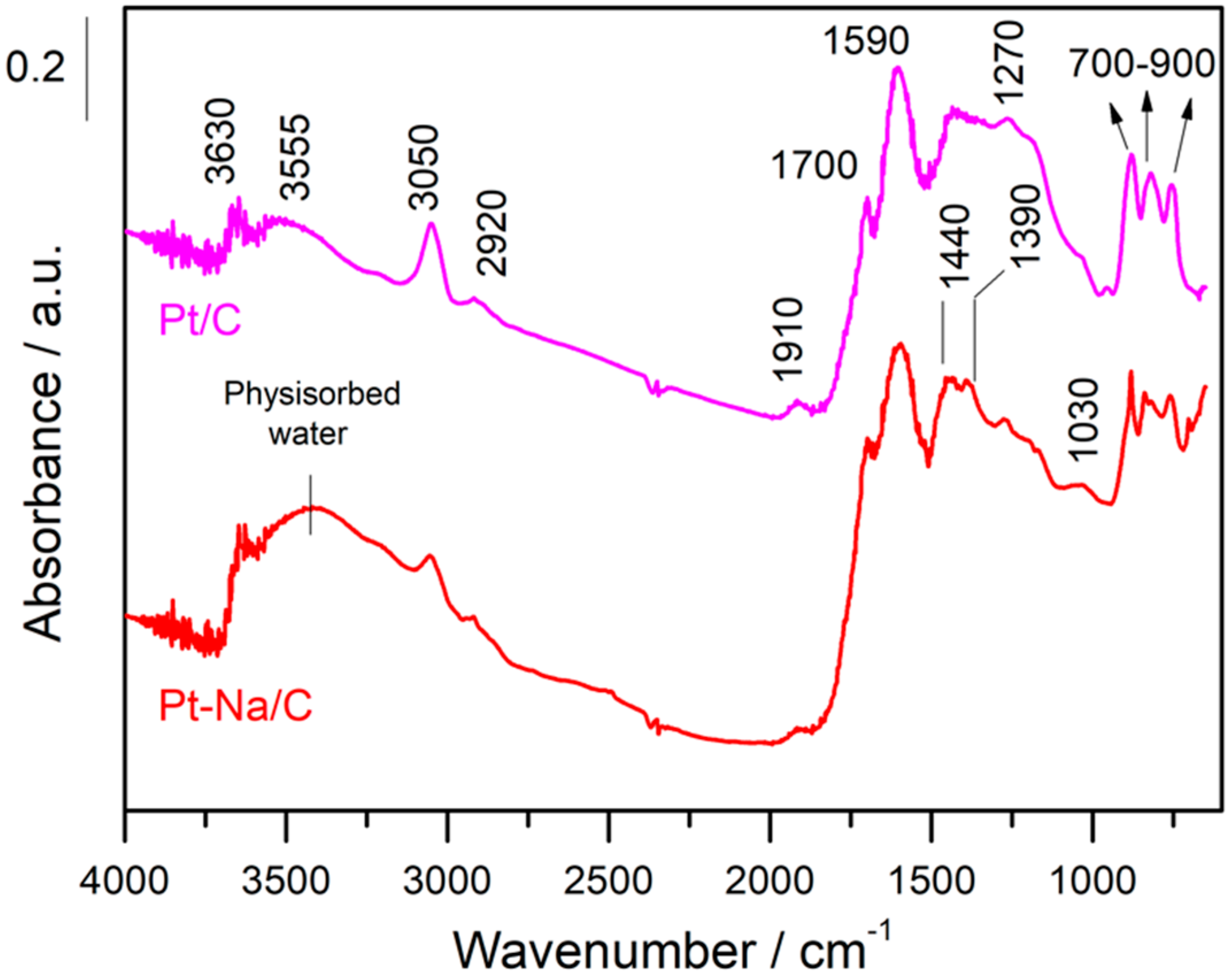
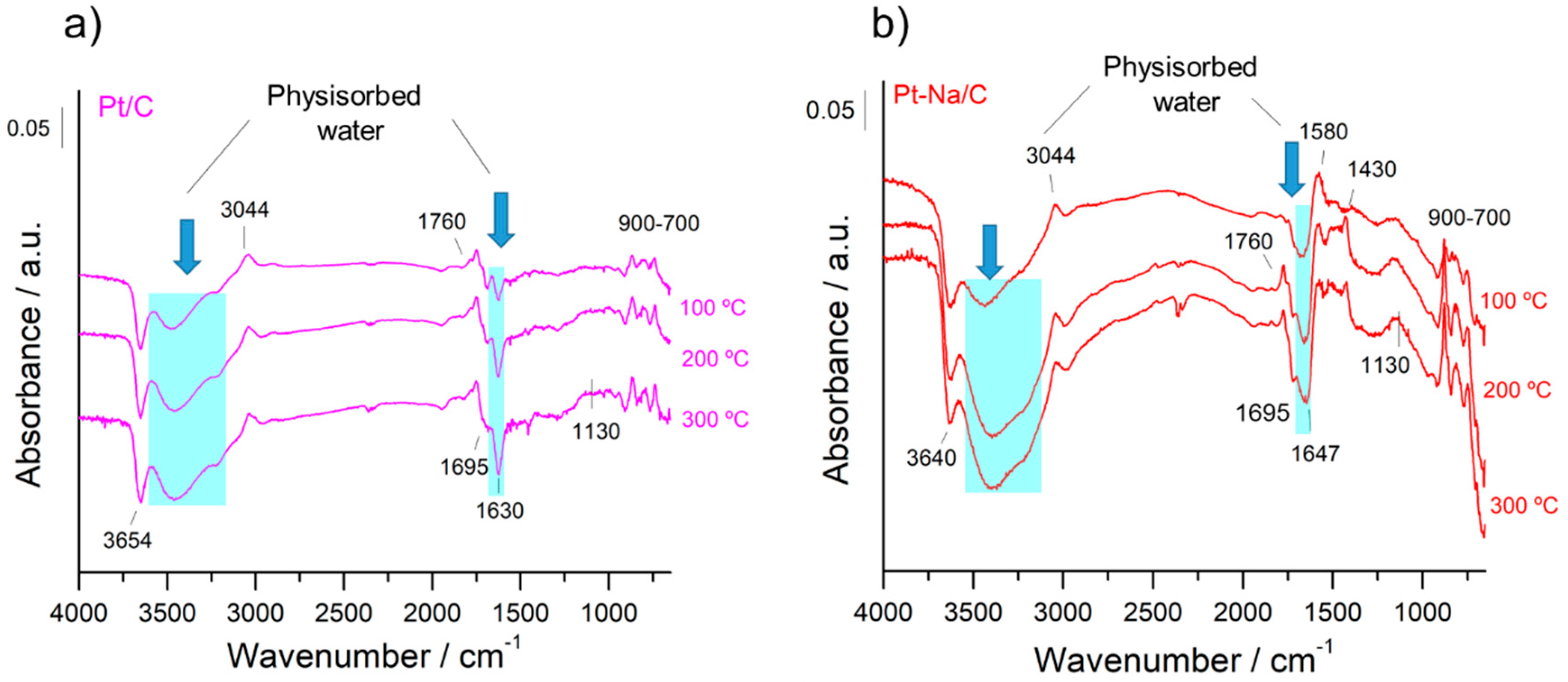
3.1. Physico-Chemical Characterisation
3.2. Operando DRIFTS-MS Catalytic Studies
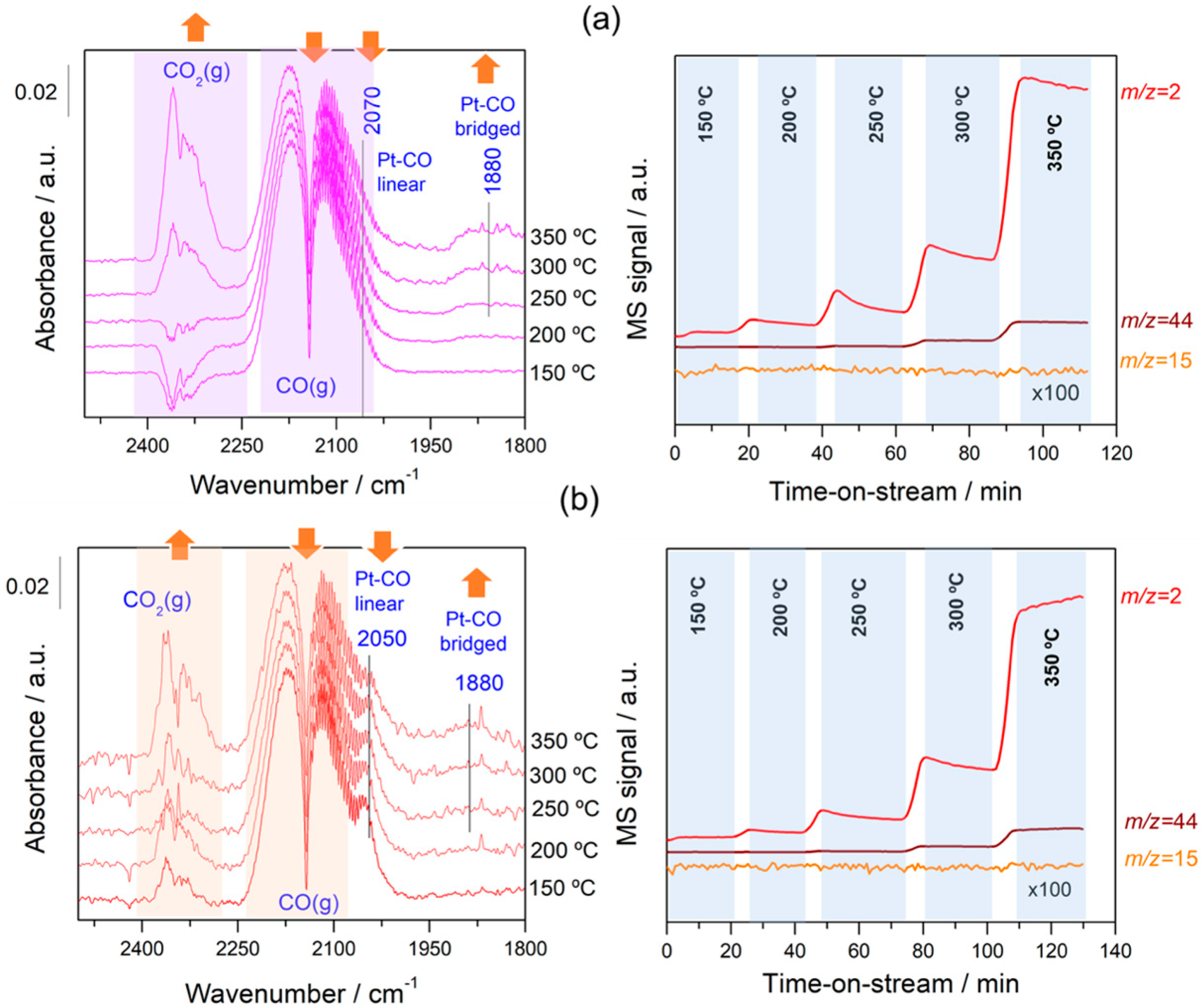
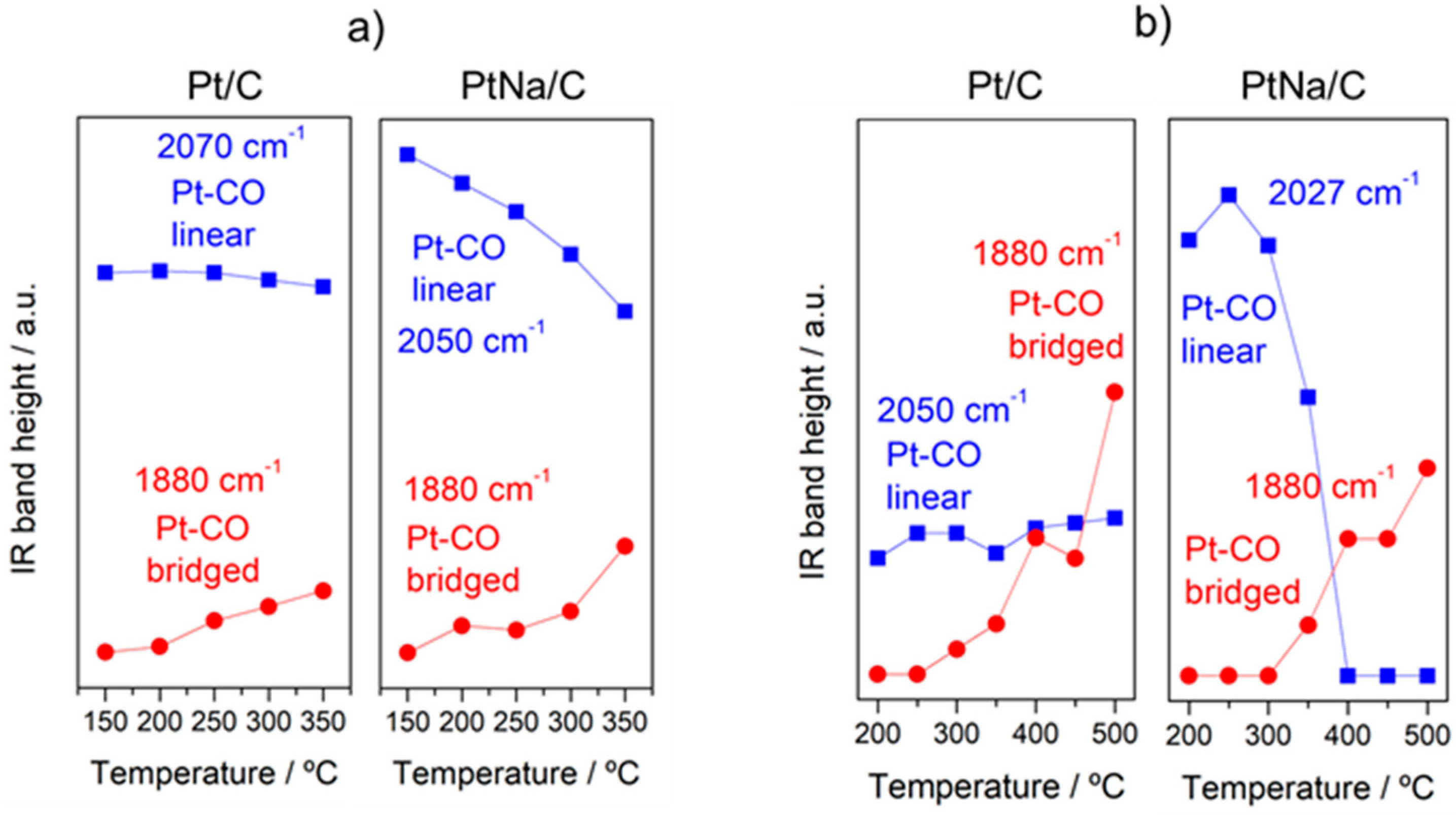
3.2.1. Activation Pre-Treatment
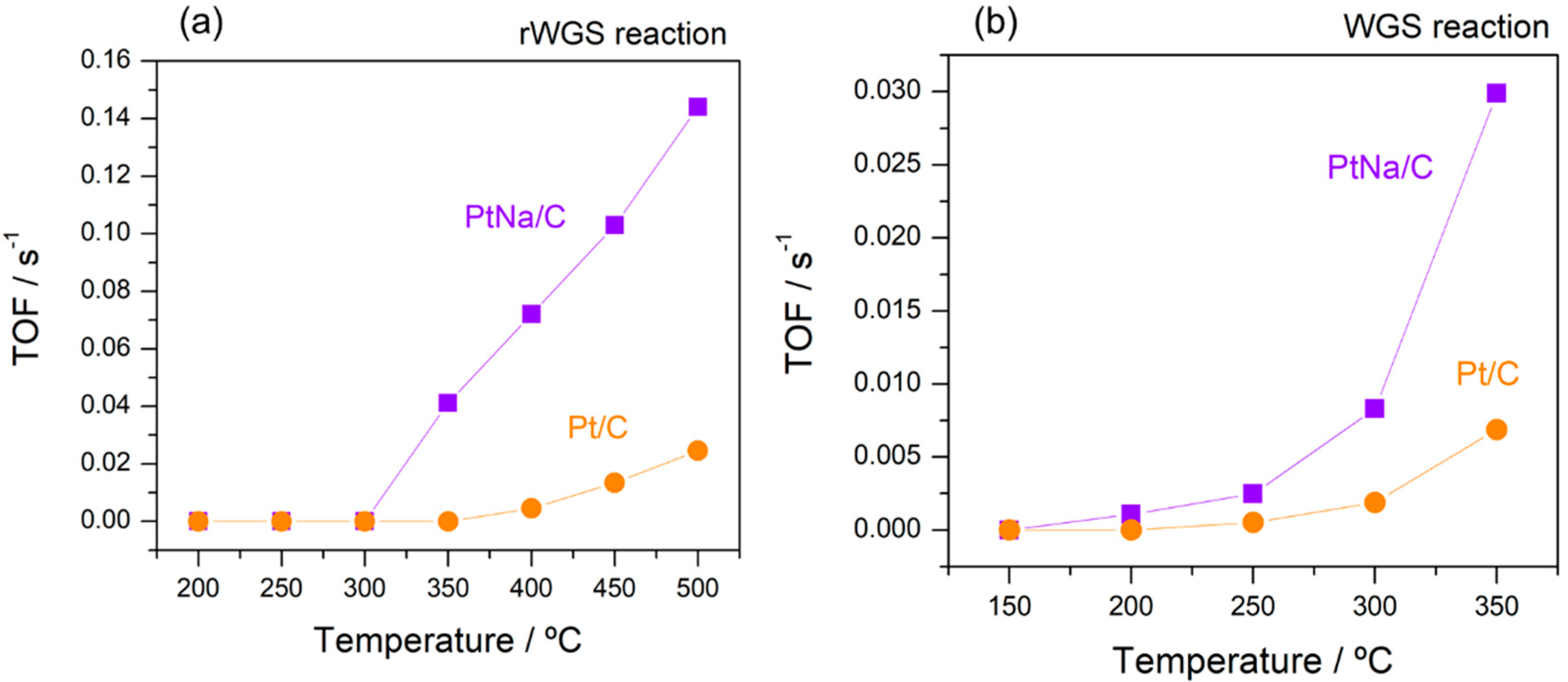
3.2.2. Forward and Reverse WGS Reaction: Promotional Effect of Na
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Takegami, H.; Kasahara, S.; Tanaka, N.; Kamiji, Y.; Iwatsuki, J.; Aita, H.; Kubo, S. R&D status in thermochemical water-splitting hydrogen production iodine-sulfur process at JAEA. Energy Procedia 2017, 131, 113–118. [Google Scholar]
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic steam reforming of biomass tar: Prospects and challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Graciani, J.; Sanz, J.F. Designing a new generation of catalysts: Water gas shift reaction example. Catal. Today 2015, 240, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-J. Sustainability study of hydrogen pathways for fuel cell vehicle applications. Renew. Sustain. Energy Rev. 2013, 19, 220–229. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Jha, A.; Jang, W.-J.; Shim, J.-O.; Rode, C.V.; Jeon, B.-H.; Bae, J.W.; Roh, H.-S. Effect of alkali and alkaline earth metal on Co/CeO2 catalyst for the water-gas shift reaction of waste derived synthesis gas. Appl. Catal. A Gen. 2018, 551, 63–70. