Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites
Abstract
:1. Introduction
2. Results and Discussion
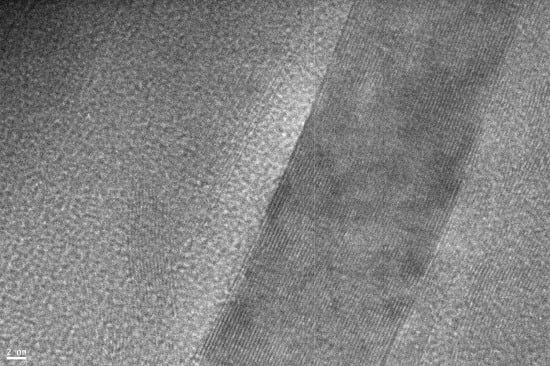
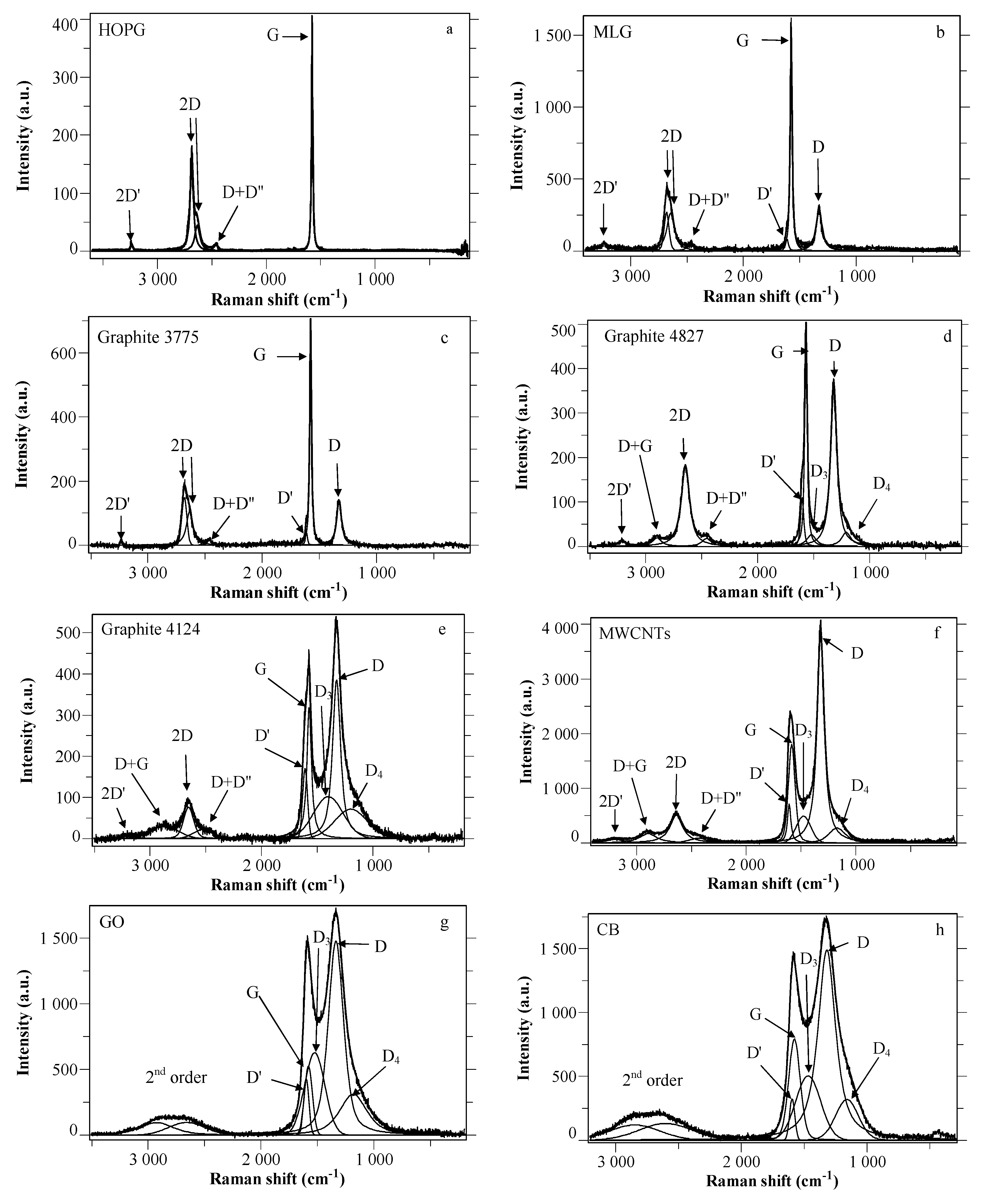
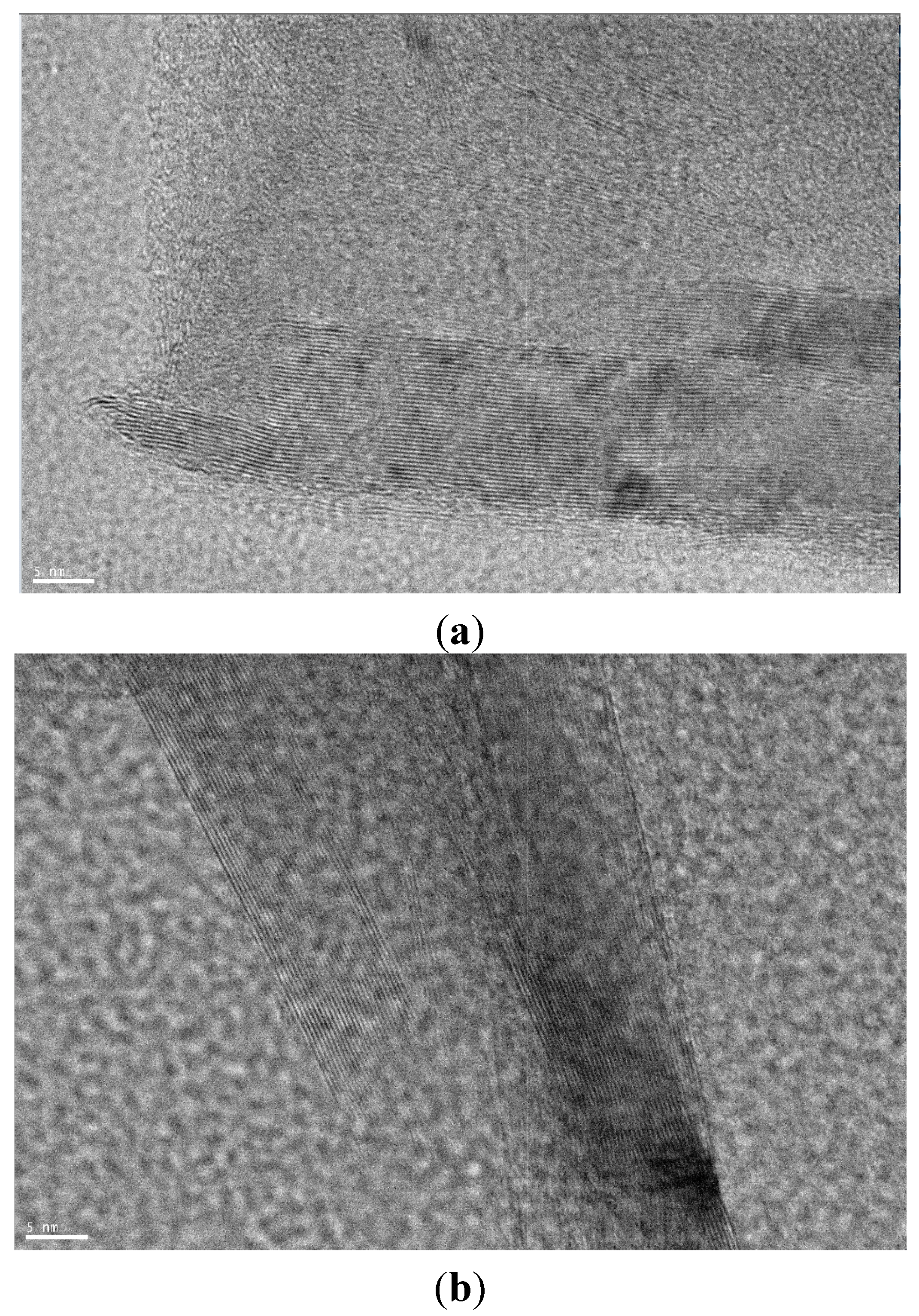
2.1. Raman Spectra of Carbon Species: An Overview
| Sample | λ = 457 nm | λ = 515 nm | λ = 515 nm | λ = 752 nm | ||||
|---|---|---|---|---|---|---|---|---|
| D (cm−1) | G’ (cm−1) | D (cm−1) | G’ (cm−1) | D (cm−1) | G’ (cm−1) | D (cm−1) | G’ (cm−1) | |
| HOPG | * | 2755 | * | 2727 | * | 2687 | * | 2656 |
| MLG | 1364 | 2750 | 1351 | 2718 | 1333 | 2677 | 1322 | 2647 |
| Graphite 3775 | 1365 | 2747 | 1353 | 2725 | 1333 | 2681 | 1314 | — |
| Graphite 4827 | 1358 | 2728 | 1349 | 2697 | 1324 | 2650 | 1313 | — |
| Graphite 4124 | 1358 | 2722 | 1346 | 2694 | 1329 | 2658 | 1311 | — |
| MWCNTs | 1360 | 2716 | 1349 | 2693 | 1325 | 2639 | 1307 | 2614 |
| GO | 1369 | — | 1361 | — | 1337 | — | 1325 | — |
| CB | 1375 | — | 1355 | — | 1332 | — | 1312 | — |
- (i)
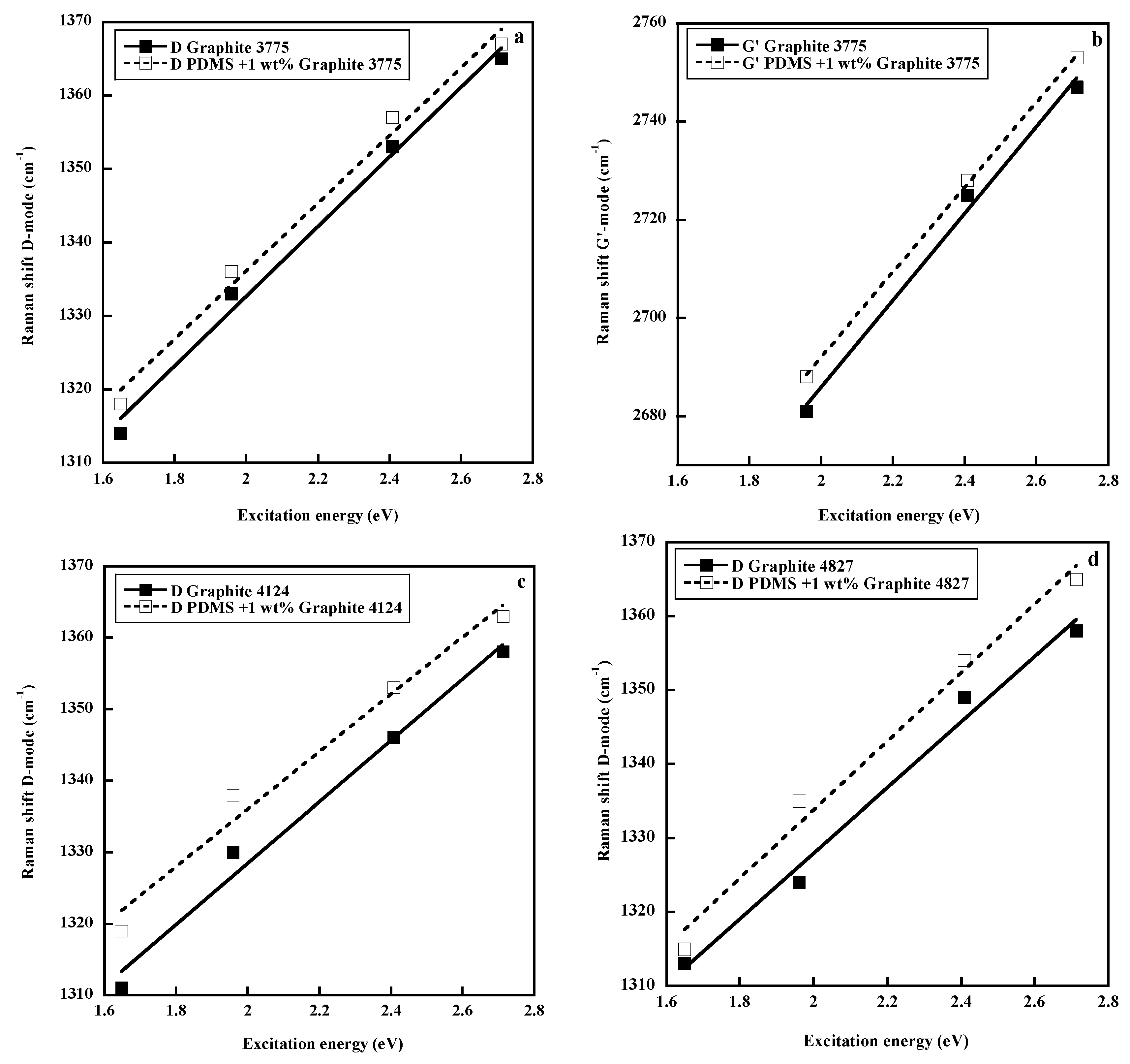
- Intensity changes: As the efficiency of the defect-induced double resonance process diminishes with increasing excitation energy, the intensities of the disorder induced first and second order Raman bands diminish relative to that of the G band. In particular, the ID/IG ratio diminishes from red to blue excitation wavelengths, so that an adaptation of the Tuinstra and Koenig relation has been proposed [46].
- (ii)
- Frequency changes: As already explained, the dispersion of the frequencies of disorder induced modes follows the slope of the corresponding phonon branches [55]. Accordingly, no dispersion is observed for the D’ and 2D’ bands (flat LO branch), blue shifts are observed for the D, 2D(G’) and D + G bands (negative slope of the TO branch) and a red shift is observed for the D + D” band (the positive slope of the LA branch prevails over the negative slope of the TO branch [55]). No clear conclusion can be drawn for the D3 and D4 bands.
