Simple Process for Sidewall Modification of Multi-Walled Carbon Nanotubes with Polymer Side Chain Radicals Generated by Ultraviolet-Induced C–Cl Bond Dissociation of Polystyrene Derivatives
Abstract
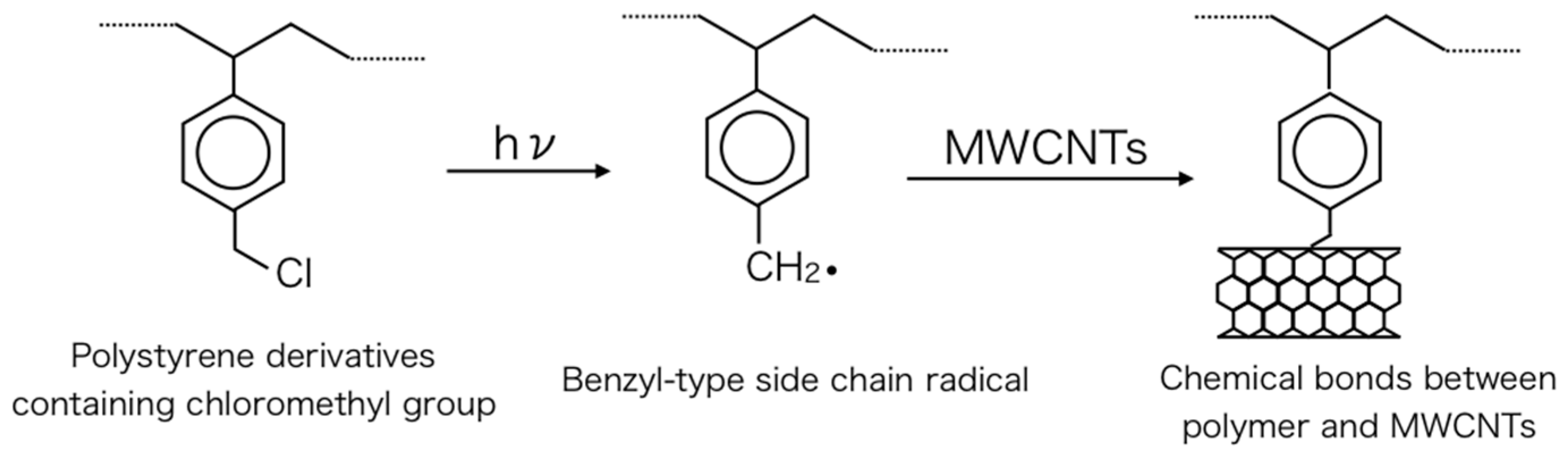
:1. Introduction
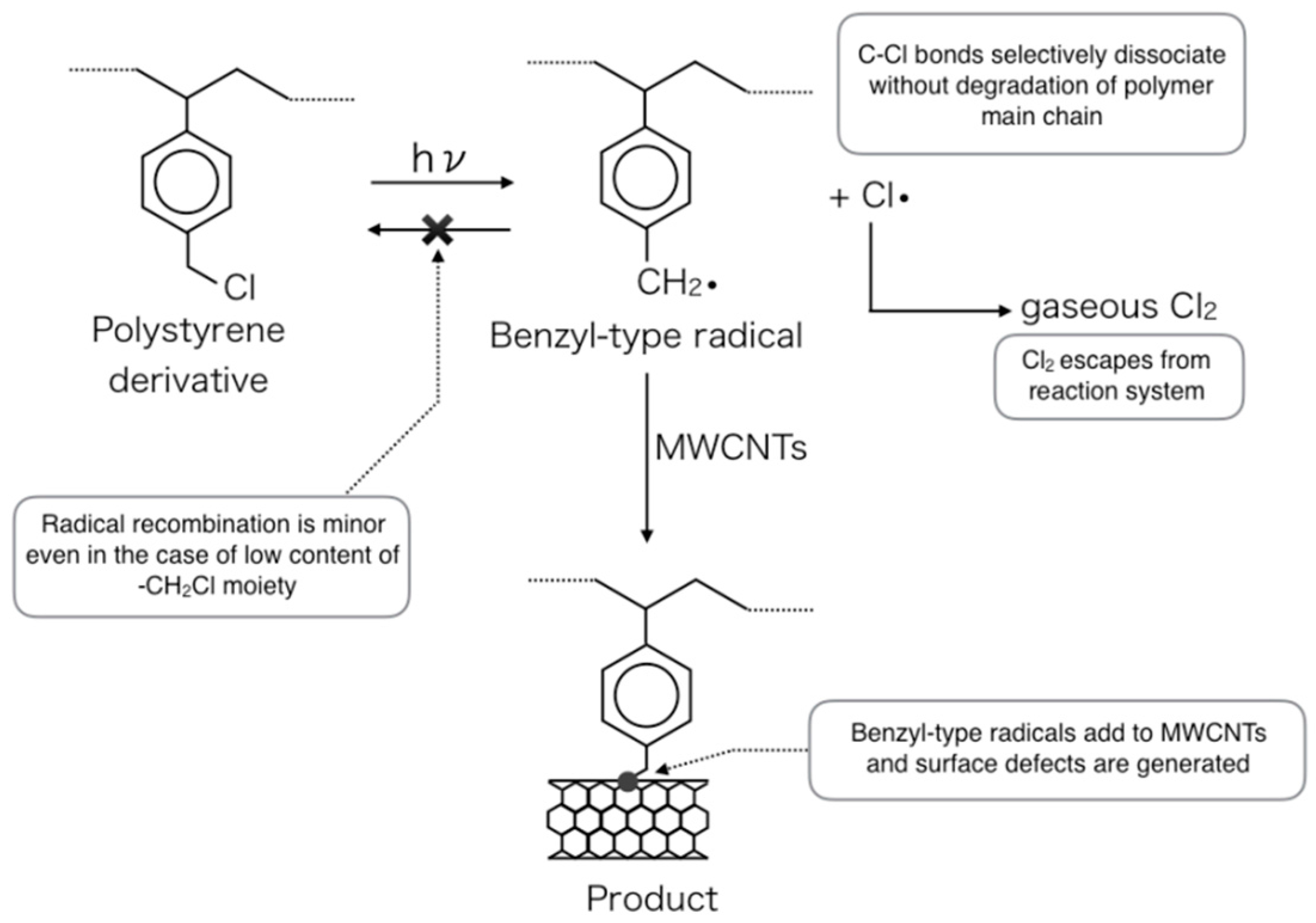
2. Results and Discussion
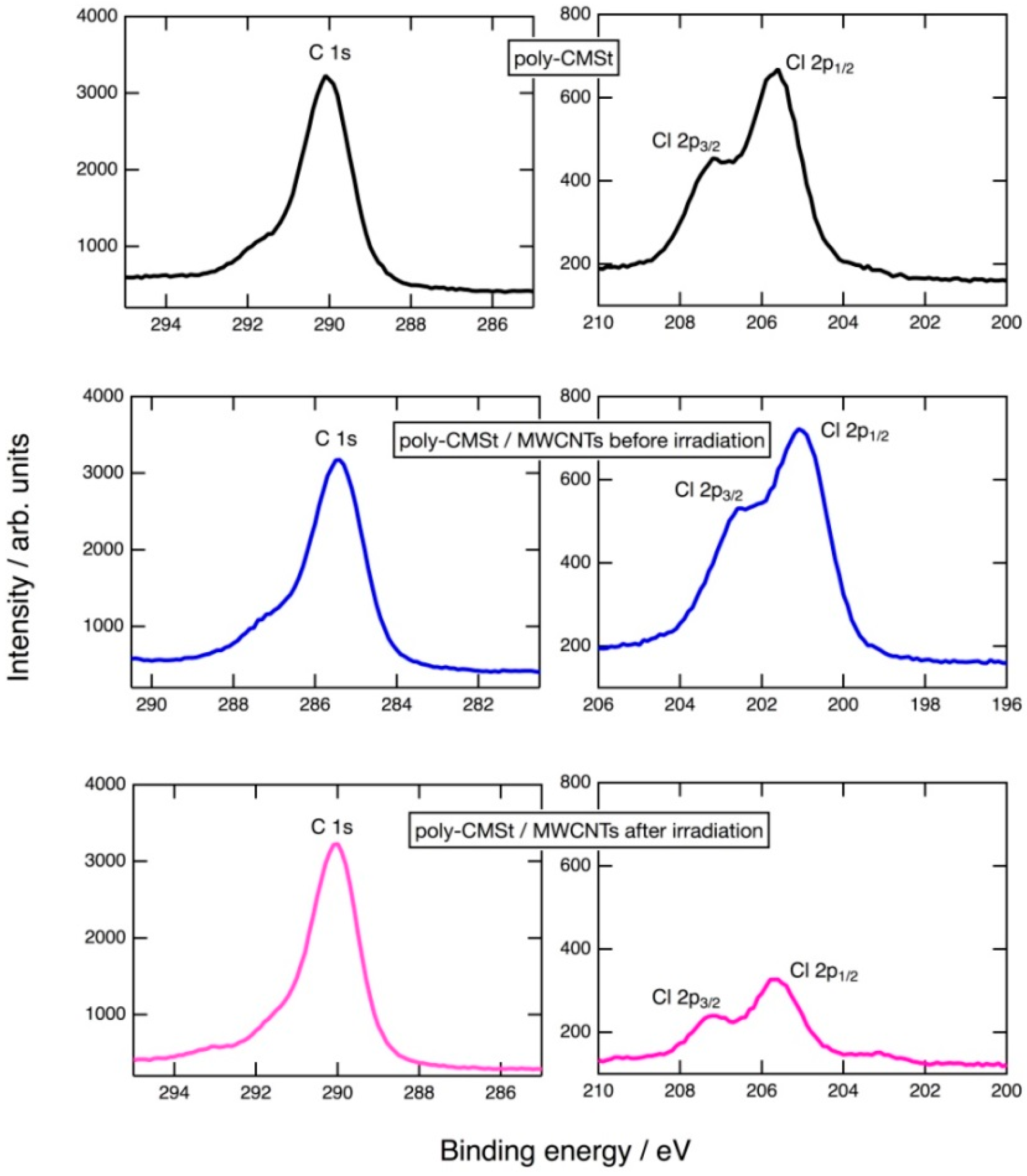
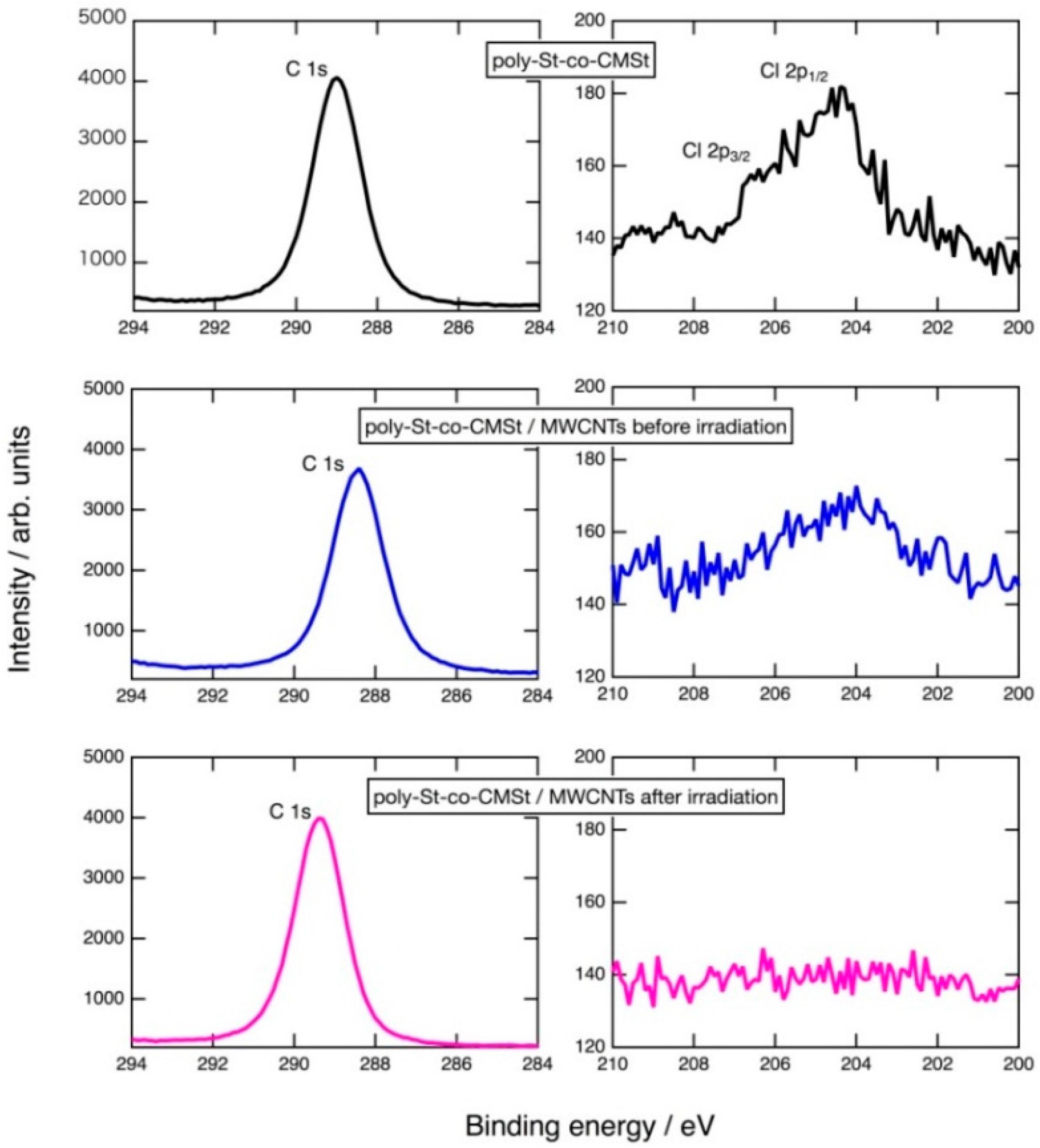
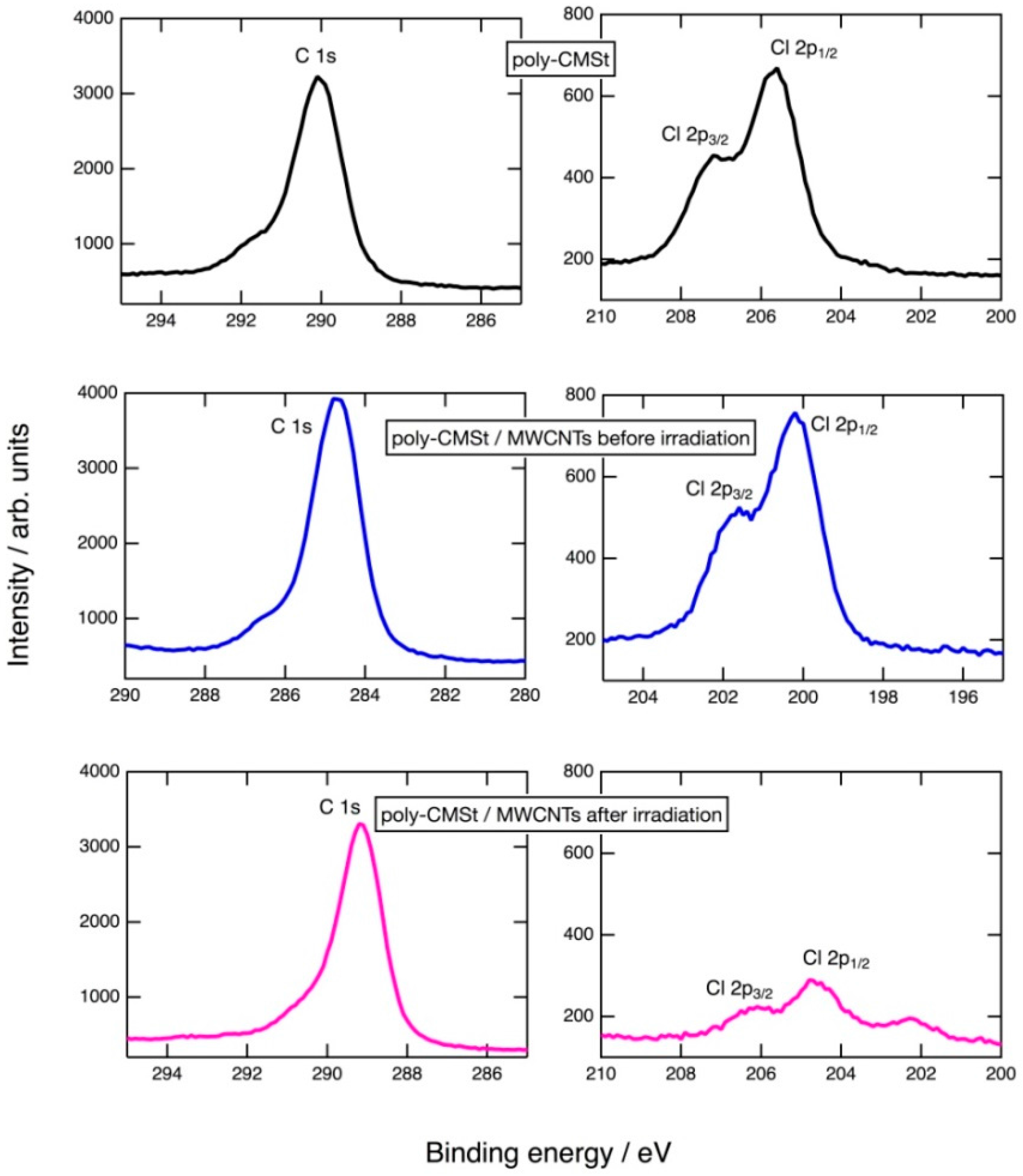
2.1. Photoinduced C–Cl Bond Dissociation of Chloromethyl Group Observed by XPS
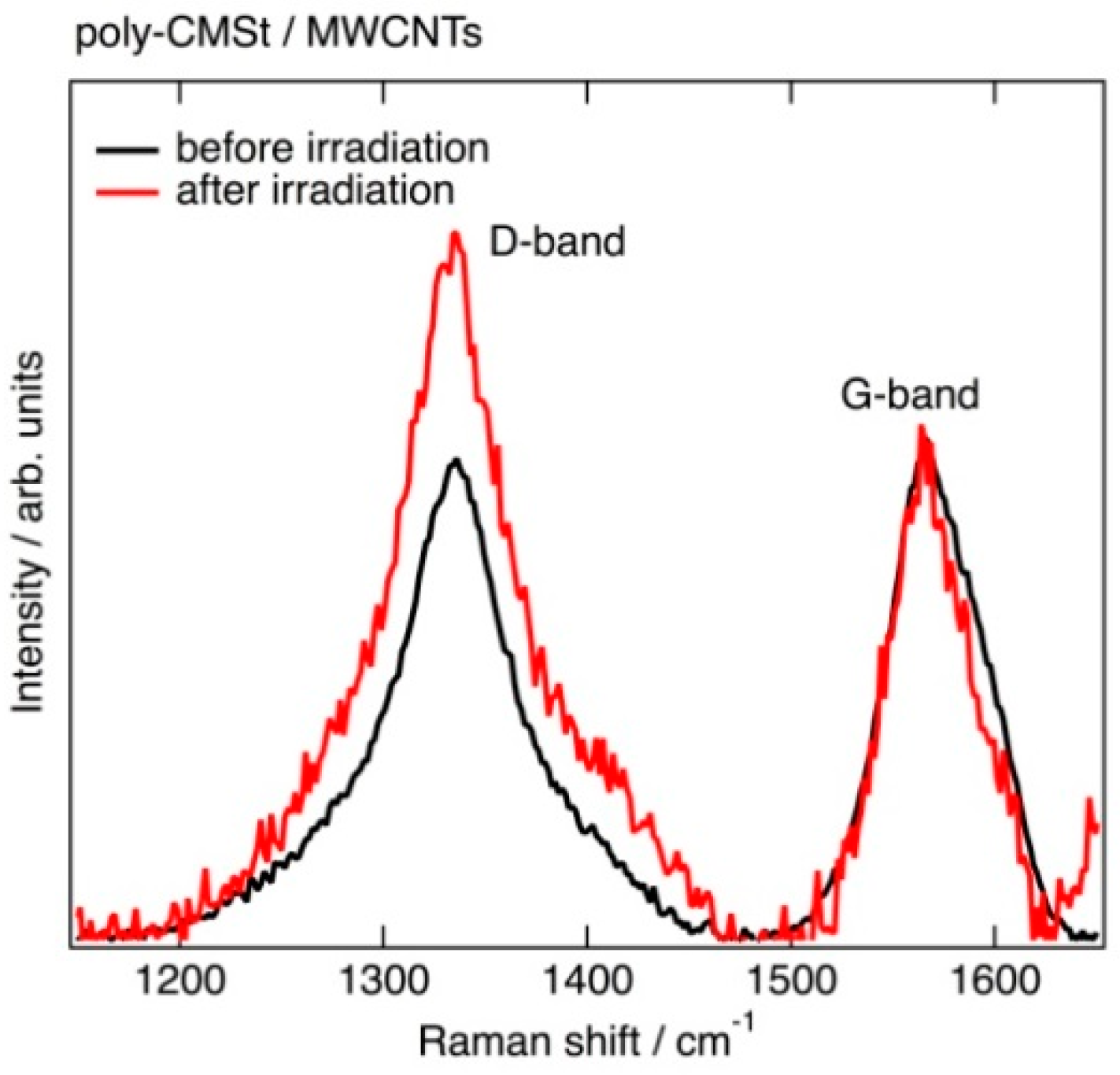
2.2. Structural Change of MWCNTs Observed by Raman Spectroscopy
2.3. Influence of Hydroxylammonium Chloride Added as Dispersant on the Reaction Efficiency
3. Materials and Methods
3.1. Materials
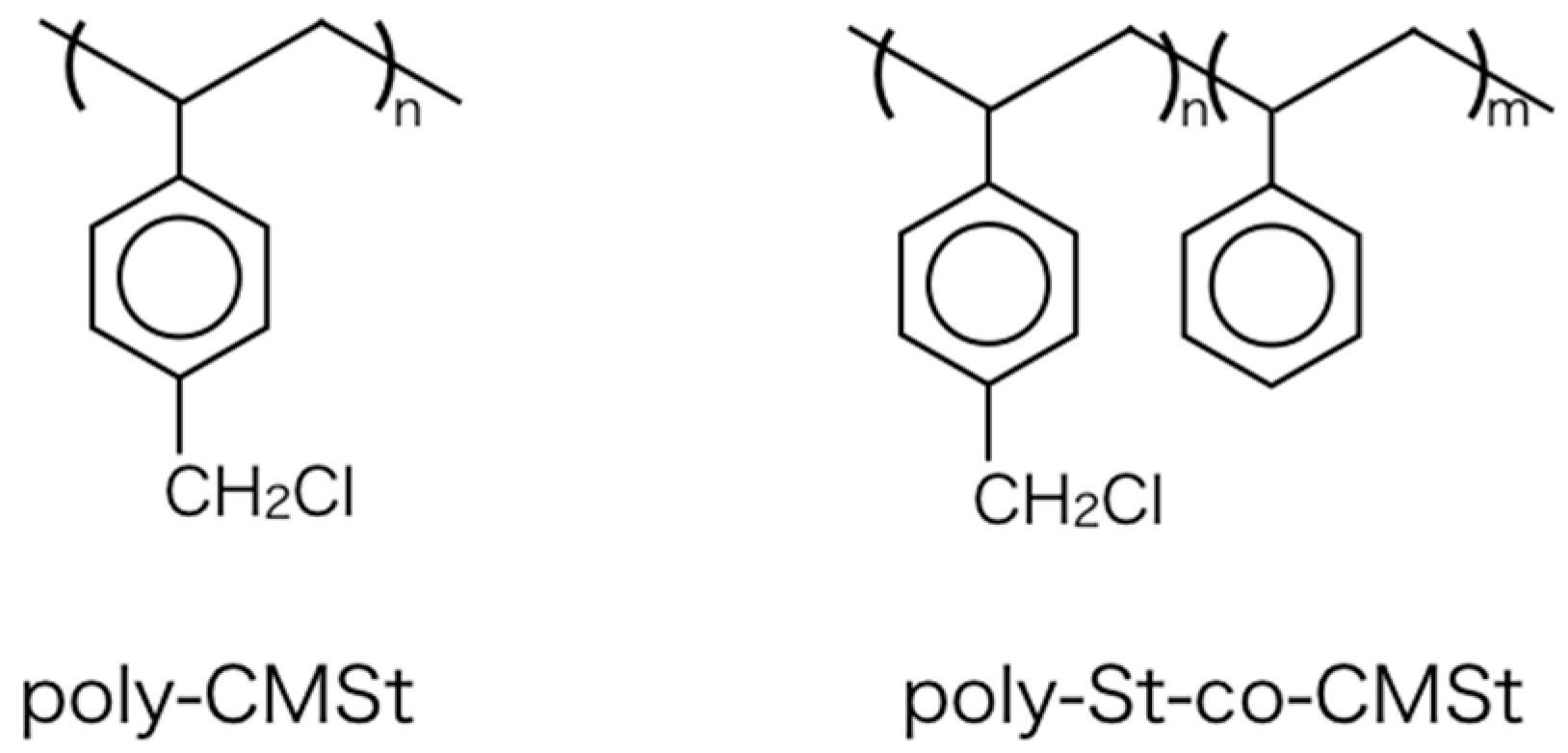
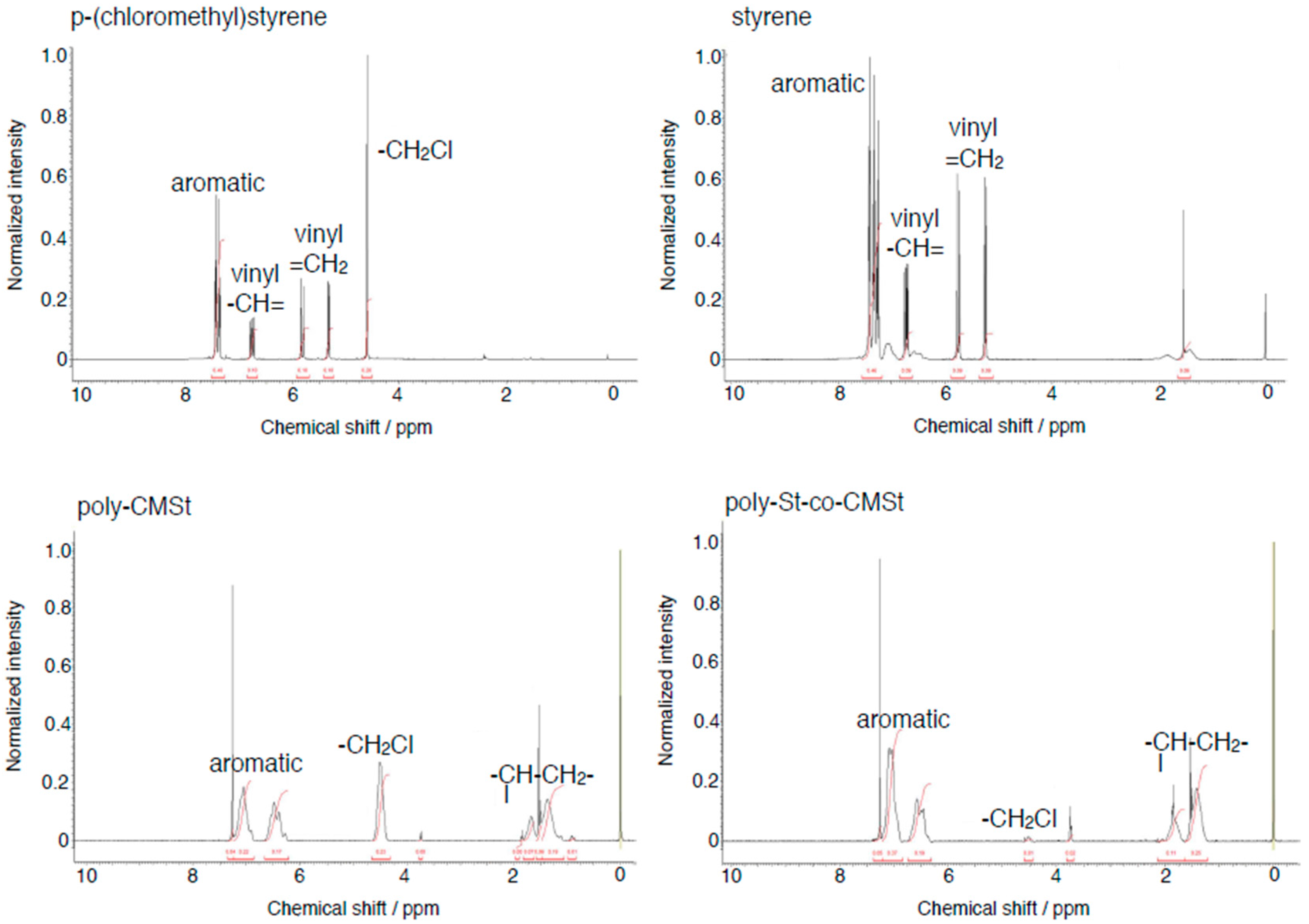
3.2. Preparation of Poly(4-shloromethyl)styrene and Styrene/(4-chloromethyl)styrene Copolymer
3.3. Irradiation of Polymer/MWCNT Mixtures with UV Light