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Buitrago-Sierra, R.; Sepúlveda-Escribano, A. CeO2-promoted Ni/activated carbon catalysts for the water-gas shift (WGS) reaction. Int. J. Hydrogen Energy 2014, 39, 17589–17599. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, M.; Lobban, L.L.; Mallinson, R.G. Structural effects of Na promotion for high water gas shift activity on Pt–Na/TiO2. J. Catal. 2011, 278, 123–132. [Google Scholar] [CrossRef]
- Pazmiño, J.H.; Shekhar, M.; Damion Williams, W.; Cem Akatay, M.; Miller, J.T.; Nicholas Delgass, W.; Ribeiro, F.H. Metallic Pt as active sites for the water-gas shift reaction on alkali-promoted supported catalysts. J. Catal. 2012, 286, 279–286. [Google Scholar] [CrossRef]
- González-Castaño, M.; Reina, T.R.; Ivanova, S.; Martínez Tejada, L.M.; Centeno, M.A.; Odriozola, J.A. O2-assisted Water Gas Shift reaction over structured Au and Pt catalysts. Appl. Catal. B Environ. 2016, 185, 337–343. [Google Scholar] [CrossRef]
- González Castaño, M.; Reina, T.R.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Pt vs. Au in water-gas shift reaction. J. Catal. 2014, 314, 1–9. [Google Scholar] [CrossRef]
- Zugic, B.; Zhang, S.; Bell, D.C.; Tao, F.; Flytzani-Stephanopoulos, M. Probing the low-temperature water-gas shift activity of alkali-promoted platinum catalysts stabilized on carbon supports. J. Am. Chem. Soc. 2014, 136, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Pierre, D.; Si, R.; Deng, W.; Ferrin, P.; Nilekar, A.U.; Peng, G.; Herron, J.A.; Bell, D.C.; Saltsburg, H.; et al. Alkali-stabilized Pt-OHx species catalyze low-temperature water-gas shift reactions. Science 2010, 329, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Zugic, B.; Bell, D.C.; Flytzani-Stephanopoulos, M. Activation of carbon-supported platinum catalysts by sodium for the low-temperature water-gas shift reaction. Appl. Catal. B Environ. 2014, 144, 243–251. [Google Scholar] [CrossRef]
- Cybulskis, V.J.; Wang, J.; Pazmiño, J.H.; Ribeiro, F.H.; Delgass, W.N. Isotopic transient studies of sodium promotion of Pt/Al2O3 for the water-gas shift reaction. J. Catal. 2016, 339, 163–172. [Google Scholar] [CrossRef]
- Evin, H.N.; Jacobs, G.; Ruiz-Martinez, J.; Thomas, G.A.; Davis, B.H. Low temperature water-gas shift: Alkali doping to facilitate formate C–H bond cleaving over Pt/Ceria catalysts—An optimization problem. Catal. Lett. 2008, 120, 166–178. [Google Scholar] [CrossRef]
- Ding, K.; Gulec, A.; Johnson, A.M.; Schweitzer, N.M.; Stucky, G.D.; Marks, L.D.; Stair, P.C. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 2015, 350, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active nonmetallic au and pt species on Ceria-Based Water-Gas shift catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Gil, S.; Romero, A.; Lucas, A.d.; Sánchez, P.; Dorado, F.; Osa, A.R.d.l.; García-Vargas, J.M.; Valverde, J.L. Nano-scale Au supported on carbon materials for the low temperature water gas shift (WGS) reaction. Catalysts 2011, 1, 155–174. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Biores. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Biores. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Fanning, P.E.; Vannice, M.A. A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon 1993, 31, 721–730. [Google Scholar] [CrossRef]
- Muckenhuber, H.; Grothe, H. A DRIFTS study of the heterogeneous reaction of NO2 with carbonaceous materials at elevated temperature. Carbon 2007, 45, 321–329. [Google Scholar] [CrossRef]
- Markus, H.; Mäki-Arvela, P.; Kumar, N.; Kul’kova, N.V.; Eklund, P.; Sjöholm, R.; Holmbom, B.; Salmi, T.; Murzin, D.Y. Hydrogenolysis of hydroxymatairesinol over carbon-supported palladium catalysts. Catal. Lett. 2005, 103, 125–131. [Google Scholar] [CrossRef]
- Wang, C.; Yi, G.; Lin, H.; Yuan, Y. Na+-intercalated carbon nanotubes-supported platinum nanoparticles as new highly effective catalysts for preferential CO oxidation in H2-rich stream. Int. J. Hydrogen Energy 2012, 37, 14124–14132. [Google Scholar] [CrossRef]
- Fraga, M.A.; Jordão, E.; Mendes, M.J.; Freitas, M.M.A.; Faria, J.L.; Figueiredo, J.L. Properties of Carbon-Supported Platinum Catalysts: Role of Carbon Surface Sites. J. Catal. 2002, 209, 355–364. [Google Scholar] [CrossRef]