2.2. Raman Spectra of Silicone Composites


3. Experimental Section
3.1. Materials
3.2. Synthesis of Silicone Rubber Composites
3.3. Raman Spectra
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rigbi, Z. Reinforcement of rubber by carbon black. Adv. Polym. Sci. 1980, 36, 21–68. [Google Scholar]
- Donnet, J.-B.; Vidal, A. Carbon black: Surface properties and interactions with elastomers. Adv. Polym. Sci. 1986, 76, 103–127. [Google Scholar]
- Donnet, J.-B. Black and white fillers and tire compounds. Rubber Chem. Technol. 1998, 71, 323–341. [Google Scholar] [CrossRef]
- Voet, A. Reinforcement of elastomers by fillers: Review of period 1967–1976. J. Polym. Sci. Macromol. Rev. 1980, 15, 327–373. [Google Scholar] [CrossRef]
- Bokobza, L. Elastomeric composites based on nanospherical particles and carbon nanotubes: A comparative study. Rubber Chem. Technol. 2013, 86, 423–448. [Google Scholar] [CrossRef]
- Maiti, M.; Bhattacharya, M.; Bhowmick, A.K. Elastomer nanocomposites. Rubber Chem. Technol. 2008, 81, 384–469. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Schön, F.; Gronski, W. Filler networking of silica and organoclay in Rubber composites: Reinforcement and dynamic-mechanical properties. Kautsch. Gummi Kunstst. 2003, 56, 166–171. [Google Scholar]
- Ramorino, G.; Bignotti, F.; Pandini, S.; Riccò, T. Mechanical Reinforcement in natural rubber/organoclay nanocomposites. Compos. Sci. Technol. 2009, 69, 1206–1211. [Google Scholar] [CrossRef]
- Galimberti, M.; Coombs, M.; Cipolletti, V.; Riccio, P.; Riccò, T.; Pandini, S.; Conzatti, L. Enhancement of mechanical reinforcement due to hybrid filler networking promoted by an organoclay in hydrocarbon-based nanocomposites. Appl. Clay Sci. 2012, 65–66, 57–66. [Google Scholar] [CrossRef]
- Galimberti, M.; Coombs, M.; Riccio, P.; Riccò, T.; Passera, S.; Pandini, S.; Conzatti, L.; Ravasio, A.; Tritto, I. The role of CNTs in promoting hybrid filler networking and synergism with carbon black in the mechanical behavior of filled polyisoprene. Macromol. Mater. Eng. 2013, 298, 241–251. [Google Scholar] [CrossRef]