3.4. Preparation of Samples for Characterization and Spectroscopic Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Kaempgen, M.; Duesberg, G.S.; Roth, S. Transparent carbon nanotube coatings. App. Surf. Sci. 2005, 252, 425–429. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, C.; Zhang, S.; Yang, M.; Wang, Z. Low Percolation thresholds of electrical conductivity and rheology in poly(ethylene terephthalate) through the networks of multi-walled carbon nanotubes. Polymer 2006, 47, 480–488. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, M.H.; Lee, J.H.; Lee, T.W.; Kim, K.J.; Lee, Y.K.; Kim, T.; Choi, H.R.; Koo, J.C.; Nam, J.D. Transparent flexible conductor of poly(methyl methacrylate) containing highly-dispersed multiwalled carbon nanotube. Org. Electron. 2008, 9, 1–13. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.; Chang, J.Y.; Theato, P. Preparation of Transparent Conductive Multilayered Films Using Active Pentafluorophenyl Ester Modified Multiwalled Carbon Nanotubes. Langmuir 2008, 24, 10467–10473. [Google Scholar] [CrossRef] [PubMed]
- Logakis, E.; Pissis, P.; Pospiech, D.; Korwitz, A.; Krause, B.; Reuter, U.; Pötschke, P. Low electrical percolation threshold in poly(ethylene terephthalate)/multi-walled carbon nanotube nanocomposites. Eur. Polym. J. 2010, 46, 928–936. [Google Scholar] [CrossRef]
- Logakis, E.; Pandis, C.; Pissis, P.; Pionteck, J.; Pöschke, P. Highly conducting poly(methyl methacrylate)/carbon nanotube composites: Investigation on their thermal, dynamic-mechanical, electrical and dielectric properties. Comp. Sci. Technol. 2011, 71, 854–862. [Google Scholar] [CrossRef]
- Zhao, L.; Kiu, W.L.; Zhang, L.D.; Yao, J.S.; Xu, W.H.; Wang, X.Q.; Wu, Y.Z. Fabrication of superhydrophobic and conductive surface based on carbon nanotubes. Coll. Surf. A 2013, 423, 69–76. [Google Scholar] [CrossRef]
- Lin, W.Y.; Shih, Y.F.; Lin, C.H.; Lee, C.C.; Yu, Y.H. The preparation of multi-walled carbon nanotube/poly (lactic acid) composites with excellent conductivity. J. Taiwan Inst. Chem. Eng. 2013, 44, 489–496. [Google Scholar] [CrossRef]