- Takagi, H.; Maruyama, K.; Yoshizawa, N.; Yamada, Y.; Sato, Y. XRD analysis of carbon stacking structure in coal during heat treatment. Fuel 2004, 83, 2427–2433. [Google Scholar] [CrossRef] [Green Version]
- Iwashita, N.; Park, C.R.; Fujimoto, H.; Shiraishi, M.; Inagaki, M. Specification for a standard procedure of X-ray diffraction measurements on carbon materials. Carbon 2004, 42, 701–714. [Google Scholar] [CrossRef]
- Santos, J.L.; Alda-Onggar, M.; Fedorov, V.; Peurla, M.; Eränen, K.; Mäki-Arvela, P.; Centeno, M.Á.; Murzin, D.Y. Hydrodeoxygenation of vanillin over carbon supported metal catalysts. Appl. Catal. A Gen. 2018, 561, 137–149. [Google Scholar] [CrossRef]
- Buitrago, R.; Ruiz-Martínez, J.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. Water gas shift reaction on carbon-supported Pt catalysts promoted by CeO2. Catal. Today 2012, 180, 19–24. [Google Scholar] [CrossRef]
- Ball, M.C.; Snelling, C.M.; Strachan, A.N.; Strachan, R.M. Thermal decomposition of solid sodium sesquicarbonate, Na2CO3·NaHCO3·2H2O. J. Chem. Soc. Faraday Trans. 1992, 88, 631–636. [Google Scholar] [CrossRef]
- Lazzarini, A.; Piovano, A.; Pellegrini, R.; Agostini, G.; Rudić, S.; Lamberti, C.; Groppo, E. Graphitization of activated carbons: A molecular-level Investigation by INS, DRIFT, XRD and Raman techniques. Phys. Procedia 2016, 85, 20–26. [Google Scholar] [CrossRef]
- Caglayan, B.S.; Soykal, İ.I.; Aksoylu, A.E. Preferential oxidation of CO over Pt–Sn/AC catalyst: Adsorption, performance and DRIFTS studies. Appl. Catal. B Environ. 2011, 106, 540–549. [Google Scholar] [CrossRef]