- Bokobza, L. Enhanced electrical and mechanical properties of multiwall carbon nanotube rubber composites. Polym. Adv. Technol. 2012, 23, 1543–1549. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Bokobza, L. Multiwall carbon nanotube elastomeric composites: A review. Polymer 2007, 48, 4907–4920. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene-based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Processing-property relationships of polycarbonate/graphene composites. Polymer 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Liao, K.-H.; Qian, Y.; Macosko, C. Ultralow percolation graphene/polyurethane acrylate nanocomposites. Polymer 2012, 53, 3756–3761. [Google Scholar] [CrossRef]
- Song, W.-L.; Veca, L.M.; Kong, C.Y.; Ghose, S.; Connell, J.W.; Wang, P.; Cao, L.; Lin, Y.; Meziani, M.J.; Qian, H.; et al. Polymeric nanocomposites with graphene sheets—Materials and device for superior thermal transport properties. Polymer 2012, 53, 3910–3916. [Google Scholar] [CrossRef]
- Scognamillo, S.; Gioffredi, E.; Piccini, M.; Lazzari, M.; Alzari, V.; Nuvoli, D.; Sanna, R.; Piga, D.; Malucelli, G.; Mariani, A. Synthesis and characterization of thermoplastic polyurethane with both graphene and graphene nanoribbon fillers. Polymer 2012, 53, 4019–4024. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chen, S.; Zhu, M. Graphene reinforced ultra high molecular weight polyethylene with improved tensile strength and creep resistance properties. Express Polym. Lett. 2014, 8, 74–84. [Google Scholar] [CrossRef]
- Syurik, J.; Alyabyeva, N.; Alekseev, A.; Ageev, O.A. AFM-based model of percolation in graphene-based nanocomposites. Compos. Sci. Technol. 2014, 95, 38–43. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, L.; Liu, J.; Cheng, J.; Luo, J.; Liang, Y.; Tao, Y.; Wang, X.; Zhao, J. Enhanced dispersion of carbon nanotube in silicone rubber assisted by graphene. Polymer 2012, 53, 3378–3385. [Google Scholar] [CrossRef]