- Isaji, S.; Bin, Y.; Matsuo, M. Electrical conductivity and self-temperature-control heating properties of carbon nanotubes filled polyethylene films. Polymer 2009, 50, 1046–1053. [Google Scholar] [CrossRef]
- Wu, Z.P.; Wang, J.N. Preparation of large-area double-walled carbon nanotube films and application as film heater. Physica E 2009, 42, 77–81. [Google Scholar] [CrossRef]
- Kang, T.J.; Kim, T.; Seo, S.M.; Park, Y.J.; Kim, Y.H. Thickness-dependent thermal resistance of a transparent glass heater with a single-walled carbon nanotube coating. Carbon 2011, 49, 1087–1093. [Google Scholar] [CrossRef]
- Jung, D.; Kim, D.; Lee, K.H.; Overzet, L.J.; Lee, G.S. Transparent film heaters using multi-walled carbon nanotube sheets. Sens. Act. A 2013, 199, 176–180. [Google Scholar] [CrossRef]
- Janas, D.; Koziol, K.K. Rapid electrothermal response of high-temperature carbon nanotube film heaters. Carbon 2013, 59, 457–463. [Google Scholar] [CrossRef]
- Kim, D.; Zhu, L.; Jeong, D.J.; Chun, K.; Bang, Y.Y.; Kim, S.R.; Kim, J.H.; Oh, S.K. Transparent flexible heater based on hybrid of carbon nanotubes and silver nanowires. Carbon 2013, 63, 530–536. [Google Scholar] [CrossRef]
- Liu, P. Modifications of carbon nanotubes with polymers. Eur. Polym. Sci. 2005, 41, 2693–2703. [Google Scholar] [CrossRef]
- Wu, H.X.; Tong, R.; Qiu, X.Q.; Yang, H.F.; Lin, Y.H.; Cai, R.F.; Qian, S.X. Functionalization of multiwalled carbon nanotubes with polystyrene under atom transfer radical polymerization conditions. Carbon 2007, 45, 152–159. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Composite A 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube-polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functional carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Khan, M.U.; Gomes, V.G.; Altawawneh, I.S. Synthesizing polystyrene/carbon nanotube composites by emulsion polymerization with non-covalent and covalent functionalization. Carbon 2010, 48, 2925–2933. [Google Scholar] [CrossRef]