- Mul, G.; Kapteijn, F.; Moulijn, J.A. A DRIFTS study of the interaction of alkali metal oxides with carbonaceous surfaces. Carbon 1999, 37, 401–410. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; Wiley & Sons: West Sussex, UK, 2005; ISBN 978-0-470-85428-0. [Google Scholar]
- Vogt, C.; Groeneveld, E.; Kamsma, G.; Nachtegaal, M.; Lu, L.; Kiely, C.J.; Berben, P.H.; Meirer, F.; Weckhuysen, B.M. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 2018, 1, 127–134. [Google Scholar] [CrossRef]
- Laletina, S.S.; Mamatkulov, M.; Shor, E.A.; Kaichev, V.V.; Genest, A.; Yudanov, I.V.; Rösch, N. Size-Dependence of the adsorption energy of co on pt nanoparticles: Tracing two intersecting trends by DFT calculations. J. Phys. Chem. C 2017, 121, 17371–17377. [Google Scholar] [CrossRef]
- Garfunkel, E.L.; Crowell, J.E.; Somorjai, G.A. The strong influence of potassium on the adsorption of carbon monoxide on platinum surfaces: A TDS and HREELS study. J. Phys. Chem. 1982, 86, 310–313. [Google Scholar] [CrossRef]
- Tong, Y.Y.; Rice, C.; Wieckowski, A.; Oldfield, E. A detailed NMR-Based model for CO on Pt catalysts in an electrochemical environment: shifts, relaxation, Back-Bonding, and the Fermi-Level local density of states. J. Am. Chem. Soc. 2000, 122, 1123–1129. [Google Scholar] [CrossRef]