- Hernández, M.; del Mar Bernal, M.; Verdejo, R.; Ezquerra, T.A.; López-Manchado, M.A. Overall performance of natural rubber/graphene nanocomposites. Compos. Sci. Technol. 2012, 73, 40–46. [Google Scholar] [CrossRef]
- Araby, S.; Zaman, I.; Meng, Q.; Kawashima, N.; Michelmore, A.; Kuan, H.-C.; Majewski, P.; Ma, J.; Zhang, L. Melt compounding with graphene to develop functional, high performance elastomers. Nanotechnology 2013, 24. [Google Scholar] [CrossRef] [PubMed]
- Araby, S.; Zhang, L.; Kuan, H.-C.; Dai, J.-B.; Majewski, P.; Ma, J. A novel approach to electrically and thermally conductive elastomers using graphene. Polymer 2013, 54, 3663–3670. [Google Scholar] [CrossRef]
- Mao, Y.; Wen, S.; Chen, Y.; Zhang, F.; Panine, P.; Chan, T.W.; Zhang, L.; Liang, Y.; Liu, L. High performance graphene oxide based rubber composites. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Stanier, D.C.; Patil, A.J.; Sriwong, C.; Rahatekar, S.S.; Ciambella, J. The reinforcement effect of exfoliated graphene oxide nanoplatelets on the mechanical and viscoelastic properties of natural rubber. Compos. Sci. Technol. 2014, 95, 59–66. [Google Scholar] [CrossRef]
- Bokobza, L.; Zhang, J. Raman spectroscopic characterization of multiwall carbon nanotubes and of composites. Express Polym. Lett. 2012, 6, 601–608. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectroscopic investigations of carbon-based materials and their composites. Comparison between carbon nanotubes and carbon black. Chem. Phys. Lett. 2013, 590, 153–159. [Google Scholar] [CrossRef]
- Bokobza, L.; Garnaud, G.; Beaunier, P.; Bruneel, J.-L. Vibrational and electrical investigations of a unuaxially stretched polystyrene/carbon nanotube composite. Vib. Spectrosc. 2013, 67, 6–13. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectroscopy as a tool for the analysis of carbon-based materials (highly oriented pyrolitic graphite, multilayer graphene and multiwall carbon nanotubes) and of some of their elastomeric composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Bokobza, L. Some issues in rubber nanocomposites: New opportunities for silicone materials. Viewpoint. Silicon 2009, 1, 141–145. [Google Scholar] [CrossRef]