- Hu, H.; Hui, K.N.; Hui, K.S.; Lee, S.K.; Zhou, W. Facile and green method for polystyrene grafted multi-walled carbon nanotubes and their electroresponse. Coll. Surf. A 2012, 396, 177–181. [Google Scholar] [CrossRef]
- Porter, G.; Wright, F.J. Primary photochemical processes in aromatic molecules. Part 3. Absorption spectra of benzyl, anilino, phenoxy and related free radicals. Trans. Faraday Soc. 1955, 51, 1469–1474. [Google Scholar] [CrossRef]
- Peng, H.; Alemany, L.B.; Margrave, J.L.; Khabashesku, V.N. Sidewall Carboxylic Acid Functionalization of Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2003, 125, 15174–15182. [Google Scholar] [CrossRef] [PubMed]
- Tsubokawa, N. Preparation and Properties of Polymer-grafted Carbon Nanotubes and Nanofibers. Polym. J. 2005, 37, 637–655. [Google Scholar] [CrossRef]
- Fenoglio, I.; Tomatis, M.; Lison, D.; Muller, J.; Fonseca, A.; Nagy, J.B.; Fubini, B. Reactivity of carbon nanotubes: Free radical generation or scavenging activity? Free Radical Biol. Med. 2006, 40, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Galano, A. Carbon Nanotubes as Free-Radical Scavengers. J. Phys. Chem. C 2008, 112, 8922–8927. [Google Scholar] [CrossRef]
- Park, J.J.; Park, D.M.; York, J.H.; Yu, W.R.; Lee, J. Functionalization of multi-walled carbon nanotubes by free radical graft polymerization initiated from photoinduced surface groups. Carbon 2010, 48, 2899–2905. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, M.; Hou, Q.; Tu, X.; Wu, X. Active Poly(4-chloromethyl styrene)-Functionalized Multiwalled Carbon Nanotubes. Macromol. Chem. Phys. 2013, 214, 1829–1835. [Google Scholar] [CrossRef]
- Sabba, Y.; Thomas, E.L. High-Concentration Dispersion of Single-Wall Carbon Nanotubes. Macromolecules 2004, 37, 4815–4820. [Google Scholar] [CrossRef]
- Collinson, E.; Swallow, A.J. The Radiation Chemistry of Organic Substances. Chem. Rev. 1956, 56, 471–568. [Google Scholar] [CrossRef]
- Saito, R.; Hoffmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman spectroscopy of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, T.; Baba, T.; Abe, S. Simple Process for Sidewall Modification of Multi-Walled Carbon Nanotubes with Polymer Side Chain Radicals Generated by Ultraviolet-Induced C–Cl Bond Dissociation of Polystyrene Derivatives. C 2016, 2, 20. https://doi.org/10.3390/c2030020
Takada T, Baba T, Abe S. Simple Process for Sidewall Modification of Multi-Walled Carbon Nanotubes with Polymer Side Chain Radicals Generated by Ultraviolet-Induced C–Cl Bond Dissociation of Polystyrene Derivatives. C. 2016; 2(3):20. https://doi.org/10.3390/c2030020
Chicago/Turabian StyleTakada, Tomoya, Takuma Baba, and Shigeaki Abe. 2016. "Simple Process for Sidewall Modification of Multi-Walled Carbon Nanotubes with Polymer Side Chain Radicals Generated by Ultraviolet-Induced C–Cl Bond Dissociation of Polystyrene Derivatives" C 2, no. 3: 20. https://doi.org/10.3390/c2030020