- Rice, C.; Tong, Y.Y.; Oldfield, E.; Wieckowski, A.; Hahn, F.; Gloaguen, F.; Léger, J.-M.; Lamy, C. In situ infrared study of carbon monoxide adsorbed onto commercial Fuel-Cell-Grade Carbon-Supported platinum nanoparticles: Correlation with 13C NMR results. J. Phys. Chem. B 2000, 104, 5803–5807. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 methanation: The effect of catalysts and reaction conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Li, D.; Ichikuni, N.; Shimazu, S.; Uematsu, T. Catalytic properties of sprayed Ru/Al2O3 and promoter effects of alkali metals in CO2 hydrogenation. Appl. Catal. A Gen. 1998, 172, 351–358. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, T.W.; Lee, S.H.; Park, E.D. Effects of Na content in Na/Ni/SiO2 and Na/Ni/CeO2 catalysts for CO and CO2 methanation. Catal. Today 2018, 303, 159–167. [Google Scholar] [CrossRef]
- Kapteijn, F.; Moulijn, J.A. Methanation of CO over alkali metal-carbon catalysts. J. Chem. Soc., Chem. Commun. 1984, 5, 278–279. [Google Scholar] [CrossRef]
- García-Moncada, N.; Bobadilla, L.F.; Poyato, R.; López-Cartes, C.; Romero-Sarria, F.; Centeno, M.Á.; Odriozola, J.A. A direct in situ observation of water-enhanced proton conductivity of Eu-doped ZrO2: Effect on WGS reaction. Appl. Catal. B Environ. 2018, 231, 343–356. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Su, X.; Chen, X.; Huang, Y.; Chen, X.; Delgado, J.J.; Zhang, T. Promoting role of potassium in the reverse water gas shift reaction on Pt/mullite catalyst. Catal. Today 2017, 281, 319–326. [Google Scholar] [CrossRef]