- Bokobza, L.; Rahmani, M. Carbon nanotubes: Exceptional reinforcing fillers for silicon rubbers. Kautsch. Gummi Kunstst. 2009, 62, 112–117. [Google Scholar]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Jorio, A. Raman spectroscopy in graphene-based systems: Prototypes for nanoscience and nanometrology. Int. Sch. Res. Netw. 2012. [Google Scholar] [CrossRef]
- May, P.; Lazzeri, M.; Venezuela, P.; Herziger, F.; Callsen, G.; Reparaz, J.S.; Hoffmann, A.; Mauri, F.; Maultzsch, J. Signature of the two-dimensional dispersion in graphene probed by double-resonant Raman scattering. Phys. Rev. B 2013, 87. [Google Scholar] [CrossRef]
- Maultzch, J.; Reich, S.; Thomsen, C. Raman scattering in carbon nanotubes revisited. Phys. Rev. B 2002, 65. [Google Scholar] [CrossRef]
- Saito, R.; Grüneis, A.; Samsonidze, G.G.; Brar, V.W.; Dresselhaus, G.; Dresselhaus, M.S.; Jorio, A.; Cançado, L.G.; Fantini, C.; Pimenta, M.A.; et al. Double resonance Raman spectroscopy of single-wall carbon nanotubes. New J. Phys. 2003, 5. [Google Scholar] [CrossRef]
- Jorio, A.; Pimenta, M.A.; Souza Filho, A.G.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Characterizing carbon nanotube samples with resonance Raman scattering. New J. Phys. 2003, 5. [Google Scholar] [CrossRef]
- Graupner, R. Raman spectroscopy of covalently functionalized single-wall carbon nanotubes. J. Raman Spectrosc. 2007, 38, 673–683. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectra of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Matthews, M.J.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Endo, M. Origin of dispersive effects of the Raman D band in carbon materials. Phys. Rev. B 1999, 59, R6585–R6588. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman spectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Pawlyta, M.; Rouzaud, J.N.; Duber, S. Raman microspectroscopy characterization of carbon black: Spectral analysis and structural information. Carbon 2015, 84, 479–490. [Google Scholar] [CrossRef]