| Sample | Metal Loading (ICP, wt. %) | Lc Parameter (XRD, Å) | Average Particle Size (XRD, nm) | Average Particle Size (TEM, nm) | Calculated Dispersion (%) | IEP (pH) | |
|---|---|---|---|---|---|---|---|
| Pt | Na | ||||||
| Carbon | - | - | 16 | - | - | - | 5.42 |
| Pt/C | 1.0 | - | 16 | 12 | 12 | 12.4 | 6.01 |
| Pt–Na/C | 1.0 | 4.8 | 20 | 40 | 36 | 3.8 | 9.96 |
| Sample | SBET (m2 g−1) | Micropore Area (m2 g−1) | External Area (m2 g−1) | Microporosity (%) | VBHJ (cm3 g−1) | Dmean (4V/A) (nm) |
|---|---|---|---|---|---|---|
| Carbon | 409 | 326 | 83 | 20.3 | 0.036 | 1.97 |
| Pt/C | 366 | 283 | 83 | 22.7 | 0.039 | 2.00 |
| Pt–Na/C | 337 | 263 | 74 | 22 | 0.036 | 1.99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.L.; Bobadilla, L.F.; Centeno, M.A.; Odriozola, J.A. Operando DRIFTS-MS Study of WGS and rWGS Reaction on Biochar-Based Pt Catalysts: The Promotional Effect of Na. C 2018, 4, 47. https://doi.org/10.3390/c4030047
Santos JL, Bobadilla LF, Centeno MA, Odriozola JA. Operando DRIFTS-MS Study of WGS and rWGS Reaction on Biochar-Based Pt Catalysts: The Promotional Effect of Na. C. 2018; 4(3):47. https://doi.org/10.3390/c4030047
Chicago/Turabian StyleSantos, José L., Luis F. Bobadilla, Miguel A. Centeno, and José A. Odriozola. 2018. "Operando DRIFTS-MS Study of WGS and rWGS Reaction on Biochar-Based Pt Catalysts: The Promotional Effect of Na" C 4, no. 3: 47. https://doi.org/10.3390/c4030047