- Saito, A.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman scattering of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Santangelo, S.; Messina, G.; Faggio, G.; Lanza, M.; Milone, C. Evaluation of crystalline perfection degree of multi-walled carbon nanotubes: Correlations between thermal kinetic analysis and micro-Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 593–602. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, L. Graphite oxide under high pressure: A Raman spectroscopic study. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.-M.; Oh, Y.-S.; Yang, Y.-H.; Lim, Y.S.; Yoon, D.Y.; Lee, C.; Kim, J.-Y.; Ruoff, R.S. Unoxidized graphene/alimina nanocomposite: Fracture- and wear-resistance effects of graphene on alumina matrix. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Mohr, M.; Maultzsch, J.; Dobardzic, E.; Milosevic, I.; Damnjanovic, M.; Bosak, A.; Krish, M.; Thomsen, C. Phonon dispersion of graphite by inelastic x-ray scattering. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef]
- Dewimille, L.; Bresson, B.; Bokobza, L. Synthesis, structure and morphology of poly(dimethylsiloxane) networks filled with in situ generated silica particles. Polymer 2005, 46, 4135–4143. [Google Scholar] [CrossRef]
- Bokobza, L.; Diop, A.L. Reinforcement of poly(dimethylsiloxane) by sol-gel in situ generated silica and titania particles. Express Polym. Lett. 2010, 4, 355–363. [Google Scholar] [CrossRef]
- Beigbeder, A.; Linares, M.; Devalckenaere, M.; Degée, P.; Claes, M.; Beljonne, D.; Lazzaroni, R.; Dubois, P. CH-π Interactions as the driving force for silicone-based nanocomposites with exceptional properties. Adv. Mater. 2008, 20, 1003–1007. [Google Scholar] [CrossRef]
- Cooper, C.A.; Young, R.J.; Halsall, M. Investigation into the deformation of carbon nanotubes and their composites through the use of Raman spectroscopy. Compos. A 2001, 32, 401–411. [Google Scholar] [CrossRef]
- Frogley, M.D.; Ravich, D.; Wagner, H.D. Mechanical properties of carbon nanoparticle-reinforced elastomers. Compos. Sci. Technol. 2003, 63, 1647–1654. [Google Scholar] [CrossRef]
- Baskaran, D.; Mays, J.W.; Bratcher, M.S. Noncovalent and nonspecific molecular interactions of polymers with multiwalled carbon nanotubes. Chem. Mater. 2005, 17, 3389–3397. [Google Scholar] [CrossRef]
- Del Corro, E.; Otero de la Roza, A.; Taravillo, M.; Baonza, V. Raman modes and Grüneisen parameters of graphite under compressive biaxial stress. Carbon 2012, 50, 4600–4606. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Young, R.J.; Kinloch, I.A. Raman spectra and mechanical properties of graphene/polypropylene nanocomposites. Int. J. Chem. Eng. Appl. 2015, 6, 1–5. [Google Scholar]
- Guimont, A.; Beyou, E.; Martin, G.; Sontag, P.; Cassagnau, P. Viscoelasticity of graphite oxide-based suspensions in PDMS. Macromolecules 2011, 44, 3893–3900. [Google Scholar] [CrossRef]
- Niu, R.; Gong, J.; Xu, D.; Tang, T.; Sun, Z.-Y. Influence of molecular weight of polymer matrix on the structure and rheological properties of graphene oxide/polydimethylsiloxane composites. Polymer 2014, 55, 5445–5453. [Google Scholar] [CrossRef]
- Huang, F.; Yue, K.T.; Tan, P.; Zhang, S.-L.; Shi, Z.; Zhou, X.; Gu, Z. Temperature dependence of the Raman spectra of carbon nanotubes. J. Appl. Phys. 1998, 84, 4022–4024. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C 2015, 1, 77-94. https://doi.org/10.3390/c1010077
Bokobza L, Bruneel J-L, Couzi M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C. 2015; 1(1):77-94. https://doi.org/10.3390/c1010077
Chicago/Turabian StyleBokobza, Liliane, Jean-Luc Bruneel, and Michel Couzi. 2015. "Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites" C 1, no. 1: 77-94. https://doi.org/10.3390/c1010077